Abstract
Permacol™ biological implant is a cross-linked porcine dermal collagen biomaterial that has been successfully used in abdominal wall repair. The purpose of this study is to examine biopsies of the implant after use in abdominal wall hernia repair and demonstrate its histological and immunohistochemical characteristics in vivo. Three male patients, ranging in age from 61 to 76 years, underwent biopsy of the implant and surrounding abdominal wall tissue at 16, 36, and 60 months after implantation. At 16 months, the implant demonstrated excellent tissue integration, cellular and neovascular response, and remodeling. The implant–host tissue interface showed seamless integration with no fibrosis. Tissue ingrowth into the material, new blood vessel formation, and remodeling of the host–tissue interface were observed. At approximately 36 months, organized remodeling of the implant into functional, predominately host tissue was generally seen. At more than 60 months, macroscopic and histological characteristics resembled functional human tissue with immunohistochemical evidence of the residual presence of porcine collagen. Permacol™ implant supports controlled and natural long-term remodeling into normal, functional host tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acellular collagen-based implants appear to offer distinct advantages over traditional methods in the repair of hernias, especially in complicated clinical scenarios of direct apposition with bowel or contaminated areas (Hiles and Record Ritchie 2009; Shaikh et al. 2007). These implants may allow host tissue regeneration and restoration of function without the intense inflammatory response that characterizes the use of a permanent synthetic mesh (Hammond et al. 2008a; Saettele et al. 2007). However, a variety of limitations of biologic materials have been surmised from human clinical trials, such as restrictions on implant placement technique (Jin et al. 2007), abandonment of tension-free hernia repair principles (Kolker et al. 2005), concerns regarding allograft harvesting methods (Gupta et al. 2006), and xenograft processing techniques (Chavarriaga et al. 2010). While these mixed results are likely due in part to differences in the available materials, such as biological source and processing methods, the optimal biologic implant characteristics and methods of processing remain in dispute.
Evidence suggests that cross-linking of collagen-based implants by means of a variety of methods can be beneficial, and that the specific clinical advantages may depend on a complex interaction of such factors as the intended surgical application, the source material, cross-linking technique, and other implant processing methods (Billiar et al. 2001; Gaertner and Bonsack 2007; Kemp and Cavallaro 1995). Despite claims that inferences as to clinical performance can be drawn directly from broad characterizations of implant source and processing (Sandor et al. 2008), ultimately the success of a particular implant in achieving a specific clinical result is determined by long-term clinical outcomes in humans. However, save for further health problems, which would require surgical intervention in the area of implantation, implant-related biopsies are both rare and of extreme importance for understanding the remodeling process and performance of biological implants in this type of clinical setting.
Permacol™ biologic implant (Covidien plc, Mansfield, MA, USA) is a hexamethylene diisocyanate cross-linked porcine dermal collagen device that has been successfully used in clinical series of abdominal wall repair and reinforcement (Shaikh et al. 2007; Catena et al. 2007; Cobb 2005; Hammond et al. 2008b; Hsu et al. 2009; O’Brien et al. 2011; Parker et al. 2006; Chand et al. 2014). However, more data are needed to characterize the sequence of physiological events that leads to long-term integration of the implant and support of the repaired tissue in humans. The purpose of this study is to examine the histological and immunohistochemical properties of Permacol™ implant and surrounding native tissue after use in abdominal hernia repair. The opportunity to biopsy any implant to allow study of its biological interaction with human tissues is rare. In the cases reported here, patients underwent further surgical procedures and biopsy with consent was possible without hazard.
Materials and methods
Explicit informed consent to biopsy the site of previous repair was obtained from each patient by the authors.
Case report 1
Explant from the anterior abdominal wall of a 76-year-old male patient, 16 months post-implantation. The patient had previously undergone panproctocolectomy for ulcerative colitis 20 years before. He underwent elective repair of a symptomatic midline incisional hernia by open, preperitoneal placement of synthetic mesh. Some 16 months before, an elective repair of a symptomatic recurrent paraileostomy hernia had been performed placing an intraperitoneal sheet of Permacol™ as reinforcement. This 10 × 10 cm sheet was 1.5 mm thick and the ileum had been brought through a central cruciate opening. The Permacol™ implant had been fixed in place with non-absorbable sutures. The edge of the ileostomy repair site was adjacent to the incisional hernia repair, and identifiable by a thickening in the tissues and the sutures. A biopsy was taken.
Case report 2
Explant from a 63-year-old male patient, 36 months post-implantation. This patient had had nine procedures for recurrent abdominal wall herniation after a childhood appendectomy complicated by peritonitis and later adhesive obstruction. A Permacol™ implant (28 × 18 cm size; 1.5 mm thick) was used in an intraperitoneal plane as a sublay and the rectus sheath closed. This healed without complication. Three years later, he developed a suddenly painful lower abdominal swelling shown on CT scan to be fluid deep to the Permacol™ repair. As 3 years had elapsed, it was felt to be a new, unconnected event perhaps traumatic in origin. This area was explored through the lower portion of the repair and found to be a resolving hematoma with serous fluid and organized fibrinous material. The fluid was drained and a biopsy of the previous repair taken. The biopsy consisted of a thick sample based on a non-absorbable polypropylene suture used to secure the implant 3 years before. The midline repair remained sound.
Case report 3
Explant from a 61-year-old male patient, 60 months post-implantation. This patient had an emergency subtotal colectomy for perforated toxic megacolon complicating ulcerative colitis. A large incisional hernia developed. An elective repair of this hernia with an onlay of Permacol™ biologic implant was performed at the same time as completion proctectomy and ileoanal pouch formation with temporary defunctioning loop ileostomy, which was closed later. Five years after the proctectomy, the patient developed adhesive small bowel obstruction with extensive intra-abdominal interloop fibrous bands. At conclusion of the laparotomy and adhesiolysis, a 5-mm full-thickness biopsy of the abdominal wall was taken prior to closure. Midline closure required a tapercut needle and was tougher than a non-reinforced wall closure.
Histology
Biopsies were fixed in a 10 % neutral buffered formalin solution. Blocks of tissue were taken from the fixed samples and were processed to paraffin wax embedding by routine automated procedures.
Two 5-μm sections were cut from each block in a transverse orientation. One section was stained with hematoxylin and eosin (H&E) and the other with a modified Picro/Miller elastin stain for collagen and elastin identification. Sections were visualized using an Olympus BX40 microscope (Olympus Optical Co., Ltd., London, UK) with a CCD color Olympus DP70 digital camera. Samples were examined for implant presence, inflammatory and immune responses, cellular penetration, cellular density, vascularization, collagen and elastin presence and configuration, tissue/implant integration, and collagen degradation.
Immunohistochemistry
Anti-human collagen type I (AbD Serotec, UK) and anti-porcine collagen type I (Novotec, France) antibodies were used to identify species-specific collagen by immunohistochemistry. Antigens within the sample were retrieved by two different methods depending on the antibody used: 1-h incubation with 0.5 % hyaluronidase at room temperature (anti-porcine) and 2-h pepsin incubation (1 mg/mL) at 37 °C (anti-human). Endogenous peroxidase activity was blocked with 3 % hydrogen peroxide for 30 min and non-specific stain was evaded by blocking the tissue with 2.5 % horse serum for 1 h. Tissue sections were incubated with primary antibodies overnight at 4 °C. ImmPress Kit reagent (Daco Laboratories, UK) against the IgG of the animal species in which the primary antibody has been raised was used to amplify the signal and DAB was the substrate chosen to develop the final colorimetric product (positive signal).
Results
Three tissue samples of explanted Permacol™ biological implant were available for examination. Macroscopically, the implants appeared completely integrated with the host tissue; all biopsy specimens were identified by non-absorbable sutures securing the implant.
Case report 1
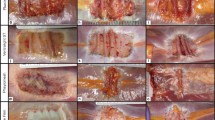
The interface between the implanted Permacol™ and the native collagen was recognizable in some places, and tissue integration between these two elements appeared to be excellent (Fig. 1). After being implanted for 16 months, Permacol™’s collagen fibers were remodeled and showed the same structural orientation as the collagen fibers from the host tissue. Probably as a consequence of the collagen remodeling, immunohistochemical analysis did not stain for porcine collagen but human collagen type I was identified throughout the whole sample.
There was no evidence of inflammatory or immune responses. There was equal vascularity in both host and implanted material, and the integration was such that, in places, it was difficult to define the junction between the two (Fig. 2). Degradation of the implant or of the adjacent native collagen was not observed. Picro Sirius red stain showed all of the implant together with the host collagen to be of appropriately good condition with no denaturation or mineralization. Neo-collagenesis was observed within the implant and all collagen present was not denatured and appeared to be of appropriately good scar quality. Elastin fibers were absent from the tissue samples examined.
Case report 2
The Permacol™ explant was taken from the abdominal wall during drainage of an organized hematoma beneath the implant. The tissue shows a well vascularized extracellular matrix (ECM) and all tissue is heavily populated with cells, mainly fibroblasts (Fig. 3). As a result, neo-collagenesis is frequently observed, a normal feature for hernia-repair surgical site. Independent of the neo-collagen observed, mature collagen showed natural birefringence, an indication of good-quality collagen (Fig. 4).
Occasionally, focal areas show remains of a chronic inflammatory response, where lymphocytes, eosinophils, and plasma cells are observed. Macrophages are visible in one localized area but at low numbers (Fig. 5). It is not clear if this is a reaction to the tissue or if the presence of these cells is related to the organized hematoma. Nevertheless, the majority of the tissue does not show evidence of a foreign body type reaction.
Histological analysis of the tissue from the Permacol™ repair site showed no evidence of the surgical implant but instead a well-populated normal collagen structure similar to surrounding tissue was observed. Permacol™ implant was not identified by immunohistochemistry specific to porcine type I collagen; however, the implant did stain positive for the presence of human type I collagen (Fig. 6). Porcine collagen was used as control to confirm that the anti-human collagen type I antibody was not cross-reacting with porcine collagen. Elastin fibers and elastin bundles were occasionally observed within the implant (Fig. 7).
Case report 3
Macroscopically, it was not possible to identify the Permacol™ implant within the biopsy. Histopathology showed a well-vascularized and cell-populated ECM (Fig. 8). Neo-collagenesis and elastogenesis were both present.
As observed in the other case reports, the implant was not easily identified, although some collagen fibers differentiated from the surrounding tissue for its thickness and were structurally similar to Permacol™ in one localized area of the sample. An immunohistochemical stain for anti-porcine collagen was performed, and within the collagenous matrix some fibers stained positively for porcine collagen type I suggesting that these fibers were part of the original implant (Fig. 9). The remaining sample did not stain for porcine collagen but stained for human collagen.
Lymphocytes were present in some localized areas, mainly in the vicinity of vessels. The observed lymphocytes and some inflammatory cells were localized away from the collagen matrix where the porcine fibers were found. Tissue reaction was restricted to one area in one extremity of the biopsy.
Discussion
The ideal prosthetic mesh would be biocompatible, resistant to infection, induce low inflammatory and low immune reactions, be able to withstand physiologic mechanical stresses and strains, be capable of restoring normal tissue functionality, withstand sterility processes, provide tissue bulk where required, be long lasting yet able to be removed if necessary, allow host tissue ingrowth with good aesthetic results, offer a rapid recovery time, and be accessible and non-expensive (Matthews et al. 2003). In the search for biologic reconstructive materials, it is sometimes assumed that a graft that provokes early and profuse neovascularization and tissue ingrowth will invariably lead to successful functional outcomes as host tissue rapidly replaces the implant. Alternatively, in certain soft tissue augmentation procedures, resistance to rapid resorption is desired (Sclafani et al. 2001). However, in abdominal wall repair and many other reconstructive procedures, where long-term strength of the repair is critical, controlled biointegration and remodeling over time may be more appropriate.
Permacol™ biological implant
Porcine dermal collagen implants stabilized against early enzymatic degradation by means of cross-linking have been advocated for use in various reconstructive applications (Abraham et al. 2000; Konstantinovic et al. 2010). Outcomes associated with the use of HMDI cross-linked porcine dermal collagen (Permacol™ implant) in a range of procedures have been generally favorable, with imaging-diagnostic confirmed results in one rotator cuff repair study with follow-up of nearly 5 years (Badhe et al. 2008). Hsu et al. examined the role of Permacol™ implant in abdominal wall reconstruction in 28 patients, documenting recurrent hernia in 3 of 28 patients on CT examination at a mean follow-up of 16 months (Hsu et al. 2009). Two additional independent studies followed a total of 29 patients in large acute and chronic abdominal wall defects for 18 months in each study, with hernia recurrence in four patients, three of which occurred within 6 months postoperatively in patients who had a complicated surgical history (Shaikh et al. 2007; Parker et al. 2006). The authors concluded that the medium-term recurrence rate was comparable to synthetic mesh repairs. Recently, a multinational retrospective study evaluated the use of Permacol™ surgical implant on 343 patients in the repair of 213 incisional and 130 ventral hernias (Chand et al. 2014). The study stated Permacol™ implant to be safe with relatively low rates of hernia recurrence, and only one patient (0.3 %) needed mesh removal.
Previous research in both animals and humans generally demonstrates that Permacol™ implant integrates with the abdominal wall in a gradual, organized fashion with minimal associated inflammatory response (Hammond et al. 2008a; Gaertner and Bonsack 2007; O’Brien et al. 2011; Macleod et al. 2005; de Castro Bras and Shurey 2012). This characteristic of Permacol™ implant in vivo is desirable in abdominal wall reconstruction, where proximity to viscera and frequently compromised wound conditions are common. O’Brien et al. report that at 24 months, Permacol™ demonstrated durability with excellent integration, vascular ingrowth and remodeling with human collagen, and elastin deposition in the implant (O’Brien et al. 2011).
In light of these studies and the present results, the early deposition of healthy host collagen, eventually leading to a fully integrated implant and potential long-term replacement by native tissue, must be sharply distinguished from encapsulation of the device. Encapsulation is classically associated with a persistent foreign body response and chronic sequestration of the implant that tends to become more severe as the duration of implantation increases (Bellon et al. 1996; Formichi et al. 1988). Clinical evidence with a carbodiimide cross-linked porcine dermal collagen device that is processed using methods that diverge from those used in producing Permacol™ implant demonstrates that encapsulation of abdominal wall implants frequently leads to postoperative wound complications and infections requiring graft removal (Chavarriaga et al. 2010). In the case of Permacol™ implant, however, the wound complications tend to be within expectations and the device can often be salvaged using topical wound care techniques (Parker et al. 2006; Chave et al. 2006).
Findings of the current study
In the current study, progressive graft incorporation and remodeling was observed in samples explanted at 16, 36, and 60 months, consistent with a gradual, controlled process of biointegration, neovascularization, and restoration of functional tissue. Histopathology showed that Permacol™ implanted for 16 months in a challenging ventral peritoneal wall position (case report 1) integrated very well with host collagen. There was equivalent vascularity in both host and implanted material, and the integration was such that, in places, it was difficult to define the interface between the two. There was no evidence of inflammatory or other detrimental activity. There was neo-collagenesis within the implanted material and remodeling of the Permacol™ but not such that it was unrecognizable as implant material. Picro Sirius red/Millers elastin stain showed all of the implant together with the host collagen to be non-denatured.
At 36 months after clinical repair of a recurrent midline hernia, Permacol™ biological implant was remodeled and well integrated with the host tissue (case report 2). Occasionally, localized inflammatory cells were present around blood vessels, but these may result from the nearby organized hematoma and not as a response to the implant. The presence of high amounts of neo-collagen suggested good healing of the wound and integration with the surrounding tissue. The tissue was well vascularized and well populated by fibroblasts. No bulging or diastasis of muscle was observed. Overall, the histology of the Permacol™ repair site was indicative of ordered and controlled integration and remodeling of the Permacol™ collagen into functional, predominantly host tissue.
After 60 months post-implantation, immunohistochemical analysis of the Permacol™ biological implant sample (case report 3) showed the presence of a small number of collagen fibers of porcine origin, suggesting that in this particular clinical situation the implant was gradually remodeled by the host tissue and, although at a low level, it was still present. This result gives evidence that Permacol™ biological implant can be used in challenging situations when a resilient material is needed.
Overall, the results reported in this manuscript suggest that Permacol™ implant remains macroscopically recognizable at 16 months, and tends to remodel into healthy native tissue by 36 months, with occasional porcine fibers still evident at 60 months. The inability to detect porcine collagen using specific antibodies in the samples at 16 and 36 months may be due to factors such as specimen fixation techniques. Prolonged preservation of the specimen in formalin, for instance, may mask the presence of such antigens. However, the finding of human collagen staining in all biopsies, together with the general absence of a recognizable implant at 36 and 60 months, suggests that remodeling of the implant in later months and years leads to replacement of porcine collagen by functional host connective tissue.
Published human abdominal wall experience
The histological observations made here may be instructively compared to earlier histological and immunohistochemical findings, at 1 to 24 months post-implantation (Hammond et al. 2008a; O’Brien et al. 2011). Hammond et al. (2008a) investigated the potential role of Permacol™ implant in reinforcing the connective tissues of the abdominal wall surrounding a temporary ileostomy site defect. In 10 of 11 biopsies, taken at stoma reversal at a median of 7 months (range 1–8 months), a clearly defined interface between the implant and host tissue was observed, with ordered deposition of host collagen parallel to the implant surface. Only limited penetration of new collagen formation and neovascularization was observed within the implant itself; however, fibroblast integration and proliferation into the native pores of the implant, along with synthesis of ECM-associated proteins such as fibronectin and laminin, seemed to be more pronounced in biopsies taken in later months. Although the population in the stoma reversal study was free of abdominal hernias at the time of implantation in all cases, and subsequently in all but one case, the finding of a well-integrated implant with organized collagen fibers staining positively for human collagen is consistent with an early stage of remodeling, in which the implant remains virtually intact in most cases. Data from O’Brien et al. (2011) provide a glimpse into the histological and immunohistochemical characteristics of Permacol™ after biopsy from the abdominal wall at 24 months, a time period that is not represented in the current study. Researchers found that the implant was still easily recognizable, but that human collagen types I and III and elastin were present throughout the explanted tissue, suggesting that a remodeling process was well underway in which the porcine collagen was being invaded and replaced by human collagen.
Performance in adverse wound conditions
In the present investigation, the wound from which the biopsy was taken at 36 months was associated with a concurrent fluid collection that required drainage. Porcine dermal collagen appears only infrequently associated with mesh removal caused by persistent postoperative seromas, in contrast to microporous synthetic mesh (Kuo et al. 2010). In the case at hand, the surgeon did not believe the implant to be a causative factor in development of the fluid collection and examination of the explanted tissue revealed no generalized foreign body response. Furthermore, healthy human collagen was observed at the implant site. The fluid collection in this case was unusual, presenting so late after surgery and being only deep to the implant. It contained fibrinous material and could have been a hematoma caused by local wall tear or trauma. Drainage stopped the pain and no re-accumulation occurred. A reaction to the implant would not have been so easily resolved. Seroma formation was reported with the use of eight-ply porcine small intestinal submucosa (SIS) in a series of ventral hernia repairs, prompting surgical treatment within approximately 6 months after the initial surgery in 17 of 41 total patients (Gupta et al. 2006). Histological analysis of explanted SIS material showed delamination of the outer layers of the device, and an acute and chronic inflammatory response. In the same study, human acellular dermal matrix was used in a subsequent series of ventral hernia repairs. Wound exploration during revision of recurrences revealed incomplete incorporation of the mesh, while 45 % of patients presented with abdominal bulging (Gupta et al. 2006). Despite the presence of the seroma in the present study at 36 months, no muscle diastasis in the explanted tissue was noted.
Reinforcement of the abdominal wall in each of these challenging cases required a material either in direct contact with the bowel, in a potentially contaminated area, or both. Biological meshes such as Permacol™ are suitable in this context providing durability of strength retention, resistance to infection, minimal intraperitoneal adhesion and fistulation, flexibility, and the opportunity to repair abdominal wall function. The histopathological analysis showed that Permacol™, implanted in challenging conditions, integrated very well with the native tissue and underwent a gradual remodeling process, leading to stabilization of the structural proteins present in the ECM which allowed healing and strengthening of the wound. Cross-linking of the collagen provides resistance to collagenases allowing incorporation before absorption of the implant and clinically relevant longevity of the repair, and in these cases, showed no tendency for encapsulation. At 16 months, the implant was undergoing a process of remodeling that included progressive deposition of new collagen, while by 36 and 60 months, the implant was almost completely replaced by functional human tissue. Clinical results with comparable long-term follow-up are needed to further delineate the role of Permacol™ implant in abdominal wall repair.
References
Abraham GA, Murray J, Billiar K et al (2000) Evaluation of the porcine intestinal collagen layer as a biomaterial. J Biomed Mater Res 51(3):442–452
Badhe SP, Lawrence TM, Smith FD et al (2008) An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Should Elb Surg 17(1 Suppl):35S–39S
Bellon JM, Bujan J, Contreras LA et al (1996) Similarity in behavior of polytetrafluoroethylene (ePTFE) prostheses implanted into different interfaces. J Biomed Mater Res 31(1):1–9
Billiar K, Murray J, Laude D et al (2001) Effects of carbodiimide crosslinking conditions on the physical properties of laminated intestinal submucosa. J Biomed Mater Res 56(1):101–108
Catena F, Ansaloni L, Gazzotti F et al (2007) Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia 11(1):57–60
Chand B, Indeck M, Needleman B et al (2014) A retrospective study evaluating the use of Permacol surgical implant in incisional and ventral hernia repair. Int J Surg S1743–9191(14):00037–00045
Chavarriaga LF, Lin E, Losken A et al (2010) Management of complex abdominal wall defects using acellular porcine dermal collagen. Am Surg 76(1):96–100
Chave H, Ahmed S, Fu B et al (2006) Salvage of infected dermal collagen implants with topical negative pressure therapy. J Wound Care 15(4):156–158
Cobb GA, Shaffer J (2005) Cross-linked acellular porcine dermal collagen implant in laparoscopic ventral hernia repair: case-controlled study of operative variables and early complications. Int Surg 90(3 Suppl):S24–29
de Castro Bras LE, Shurey S, Sibbons PD (2012) Evaluation of crosslinked and non-crosslinked biologic prostheses for abdominal hernia repair. Hernia 16(1):77–89
Formichi MJ, Guidoin RG, Jausseran JM et al (1988) Expanded PTFE prostheses as arterial substitutes in humans: late pathological findings in 73 excised grafts. Ann Vasc Surg 2(1):14–27
Gaertner WB, Bonsack ME, Delaney JP (2007) Experimental evaluation of four biologic prostheses for ventral hernia repair. J Gastrointest Surg 11(10):1275–1285
Gupta A, Zahriya K, Mullens PL et al (2006) Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia 10(5):419–425
Hammond TM, Chin-Aleong J, Navsaria H et al (2008a) Human in vivo cellular response to a cross-linked acellular collagen implant. Br J Surg 95(4):438–446
Hammond TM, Huang A, Prosser K et al (2008b) Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia 12(5):475–481
Hiles M, Record Ritchie RD, Altizer AM (2009) Are biologic grafts effective for hernia repair?: a systematic review of the literature. Surg Innov 16(1):26–37
Hsu PW, Salgado CJ, Kent K et al (2009) Evaluation of porcine dermal collagen (Permacol) used in abdominal wall reconstruction. J Plast Reconstr Aesthet Surg 62(11):1484–1489
Jin J, Rosen MJ, Blatnik J et al (2007) Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg 205(5):654–660
Kemp PD, Cavallaro JF, Hastings DN (1995) Effects of carbodiimide crosslinking and load environment on the remodeling of collagen scaffolds. Tissue Eng 1(1):71–79
Kolker AR, Brown DJ, Redstone JS et al (2005) Multilayer reconstruction of abdominal wall defects with acellular dermal allograft (AlloDerm) and component separation. Ann Plast Surg 55(1):36–41, discussion 41–32
Konstantinovic ML, Ozog Y, Spelzini F et al (2010) Biomechanical findings in rats undergoing fascial reconstruction with graft materials suggested as an alternative to polypropylene. Neurourol Urodyn 29(3):488–493
Kuo YC, Mondschein JI, Soulen MC et al (2010) Drainage of collections associated with hernia mesh: is it worthwhile? J Vasc Interv Radiol 21(3):362–366
Macleod TM, Williams G, Sanders R et al (2005) Histological evaluation of Permacol as a subcutaneous implant over a 20-week period in the rat model. Br J Plast Surg 58(4):518–532
Matthews BD, Pratt BL, Pollinger HS et al (2003) Assessment of adhesion formation to intra-abdominal polypropylene mesh and polytetrafluoroethylene mesh. J Surg Res 114(2):126–132
O’Brien JA, Ignotz R, Montilla R et al (2011) Long-term histologic and mechanical results of a Permacol abdominal wall explant. Hernia 15(2):211–215
Parker DM, Armstrong PJ, Frizzi JD et al (2006) Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg 63(4):255–258
Saettele TM, Bachman SL, Costello CR et al (2007) Use of porcine dermal collagen as a prosthetic mesh in a contaminated field for ventral hernia repair: a case report. Hernia 11(3):279–285
Sandor M, Xu H, Connor J et al (2008) Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A 14(12):2021–2031
Sclafani AP, Romo T 3rd, Jacono AA et al (2001) Evaluation of acellular dermal graft (AlloDerm) sheet for soft tissue augmentation: a 1-year follow-up of clinical observations and histological findings. Arch Facial Plast Surg 3(2):101–103
Shaikh FM, Giri SK, Durrani S et al (2007) Experience with porcine acellular dermal collagen implant in one-stage tension-free reconstruction of acute and chronic abdominal wall defects. World J Surg 31(10):1966–1975
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sibbons, P.D., Pullan, R.D. & de Castro Brás, L.E. Biopsies of Permacol™ implant in humans after use in abdominal wall repair: histological and immunohistochemical analysis. Comp Clin Pathol 24, 831–840 (2015). https://doi.org/10.1007/s00580-014-1990-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-014-1990-y