Abstract
Numerous biologic meshes exist for soft tissue repair applications such as hernia repair/abdominal wall reconstruction. These materials can be classified based on the species and type of tissue from which they are derived, as well as the processing that the tissue undergoes. The impact of these variables on the mechanical properties and remodeling characteristics of biologic meshes are not well understood. Recent studies have documented the baseline physical, mechanical, and thermal properties of several biologic meshes, along with in vitro studies of the impact of repetitive loading and enzyme exposure on baseline mechanical properties. Porcine models have also described the mechanical strength and host tissue response of several biologic meshes in an in vivo setting. Additionally, a recent clinical trial has documented the remodeling characteristics of several types of biologic meshes after implantation in human subjects. The results of these studies have consistently shown that the effects of crosslinking are species/tissue dependent or related to the specific chemical compounds utilized to achieve crosslinking and the number of additional bonds ultimately introduced into these tissues. Additionally, differences have been observed between non-crosslinked materials, suggesting that widespread generalizations should not be made even amongst non-crosslinked materials. Differences due to species, tissue type, and other processing conditions such as decellularization and sterilization are likely as influential as the presence or absence of intentional crosslinking and should be explored further in future studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biologic mesh
- Collagenase
- Decellularization
- Enzymatic degradation
- Extracellular matrix
- Hernia repair
- Porcine model
- Remodeling
- Repetitive loading
- Tensile strength
Current State of the Art
At least thirty types of biologic meshes exist for soft tissue repair applications such as hernia repair/abdominal wall reconstruction, breast reconstruction, wound healing, urogenital/pelvic floor reconstruction, and musculoskeletal reconstruction [1–6]. Of these, fifteen are commonly utilized for hernia repair applications and are fully described in Table 7.1. Biologic meshes are touted to possess many advantages over permanent synthetic meshes. Since biologic meshes are derived from biological tissues, these materials are eventually degraded and remodeled by the host, providing the benefit of a temporary scaffold at the repair site with low risk of long-term inflammation and fibrosis. In addition, biologic meshes can be utilized in clean-contaminated or contaminated settings where synthetic meshes may be contraindicated. It is believed that revascularization of these materials during the remodeling process effectively clears pathogens from the mesh. Despite these potential advantages, there are also some disadvantages associated with biologic mesh use, namely the high cost of these materials compared to synthetic meshes, variability in biologic mesh properties due to donor characteristics, and production of these materials in limited sizes and geometries. Furthermore, biologic meshes may be problematic for patients with religious or ethical concerns surrounding the use of human or animal tissue-derived products [7].
In the future, biologic mesh designs may expand to include antibacterial coatings to reduce or inhibit microbial colonization. This could be particularly useful in clean-contaminated or contaminated settings. One such mesh, XenMatrix™ AB Surgical Graft (C.R. Bard/Davol, Inc., Warwick, RI), has recently received 510 k approval from the FDA. This mesh is comprised of acellular porcine dermis, coated with a resorbable polymer (l-tyrosine succinate) that serves as a carrier for two antimicrobial agents, derivatives of rifamycinB and tetracycline (180 μg/cm2 each). According to the Instructions for Use (IFU), preclinical studies have demonstrated that these antimicrobial agents reduce or inhibit microbial colonization of the mesh when compared to a control mesh. However, data have not yet been acquired in human subjects.
Classification of Biologic Mesh
Biologic meshes are typically classified according to three major categories as shown in Table 7.1: (1) species of origin, (2) tissue type, and (3) processing conditions. These materials are derived from a variety of species (i.e., human, bovine, porcine, and equine) and tissue types (i.e., dermis, pericardium, and small intestine submucosa). The species and type of tissue from which a biologic mesh is derived determine the structure, composition, and mechanical properties of the resulting biologic mesh and can have important implications when implanted in human subjects. However, more attention has historically been paid to the method by which the original tissue is processed to become a biologic mesh, particularly the crosslinking process.
At a minimum, all biologic meshes undergo a decellularization process to remove cells and cellular debris, leaving behind the extracellular matrix (ECM) component of the original. This is an extremely important aspect of biologic mesh development since the recipient’s immune response is directly influenced by the efficacy of the decellularization process. Residual cellular debris can lead to an inflammatory response and should be eliminated to the extent possible without damaging the structure or composition of the ECM. Numerous decellularization techniques exist such as treatments with enzymes [8, 9], solvents [10–12], acids/bases, detergents [11–13], hypertonic/hypotonic solutions [14, 15], chelating agents [16, 17], and toxins [4]. The decellularization technique must be optimized for the species and tissue type from which the mesh is derived, and the details of the decellularization process are often withheld by the manufacturer as proprietary.
In addition to decellularization, some biologic meshes are also intentionally crosslinked through chemical treatments or dehydration. Crosslinking is typically done to improve the strength of the mesh and/or to prevent rapid degradation of the mesh in vivo. Crosslinking can be accomplished through a variety of chemicals such as carbodiimides [18–21], glutaraldehyde [22–24], or hexamethylene diisocyanate [22]. Variables such as crosslinking agent, concentration, temperature, pH, and exposure time all contribute to the number and type of new bonds that are introduced into the tissue [18, 22, 25].
Xenogeneic meshes are terminally sterilized using gamma irradiation, ethylene oxide, or e-beam treatments, while allogeneic meshes are subjected only to a final disinfection process such as ethanol or peracetic acid treatment. Inadvertent crosslinking may occur during the sterilization process, which can have unfavorable consequences, such as reducing cellular infiltration and scaffold degradation.
Preservation of the tissue for long-term packaging and storage is the final step in the processing of biologic meshes. Some are dehydrated, while others are stored in a hydrated state or even submerged in a preservation fluid. These conditions can lead to unintended disruption of the structure and composition of the ECM, which may influence the remodeling process in vivo.
In summary, there are a tremendous number of variables due to the number of species, tissue types, and processing conditions involved in the production of biologic mesh materials. Furthermore, the details of many of the processing techniques are withheld by the manufacturers as proprietary, making it even more challenging to directly compare biologic mesh products and scientifically determine the effect of a single variable. Human tissue-derived biologic meshes are also plagued by the added variables of donor age, sex, comorbidities, and anatomical location from which the tissue is procured.
Evidence-Based Critical Appraisal
Characterization of Biologic Meshes
The physical, thermal, and mechanical characteristics of twelve biologic meshes were evaluated in a recent study via laser micrometry, differential scanning calorimetry, suture retention strength testing, tear resistance testing, and ball burst testing [26]. The results were compared based on species, tissue type, and processing conditions, namely crosslinking. These tests were designed to fully characterize the pre-implantation properties of biologic meshes and to test the hypothesis that crosslinked materials possess greater pre-implantation strength than non-crosslinked materials.
The results of this study revealed a wide variety of pre-implantation characteristics between different types of biologic meshes. In contrast to the hypothesis, crosslinked meshes exhibited lower mechanical strengths than non-crosslinked meshes in all three mechanical tests performed (i.e., suture retention strength testing, tear resistance testing, and ball burst testing). This was especially true of the porcine dermis-derived meshes. The bovine pericardium-derived meshes exhibited similar mechanical strengths between the crosslinked and non-crosslinked meshes, indicating little effect of crosslinking on the mechanical characteristics of bovine pericardium tissue. It was expected that the human dermis-derived meshes would exhibit similar mechanical strengths since all are derived from the same species/type of tissue and none are crosslinked. However, the three human dermis-derived meshes exhibited a wide range of mechanical strengths. These results indicate that other factors such as donor variables (i.e., age, sex, tissue procurement site, comorbidities, etc.) or conditions during the decellularization and decontamination processes significantly influenced the resulting properties of the human dermis-derived meshes.
Repetitive Loading
A subset of biologic meshes was further evaluated in another study involving repetitive loading experiments [27]. Nine types of biologic meshes were subjected to cycles of uniaxial tensile loading, and series of 10, 100, and 1000 cycles were completed for each mesh type. It was hypothesized that crosslinked materials resist damage during repetitive loading and maintain baseline strength while non-crosslinked materials sustain damage during repetitive loading and exhibit a significant reduction in strength.
Consistent with this hypothesis, one of the crosslinked porcine dermis meshes (Permacol™ ) was significantly stronger than the non-crosslinked porcine dermis meshes (Strattice™ and XenMatrix™ ) at baseline and after 10, 100, or 1000 cycles of loading. However, the other crosslinked porcine dermis mesh (CollaMend™ ) exhibited similar results as the non-crosslinked porcine dermis meshes at baseline and after 10, 100, or 1000 cycles, indicating that the particular crosslinking agent or conditions utilized to achieve decellularization, crosslinking, and/or sterilization significantly influenced the properties of this material. Additionally, both crosslinked porcine dermis meshes (Permacol™ and CollaMend™ ) and one of the non-crosslinked porcine dermis meshes (XenMatrix™) maintained their baseline tensile strength even after exposure to repetitive loading conditions, while the other non-crosslinked porcine dermis (Strattice™ ) exhibited a significant decrease in tensile strength with increasing number of cycles. As expected, the crosslinked bovine pericardium mesh (PeriGuard®) was significantly stronger than the non-crosslinked bovine pericardium mesh (Veritas®) at baseline and after 10, 100, or 1000 cycles of loading. However, both bovine pericardium meshes maintained their baseline tensile strength even after 1000 cycles of loading, regardless of the presence of crosslinking. These results contrast those of porcine dermis-derived meshes, indicating that variables such as species, tissue type, and processing conditions may all play a role in determining the final properties of these materials. As in the previous study, wide variation was observed between the human dermis-derived meshes, pointing to donor variables, in addition to processing conditions, as particularly problematic for human tissue-derived meshes.
In general, crosslinked meshes resisted damage during repetitive loading and maintained baseline tensile strength, while non-crosslinked meshes sustained damage during repetitive loading and exhibited significant reduction in tensile strength. However, widespread generalizations should not be made, as this study demonstrated exceptions, particularly for porcine-dermis-derived products.
Resistance to Enzymatic Degradation
In another study, the same subset of biologic meshes was also exposed to collagenase enzymes in vitro in order to assess the impact of enzymatic degradation on the uniaxial tensile strength of these materials [28]. It was hypothesized that crosslinked materials resist enzymatic degradation and maintain baseline strength while non-crosslinked materials undergo enzymatic degradation and exhibit a significant reduction in strength.
Nine types of biologic mesh materials were exposed to collagenase solution at 37 °C. After 30 hours of exposure, both crosslinked and non-crosslinked porcine dermis meshes exhibited significantly reduced tensile strength compared to their respective baseline tensile strengths, indicating significant enzymatic degradation. This result was observed regardless of crosslinking. Even so, one of the crosslinked porcine dermis meshes (Permacol™) maintained significantly greater tensile strength than the two non-crosslinked porcine dermis meshes (Strattice™ and XenMatrix™) and the other crosslinked porcine dermis mesh (CollaMend™) throughout the exposure period. On the other hand, one of the non-crosslinked porcine dermis meshes (XenMatrix™) was so significantly degraded that it was difficult to measure the tensile strength of the specimens beyond 12 hours of exposure to collagenase solution.
Similarly, the non-crosslinked bovine pericardium mesh (Veritas®) exhibited significantly reduced tensile strength compared to its baseline tensile strength after just 6 hours of exposure to collagenase solution, indicating significant and rapid in vitro degradation of this particular material. However, the crosslinked bovine pericardium mesh (PeriGuard®) maintained its baseline tensile strength and did not show any evidence of degradation even after 30 hours of exposure to collagenase. The crosslinked bovine pericardium mesh (PeriGuard®) also maintained significantly greater tensile strength than its non-crosslinked counterpart (Veritas®) throughout the exposure period, as expected.
The human dermis-derived meshes displayed wide variation in baseline properties and in their ability to resist enzymatic degradation. Although the baseline tensile strength of FlexHD® was lower than that of AlloMax™, FlexHD® resisted enzymatic degradation more effectively. AlloMax™ was so significantly degraded after just 12 hours of exposure that tensile strength could not be reliably measured beyond that point.
The results of this study demonstrated that in general, crosslinking did not improve the resistance of porcine dermis-derived meshes to enzymatic degradation. However, crosslinking did significantly improve the resistance of bovine pericardium-derived meshes to enzymatic degradation . These results suggest that the effects of crosslinking may be species/tissue dependent or related to the specific chemical compounds utilized to achieve crosslinking and the number of additional bonds ultimately introduced into these tissues. Additionally, differences were observed between non-crosslinked materials, suggesting that widespread generalizations of all non-crosslinked materials should not be made. Differences due to species, tissue type, and other processing conditions appear to be extremely influential.
Porcine Model of Ventral Hernia Repair
The mechanical strength of a hernia repair site and the host tissue response to six types of biologic meshes were evaluated in several recent studies using a well-established porcine model of ventral hernia repair [29–31]. This model was designed to fully characterize the post-implantation properties of biologic meshes and to test the hypotheses that (1) crosslinked materials augment the strength of native tissue, leading to a stronger hernia repair and that (2) crosslinked materials resist degradation, thereby reducing cellular infiltration and overall tissue remodeling compared to non-crosslinked materials.
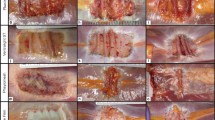
In this study, a total of four hernia defects (5 cm each) were surgically created in each animal, one in each abdominal quadrant. The musculature and fascia of the abdominal wall were incised and left open, creating the abdominal wall defects. The subcutaneous fat, areolar tissue, and skin were closed in separate layers to prevent wound dehiscence. The defects were allowed to mature for 21 days and were then repaired with biologic mesh positioned in the retromuscular/preperitoneal space. Animals were subsequently survived for 1, 6, or 12 months. Mesh-tissue composites were procured at the end of the survival period and subjected to uniaxial tensile testing to provide a measure of the biomechanics of the repair site. Specimens were also stained with hematoxylin and eosin (H&E) and semi-quantitatively assessed for six characteristics of tissue response: cellular infiltration, cell types, scaffold degradation, ECM deposition, neovascularization, and fibrosis. Possible scores in each category ranged from 0 to 3, with higher scores representing more favorable characteristics of tissue response. A composite score was also generated from the mean of the six component scores and was utilized as an overall measure of tissue remodeling.
Mechanical testing of the mesh-tissue composites revealed that all repair sites exhibited similar tensile strengths at all time points regardless of biologic mesh type or presence/absence of crosslinking. Furthermore, the strength of the native tissue of the porcine abdominal wall was not significantly augmented by any of the biologic meshes, including crosslinked materials.
Histological analysis revealed that in the short-term, crosslinking of biologic meshes impacted characteristics of tissue remodeling such as cellular infiltration and neovascularization. As shown in Fig. 7.1, non-crosslinked meshes exhibited higher scores at earlier time points than crosslinked meshes. However, at later time points, scores for crosslinked materials tended to reach levels similar to non-crosslinked materials. Thus, crosslinking did not appear to significantly influence cellular infiltration over the long-term as anticipated. Other processing conditions such as differences in decellularization and sterilization techniques may have impacted tissue remodeling characteristics more substantially and should be evaluated in future studies.
Biologic Meshes Explanted from Human Subjects
The remodeling characteristics of biologic meshes after implantation in human subjects for abdominal wall reconstruction are not well understood. Thus, two recent studies have evaluated biopsies of biologic meshes procured from human subjects during abdominal re-exploration [32, 33].
In the study by Cavallo et al., biopsies were obtained from forty human subjects [32]. Mesh type was identified in 37 out of 40 biopsies and included 23 human dermis-derived biologic meshes, 11 porcine dermis-derived biologic meshes, and 3 bovine dermis-derived biologic meshes. After procurement, the specimens were stained with hematoxylin and eosin (H&E) and semi-quantitatively assessed for six characteristics of tissue response: cellular infiltration, cell types, scaffold degradation, ECM deposition, neovascularization, and fibrosis. Possible scores in each category ranged from 0 to 3, with higher scores representing more favorable characteristics of tissue response. A composite score was also generated from the mean of the six component scores and was utilized as an overall measure of tissue remodeling.
Cellular infiltration, ECM deposition, and neovascularization scores were 2 for 80%, 64%, and 64% of the specimens, respectively, indicating that the majority of cells, host ECM deposition, and vasculature infiltrated beyond the periphery and began to penetrate deeper into the mesh, even reaching the center of the biopsy in some cases. Cell types scores were <3 in 57% of the specimens, indicating that the majority of meshes showed evidence of inflammatory infiltrate. Only 43% of the specimens scored ≥3 for cell types, indicating the presence of fibroblasts only without any inflammatory cells. Scaffold degradation and fibrosis scores were ≥2 in 56% and 70% of cases, respectively, indicating that the majority of the meshes were significantly degraded with mild fibrous encapsulation of less than 25% of the mesh periphery. In general, the biologic mesh biopsies indicated favorable host remodeling scores with cells (primarily fibroblasts), host ECM deposition, and new vasculature beginning to reach the center of the biopsies, which were almost fully degraded with minimal inflammatory or fibrous reaction.
When the meshes were subdivided by mesh type, it was revealed that human dermis-derived meshes exhibited significantly improved cellular infiltration, ECM deposition, scaffold degradation, and neovascularization scores compared to porcine dermis-derived meshes and trended toward improved scores compared to bovine dermis-derived meshes. Thus, the species of origin appears to significantly impact remodeling of biologic meshes when implanted in human subjects.
In the study by De Silva et al., biopsies were obtained from fourteen (n = 14) human subjects who underwent biologic mesh repairs placed as an intraperitoneal underlay [33]. Mesh type was identified in all biopsies with n = 7 crosslinked porcine dermis (Permacol™) and n = 7 non-crosslinked porcine dermis (Strattice™). After procurement, the specimens were stained with hematoxylin and eosin (H&E) and Masson’s trichrome and evaluated for acute and chronic inflammatory response, foreign body reaction, fibrous capsule formation, cellular infiltration, neovascularization, and degradation/remodeling. Possible scores in each category were 0 (none), 1 (minimal), 2 (mild), 3 (moderate), or 4 (extensive).
The crosslinked porcine dermis specimens exhibited mild foreign body reaction, moderate fibrous capsule formation, no neovascularization, no cellular infiltration, and no quantifiable new collagen deposition. The non-crosslinked porcine dermis specimens exhibited similar characteristics with mild to moderate foreign body reaction, mild to moderate fibrous encapsulation, no neovascularization. However, non-crosslinked grafts did demonstrate some neo-cellularization at the periphery of the mesh, albeit without any quantifiable new collagen deposition. Regardless of crosslinking, the porcine dermis-based biopsies showed no evidence of significant remodeling at the time of explantation. Although the findings of the study questioned the concept of biologic mesh remodeling, this finding might be a factor of the underlay mesh positioning.
Conclusions
A large number of biologic meshes are currently available. Those meshes are touted to possess many advantages over permanent synthetic meshes. It is believed that revascularization of these materials during the remodeling process effectively clears pathogens from the mesh. Mesh remodeling has proven to be inconsistent. Crosslinking is not the only factor that determines the properties or performance of biologic meshes. Other aspects of the tissue treatment process (i.e., decellularization method, crosslinking technique, extent of crosslinking, sterilization process, and packaging conditions) or species/tissue from which these meshes are derived all contribute and should be explored in more detail in future studies. Overall, biologic mesh use appears to have peaked several years ago and recent disappointing clinical data and high cost have begun to limit its utilization.
References
Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26:507–23.
Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77.
Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93.
Badylak SF. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann Biomed Eng. 2014;42:1517–27.
Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–81.
Cook JL, Fox DB, Kuroki K, Jayo M, De Deyne PG. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148–56.
Jenkins ED, Yip M, Melman L, Frisella MM, Matthews BD. Informed consent: cultural and religious issues associated with the use of allogeneic and xenogeneic mesh products. J Am Coll Surg. 2010;210:402–10.
Meyer SR, Chiu B, Churchill TA, Zhu L, Lakey JR, Ross DB. Comparison of aortic valve allograft decellularization techniques in the rat. J Biomed Mater Res Part A. 2006;79:254–62.
Lynch AP, Ahearne M. Strategies for developing decellularized corneal scaffolds. Exp Eye Res. 2013;108:42–7.
Horowitz B, Bonomo R, Prince AM, Chin SN, Brotman B, Shulman RW. Solvent/detergent-treated plasma: a virus-inactivated substitute for fresh frozen plasma. Blood. 1992;79:826–31.
Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–40.
Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–49.
Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, et al. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B Appl Biomater. 2011;96:199–206.
Gillies AR, Smith LR, Lieber RL, Varghese S. Method for decellularizing skeletal muscle without detergents or proteolytic enzymes. Tissue Eng Part C Methods. 2011;17:383–9.
Gratzer PF, Harrison RD, Woods T. Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng. 2006;12:2975–83.
Zhang AY, Bates SJ, Morrow E, Pham H, Pham B, Chang J. Tissue-engineered intrasynovial tendons: optimization of acellularization and seeding. J Rehabil Res Dev. 2009;46:489–98.
Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405.
Damink LHHO, Dijkstra PJ, vanLuyn MJA, vanWachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–73.
Abraham GA, Murray J, Billiar K, Sullivan SJ. Evaluation of the porcine intestinal collagen layer as a biomaterial. J Biomed Mater Res. 2000;51:442–52.
Billiar K, Murray J, Laude D, Abraham G, Bachrach N. Effects of carbodiimide crosslinking conditions on the physical properties of laminated intestinal submucosa. J Biomed Mater Res. 2001;56:101–8.
Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials. 1996;17:679–84.
Khor E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials. 1997;18:95–105.
Courtman DW, Errett BF, Wilson GJ. The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices. J Biomed Mater Res. 2001;55:576–86.
HardinYoung J, Carr RM, Downing GJ, Condon KD, Termin PL. Modification of native collagen reduces antigenicity but preserves cell compatibility. Biotechnol Bioeng. 1996;49:675–82.
Gratzer PF, Lee JM. Control of pH alters the type of cross-linking produced by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) treatment of acellular matrix vascular grafts. J Biomed Mater Res. 2001;58:172–9.
Deeken CR, Eliason BJ, Pichert MD, Grant SA, Frisella MM, Matthews BD. Differentiation of biologic scaffold materials through physiomechanical, thermal, and enzymatic degradation techniques. Ann Surg. 2012;255:595.
Pui CL, Tang ME, Annor AH, Ebersole GC, Frisella MM, Matthews BD, et al. Effect of repetitive loading on the mechanical properties of biological scaffold materials. J Am Coll Surg. 2012;215:216–28.
Annor AH, Tang ME, Pui CL, Ebersole GC, Frisella MM, Matthews BD, et al. Effect of enzymatic degradation on the mechanical properties of biological scaffold materials. Surg Endosc. 2012;26:2767–78.
Deeken CR, Melman L, Jenkins ED, Greco SC, Frisella MM, Matthews BD. Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair. J Am Coll Surg. 2011;212:880–8.
Jenkins ED, Melman L, Deeken CR, Greco SC, Frisella MM, Matthews BD. Biomechanical and histologic evaluation of fenestrated and nonfenestrated biologic mesh in a porcine model of ventral hernia repair. J Am Coll Surg. 2011;212:327–39.
Cavallo JA, Greco SC, Liu J, Frisella MM, Deeken CR, Matthews BD. Remodeling characteristics and biomechanical properties of a crosslinked versus a non-crosslinked porcine dermis scaffolds in a porcine model of ventral hernia repair. Hernia. 2013;19(2):207–18.
Cavallo JA, Roma AA, Jasielec MS, Ousley J, Creamer J, Pichert MD, et al. Remodeling characteristics and collagen distribution in biological scaffold materials explanted from human subjects after abdominal soft tissue reconstruction: an analysis of scaffold remodeling characteristics by patient risk factors and surgical site classifications. Ann Surg. 2013.
De Silva GS, Krpata DM, Gao Y, Criss CN, Anderson JM, Soltanian HT, et al. Lack of identifiable biologic behavior in a series of porcine mesh explants. Surgery. 2014;156:183–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Deeken, C.R. (2016). Biologic Mesh: Classification and Evidence-Based Critical Appraisal. In: Novitsky, Y. (eds) Hernia Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-27470-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-27470-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27468-3
Online ISBN: 978-3-319-27470-6
eBook Packages: MedicineMedicine (R0)