Abstract
Background
In a recent propensity score study, we established that overall- and disease-free survival were worse after use of a colonic stent (CS) than after emergency surgery for colonic obstruction. The present study sought to explain the association between CS use and poor survival by analyzing pathological specimens.
Methods
From January 1998 to December 2011, all patients with left obstructive colon cancer and having been operated on with curative intent were included in the study. The primary end point involved a comparison of pathological data from the CS- and the surgery-only groups in a case-matched analysis (with the groups matched for the T stage). In a series of secondary analyses, we studied a range of factors known to be associated with adverse outcomes (microscopic perforation, vascular embolism, perineural invasion, and lymph node invasion) in the study population as a whole (in order to evaluate stenting as an adverse factor) and in the CS group alone (in order to identify factors associated with a poor prognosis in this specific group of patients).
Results
A total of 84 patients were included in the study (50 in the CS group). Stenting was mentioned in only 70 % of the pathology lab reports (n = 35/50). Twenty-five patients in the CS group were matched with 25 patients of the surgery-only group. Tumor ulceration (p < 0.0001), peritumor ulceration (p < 0.0001), perineural invasion (p = 0.008), and lymph node invasion (p = 0.005) were significantly more frequent in the CS group. In a multivariate analysis of the CS group, T4 status and tumor size were significant risk factors for microscopic perforation, perineural invasion, and lymph node invasion.
Conclusion
The CS- and surgery-only groups differed significantly in terms of ulceration at or near the tumor, perineural invasion, and lymph node invasion. Explanation of the adverse outcomes associated with CS use will probably require further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sixteen percent of cases of colon cancer are revealed by obstruction in an emergency context and treatment of this condition remains a challenge [1]. Several treatment options for colonic obstruction are available. Loop colostomy, Hartmann’s procedure, one-stage surgery, and colonic stenting have been selected as options for the management of colonic obstruction [2]. The choice between different surgical options depends on the general state of the patient and of the tumor and on the presence of peritonitis. Use of a colonic stent (CS) can be an alternative to emergency surgery as long as there is no tumor-related or diastatic colonic perforation or risk factors for perforation [3]. The efficacy and safety of a CS has been reported in several systematic reviews, a nonsystematic review [4–6], and a meta-analysis [7]. In our experience, a CS enables an ideal CS-elective surgery sequence in 70 % of cases. In a recent propensity score study, we established that overall- and disease-free survival were worse after use of a CS than after emergency surgery [8]. Moreover, all the multicenter, randomized, controlled trials, including those of self-expanding metallic stents, have closed prematurely because of the high morbidity (perforation) observed in the stent groups [9–12]. The reason for the adverse outcomes with CS use is unknown. Macroscopic colonic perforation cannot be the only associated factor, since we found that patients lacking macroscopic perforation (in an ideal sequence) also had a worse prognosis than patients operated on in an emergency setting. Although Maruthachalam et al. [13] found that CS use was associated with a higher circulating cell count, the latter’s impact on survival is unclear. We hypothesized that the pathological characteristics of obstructive tumors treated with or without use of a CS could provide an explanation for the observed difference in survival. Hence, the purpose of the present study was to analyze the impact of CS use on the characteristics of pathology lab specimens.
Patients and methods
Inclusion and exclusion criteria
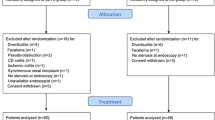
From January 1998 to December 2011, all patients (1) with left obstructive colon cancer (LOCC), (2) with no macroscopic perforation or peritonitis, and (3) operated on with curative intent were included in the study, regardless of the treatment sequence (emergency surgery or use of a CS as a bridge to surgery). Patients operated on for right colonic obstruction or colonic perforation (whether tumor-related or diastatic) were not included in the study. Data from some of the cases have already been published in our previous study of the long-term outcomes after CS use [8].
Study design and period of inclusion
This was a retrospective, two-center study performed in two hospitals in France (Amiens University Medical Center and Beauvais Medical Center). From 1998 to 2004, patient data were extracted from operating theatre registers, pathology lab reports, and medical records. In the operating theatre registers and operative notes, all patients with a CS were clearly identified. We used data from a prospective, colorectal-dedicated databases maintained from 2004 to 2011 by each department involved in the study. Patients with CS were identified in these databases. The pathologist was blinded to the surgical procedures. The study’s protocol was approved by the local institutional review board.
Population definition
All cases of LOCC treated with curative intent were included in the study. Patients who received a CS as a bridge to surgery (i.e., as the first step in treatment, prior to surgery) formed the CS group and the group of patients operated on in an emergency setting formed the surgery-only group. To be sure that the groups were similar, we excluded macroscopic perforations and matched populations on T stage.
End points
Primary end point
The study’s primary end point involved a comparison of the pathological data from patients in the CS- and surgery-only groups in a case-matched analysis (with the groups matched for the T stage). The pathological parameters studied included the tumor and peritumor perforation rates, the tumor and peritumor ulceration rates, the pericolic abscess rate, and the presence or absence of stromal inflammation, vascular embolism, perineural invasion, and lymph node invasion.
Secondary end points
The secondary end points were conventionally described pathological features known to be associated with poor survival in colonic cancer (perforation, vascular embolism, perineural invasion, and lymph node invasion) [14]. The secondary analyses were performed for the study population as a whole (i.e., the CS- and the surgery-only groups), in order to study stenting as a factor associated with adverse outcomes, and for the CS group alone, in order to identify factors associated with a poor prognosis in this particular group of patients. The following items were analyzed: CS use, the presence of metastases, the T stage, the degree of tumor differentiation, the tumor site, and the tumor’s longest dimension (referred to henceforth as tumor size). Uni- and multivariate analyses were performed on the study population as a whole (i.e., with no case-matching).
Treatment
Choice of emergency procedure
The two participating medical centers had developed different strategies for the management of LOCC because of their respective skills and the technical equipment required for CS insertion, as explained elsewhere [8]. In both institutions, emergency procedures were always performed within hours of diagnosis of obstruction. Stenting was not used in patients presenting either cecal perforation at the time of diagnosis or a risk of perforation (i.e., a cecal diameter of more than 9 cm on a CT scan).
Emergency and planned procedures
Each patient underwent an emergency preoperative battery of clinical biochemical assays and a contrast-enhanced abdominal CT scan or a contrast-enhanced abdominal X-ray.
Stenting procedure
The procedure used at Amiens University Hospital (with radiographic/endoscopic guidance but no balloon dilatation) has been described in detail elsewhere [15].
Pathological examination
In both institutions, the unopened, unpinned specimen was delivered to the pathology lab within 2 or 3 h of resection (except during the weekend and at night). The specimen was opened and analyzed by the pathologist. The pathologist’s report on macroscopic features was standardized (specimen measurements, tumor size and description, stent site, the width of the resection margins, ulceration, perforation, and lymph nodes status). The stent was removed prior to tissue fixation and a number of photographs were taken.
The specimen was fixed in 4 % formalin for between 24 and 48 h. After the preparation of parallel slices (3–4 mm thick), perpendicular to the bowel’s long axis, blocks of interest were selected. Ulceration was analyzed along the entire length of the specimen and all lymph nodes were studied. One block of normal colonic tissue was studied. The blocks were included in paraffin and stained with hematoxylin–eosin–saffron.
Statistical analysis
Chi-squared tests or Fisher’s exact tests were used to compare categorical variables, and Student’s t- or Mann–Whitney tests were used to compare quantitative variables. A case-matched model was built in which each patient in the CS group was matched with a patient in the surgery-only group as a function of the T stage. Patients who did not match a patient in the other group were excluded from this analysis. A McNemar test was used to compare the results of the case-matched analysis. Furthermore, factors known to be associated with poor outcomes (microscopic perforation, vascular embolism, perineural invasion, and lymph node invasion) were analyzed for the study population as a whole (without case-matching). To identify possible correlations between variables, a multivariate logistic regression analysis was performed. A p value <0.05 was considered to be statistically significant. All variables with a p value <0.05 were included in the multivariate model. All statistical analyses were performed using SPSS for Windows® software ver. 15.0 (SPSS, Inc., Chicago, IL, USA).
Results
A total of 84 patients (49 males) were included in the study (50 in the CS group and 34 in the surgery-only group). The study population’s mean (±SD) age was 72.1 ± 13.6 years (range = 35–97). All the obstructive tumors were located in the left colon. The use of a CS was mentioned in only 70 % of the pathology lab reports (n = 35) (Table 1). In order to compare the pathological data, 25 patients from the CS group were matched with 25 patients from the surgery-only group as a function of the T stage (Table 1).
End points
Primary end point
A univariate analysis of the CS- and surgery-only groups revealed that tumor ulceration (96 vs. 60 %, respectively; p < 0.0001), peritumor ulceration (68 vs. 0 %, respectively; p < 0.0001), perineural invasion (60 vs. 20 %, respectively; p = 0.008), and lymph node invasion (52 vs. 12 %, respectively; p = 0.005) were significantly more frequent in the CS group. There were no significant intergroup differences in terms of the frequency of tumor perforation (24 vs. 12 %, respectively; p = 0.4), peritumor perforation (0 vs. 12 %, respectively; p = 0.2), abscess (16 vs. 16 %, respectively; p = 1), stromal inflammation (46.7 vs. 60 %, respectively; p = 0.5), vascular embolism (32 vs. 16 %, respectively; p = 0.3), or tumor differentiation (well/moderately/poorly differentiated; 60/32/8 vs. 68/32/0, respectively; p = 0.5). There was no correlation between pathological data and time between stent insertion and surgery (Table 2).
Secondary end points
Microscopic tumor perforation
The study population as a whole (CS + surgery-only groups) We found that tumor size was a risk factor for microscopic tumor perforation. The mean tumor size was 47.5 ± 19.5 mm (range = 10–110) in patients with no perforation and 61 ± 31 mm (range = 30–130) in patients with perforation (p = 0.05). No other risk factors were linked to perforation since the associations with CS use (p = 0.9), the presence of metastases (p = 0.4), the T stage (p = 0.2), and the degree of differentiation (p = 0.3) were not statistically significant (Table 3).
The CS group In univariate analysis, we found that T4 tumor status (p = 0.05) and tumor size (49.08 ± 22.06 mm in patients with microscopic tumor perforation vs. 71.4 ± 39.07 mm in patients with no microscopic tumor perforations, respectively; p = 0.03) were risk factors for perforation (Table 3). The presence of metastases (p = 0.2) and the degree of differentiation (p = 0.9) were not associated with a risk of perforation. In multivariate analysis, tumor size (p = 0.028) and T4 tumor status (p = 0.029) were confirmed as risk factors for perforation (Table 4).
Vascular embolism
The study population as a whole (CS + surgery-only groups) None of the tested factors was significantly associated with vascular embolism (Table 5).
The CS group In univariate analysis, T4 tumor status was found to be a risk factor for vascular embolism (p = 0.02), as was tumor size (55.8 ± 28.9 mm in patients with vascular embolism vs. 42.5 ± 14.3 mm in patients with no vascular embolisms, respectively; p = 0.04) (Table 6). In multivariate analysis, none of the tested factors was significantly associated with vascular embolism (Table 4).
Perineural invasion
The study population as a whole (CS + surgery-only groups) Use of a CS was the only identified risk factor for perineural invasion (p = 0.03) (Table 7).
The CS group In the CS group, T4 tumor status (p = 0.006) and tumor size (59.9 ± 29.2 mm in patients with perineural invasion vs. 43.8 ± 20.5 mm in patients with no perineural invasion, respectively; p = 0.03) were identified as risk factors for perineural invasion (Table 6). In multivariate analysis, tumor size (p = 0.03) and T4 tumor status (p = 0.006) were confirmed as risk factors for perineural invasion (Table 4).
Lymph node invasion
The study population as a whole (CS + surgery-only groups) We found that CS was a risk factor for lymph node invasion (p = 0.002) (Table 7).
The CS group In the CS group, T4 tumor status (p = 0.04) and tumor size (60.3 ± 28.9 mm in patients with lymph node invasion vs. 43.4 ± 20.7 mm in patients with no lymph node invasion, respectively, p = 0.02) were risk factors for lymph node invasion (Table 7). In multivariate analysis, tumor size (p = 0.048) and T stage (p = 0.039) were confirmed as risk factors for lymph node invasion (Table 4).
Discussion
Although implantation of a CS is a frequently implemented alternative to emergency surgery in the management of colonic obstruction, little is known about the CSs impact on long-term oncological outcomes. We recently reported poor survival in a series of patients treated with a CS as a bridge to surgery. Hence, we hypothesized that a stent might influence the colonic tumor’s pathological characteristics and that could partially account for the poor overall survival in patients with a CS [8].
In the present study, we were surprised to find that use of a CS was not mentioned in 30 % of the pathology lab reports of patients who had stent insertion, despite the potential importance of this factor. This information shows that the real importance of a CS is probably underestimated.
Univariate analysis revealed that tumor ulceration, peritumor ulceration, perineural invasion, and lymph node invasion occurred more frequently in the CS- than in the surgery-only groups. However, in multivariate analysis, only tumor ulceration differed significantly between the two groups.
The correlation between tumor perforation and adverse outcomes was not surprising since all the prospective studies on CSs were closed prematurely because of a high rate of macroscopic perforation in the stent groups. Nevertheless, we observed that patients with a CS had a worse prognosis, even when those with macroscopic perforation were excluded from the analysis [8]. In the present study, neither uni- nor multivariate analysis found significant differences between the CS- and surgery-only groups in terms of the frequency of microscopic tumor or peritumoral perforation. These results show that colonic perforation per se cannot explain the intergroup difference in survival; other factors must be identified.
The higher tumor ulceration rate in the CS group was not surprising since the CS pushes the tumor against the colon wall. Since perineural invasion and lymph node invasion are known to be prognostic factors in colon cancer [14], one can legitimately hypothesize that these factors can explain (at least in part) the adverse impact of CS use on survival [8]. Indeed, we found that CS use was significantly associated with perineural invasion and lymph node invasion. We found no correlation between pathological data and time between stent insertion and surgery.
In a recent study, Kim et al. [16] compared survival in 25 patients with CS implantation (as a bridge to surgery) to that in 70 patients who had undergone emergency surgery. There were no intergroup differences in overall- (p = 0.385) or cancer-related survival (p = 0.233). The researchers also analyzed pathological data; although there were no significant CS- versus surgery-only differences for differentiation (p = 0.452) or lymph node invasion (p = 0.609), perineural invasion was more frequent in the CS group (p = 0.033) [16]. Interestingly, Kim et al. [16] compared groups of patients with and without perineural invasion and did not find any significant differences in terms of overall- (p = 0.527) or disease-free survival (p = 0.084). These outcomes have to be weighed against the large number of literature reports of a correlation between perineural invasion and survival [14, 17].
It has also been suggested that by compressing a tumor, a CS increases the number of circulating cells. Maruthachalam et al. [13] compared levels of CK20 mRNA after a staging colonoscopy and after CS insertion. Blood samples were collected before and immediately after the procedure. The mean CK20 mRNA level was significantly higher (p = 0.007) after CS insertion than after staging colonoscopy. The authors suggested that CS insertion increased the rate of CK20 mRNA, whereas insufflation of air into the colon did not [13]. Even though large-scale clinical trials are needed to check the relevance of circulating cell counts in routine clinical practice, Steinert et al.’s systematic review [18] stated that circulating cells have clinical relevance and prognostic significance. The outcomes reported by Maruthachalam et al. [13] should be considered as the potential explanation for the adverse outcomes associated with CS insertion [18].
Even though the use of a CS as a “bridge to surgery” appears to be associated with shorter overall survival, stenting may be of value in a palliative setting, although patients with a T4 tumor or a large tumor should still be managed carefully in this context, since both factors were associated with microscopic perforation in the present study.
We also studied pathological parameters with known prognostic value. In univariate analysis, there was a higher frequency of CS use in the groups of patients with perineural invasion or lymph node invasion. However, this difference was no longer observed in multivariate analysis. In the CS group, tumor size and the presence of a T4 tumor were risk factors for perineural invasion and lymph node invasion. The higher rate of perineural invasion and lymph node invasion in the CS group (observed in univariate analysis) cannot be explained by inflammation in the vicinity of the stent, since Pages et al. [14] failed to observe any correlation between vascular embolism, perineural invasion, lymph node invasion, and inflammation marker levels.
We decided to focus on pathological features in an attempt to explain the adverse impact of CS on survival. Tumor ulceration, peritumor ulceration, perineural invasion, and lymph node invasion were significantly more frequent in the CS- than in the surgery-only groups. Further investigations are needed to explain the adverse outcomes associated with CS insertion.
References
Champault G, Adloff M, Arnaud JP, Branche D, Baulieux J, Boutelier P, Descottes B, Deliere T, Edelmann G, Escat J, Fourtanier G, Hollender L, Hourlier M, Khayat M, Lagache G, Maillet P, Meyer C, Moller F, Montete C, Patel JC, Pailler JL, Penchet A, Rignault D, Soulier Y, Stoppa R, Triboulet JP, Varon F, Verhaeghe P (1983) Colonic occlusions. Retrospective cooperative study of 497 cases. J Chir 120:47–56
Ansaloni L, Andersson RE, Bazzoli F, Catena F, Cennamo V, Di Saverio S, Fuccio L, Jeekel H, Leppaniemi A, Moore E, Pinna AD, Pisano M, Repici A, Sugarbaker PH, Tuech JJ (2010) Guidelines in the management of obstructing cancer of the left colon: consensus conference of the World Society of Emergency Surgery (WSES) and Peritoneum and Surgery (PnS) Society. World J Emerg Surg 5:29
Trompetas V (2008) Emergency management of malignant acute left-sided colonic obstruction. Ann R Coll Surg Engl 90:181–186
Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99:2051–2057
Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ (2007) Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 246:24–30
Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX (2012) Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc 26:110–119
Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, Regimbeau JM (2013) Is stenting as “a Bridge to Surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. doi:10.1097/SLA.0b013e31827e30ce
Alcantara M, Serra-Aracil X, Falco J, Mora L, Bombardo J, Navarro S (2011) Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 35:1904–1910
Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL (2011) Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25:1814–1821
van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA (2008) Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy 40:184–191
van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P (2011) Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 12:344–352
Maruthachalam K, Lash GE, Shenton BK, Horgan AF (2007) Tumour cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Brehant O, Fuks D, Bartoli E, Yzet T, Verhaeghe P, Regimbeau JM (2009) Elective (planned) colectomy in patients with colorectal obstruction after placement of a self-expanding metallic stent as a bridge to surgery: the results of a prospective study. Colorectal Dis 11:178–183
Kim HJ, Choi GS, Park JS, Park SY, Jun SH (2013) Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis 28(3):407–414
Ueno H, Mochizuki H, Shirouzu K, Kusumi T, Yamada K, Ikegami M, Kawachi H, Kameoka S, Ohkura Y, Masaki T, Kushima R, Takahashi K, Ajioka Y, Hase K, Ochiai A, Wada R, Iwaya K, Nakamura T, Sugihara K (2012) Multicenter study for optimal categorization of extramural tumor deposits for colorectal cancer staging. Ann Surg 255:739–746
Steinert EM, Schwartz RH, Singh NJ (2012) At low precursor frequencies, the T-cell response to chronic self-antigen results in anergy without deletion. Eur J Immunol 42(11):2875–2880
Disclosures
Charles Sabbagh, Denis Chatelain, Nathalie Trouillet, François Mauvais, Sif Bendjaballah, François Browet, and Jean-Marc Regimbeau have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabbagh, C., Chatelain, D., Trouillet, N. et al. Does use of a metallic colon stent as a bridge to surgery modify the pathology data in patients with colonic obstruction? A case-matched study. Surg Endosc 27, 3622–3631 (2013). https://doi.org/10.1007/s00464-013-2934-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-2934-3