Abstract
Introduction
Stenting of obstructing colorectal cancers obviates the need for emergency surgery, reducing initial morbidity and mortality rate associated with emergency surgery and facilitates full staging of the neoplastic process with an opportunity to optimize the patient for surgery. Some recent publications have suggested however that this approach may be associated with higher local recurrence rates. We examined our outcomes following colonic stenting as a bridge to resection.
Methods
A database was reviewed (2006–2018) of patients presenting with acute colorectal obstruction that proceeded to endoscopic stenting. We assessed the bridge to surgery strategy, its success, complication rate, and impact on recurrence and survival.
Results
Of a total of 103 patients who presented with acute malignant large bowel obstruction over this time period, 26 patients had potentially curable disease at presentation and underwent stenting as a bridge to surgery. The technical success rate for stenting in those managed as a bridge to surgery was 92% (n = 24/26) with 7.69% (n = 2/26) having a complication. There was one stent-related perforation. Median follow-up of this cohort was 31 months, with a 5-year overall survival of 53.5%.
Conclusion
Colorectal stenting as a bridge to resection is a successful management strategy for those presenting with obstructing colorectal obstruction. Selective use is associated with lower rates of stoma formation, greater rates of laparoscopic resections with low complication rates, and acceptable oncological outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, 15% of colorectal cancers present with acute colonic obstruction, representing a life-threatening condition requiring immediate intervention [1]. Current management options include emergency resection, placement of self-expandable metal stents (SEMS), or diverting stoma construction [2]. Emergency colorectal surgery is associated with high morbidity and mortality rates [3]. In addition, there are concerns over adequacy of oncological resection and long-term outcomes when compared with those having elective surgery [2, 3].
SEMS placement has been reported as a viable alternative, facilitating decompression of the acute colonic obstruction, while being a bridge to surgery (BTS) in suitable cases [4]. In 1994, Tejero et al. were the first to publish their experience of SEMS placement as a bridge to definitive surgery in patients with colonic obstruction [5]. The main advantage of this approach is the early decompression of the colon, thereby reducing morbidity and mortality associated with emergency surgery, when patients are typically in poor clinical condition [2]. This provides time to improve the patient’s clinical and nutritional status, while accurately staging and educating the patient regarding their condition [6]. BTS can also improve the opportunity for subsequent laparoscopic resection, with reduced stoma formation rates [7]. It can also spare those frail and/or elderly patients or those with extensive disease that would not be suitable to undergo a major resection. It offers considerable palliative relief with good degrees of success [8, 9].
Tekkis et al. demonstrated that emergency surgery is associated with considerably higher postoperative mortality (20% vs. 12.8% elective surgery) [10]. The World Society of Emergency Surgery (WSES) have concluded that SEMS may represent a valid option in selected cases of malignant large bowel obstruction when performed by individuals of adequate expertise in tertiary referral hospitals [11].
The major concern over SEMS is the fear of tumor perforation, which could cause cancer cell dissemination and increased rates of tumor recurrence after curative resection [12]. Other stent-related issues include stent migration, bleeding, or procedural related pain [13]. However, recent studies have shown the technical and clinical success of SEMS in obstructing left colonic tumors of 90.5% and 81%, respectively, with low complication rates [14].
The use of SEMS as a bridge to surgery in the setting of obstructing colorectal neoplasms is a well-established treatment strategy; however, oncological outcome data is still emerging. A multi-center randomized controlled trial from the Netherlands observed increased morbidity and mortality in patients having SEMS as a bridge to surgery and was discontinued early [15]. We report surgical and survival data on those having SEMS placement in obstructing colonic tumor and proceeding to curative resection.
Methods
Treatment strategy
Since the introduction of colorectal stenting to our unit in 2006, it has been our policy to place a SEMS in all patients presenting with an acute large bowel obstruction due to a tumor, in the absence of clinical peritonitis or radiological evidence of perforation or contraindications to stenting.
All patients must have an abdominal computed tomography (CT) to confirm the presence of obstruction and to demonstrate the level of obstruction and length of the stricture. Following SEMS insertion, a CT thorax, carcinoembryonic antigen (CEA) level and full colonoscopy with biopsy is performed followed by multidisciplinary discussion. Eligible patients then proceed to curative resection/neoadjuvant therapy, while those with disseminated disease or with significant comorbidities or poor functional status receive palliative care.
Stenting technique
Following preparation of the distal colon with phosphate enemas and administration of prophylactic antibiotics, all SEMS insertions were performed under conscious sedation either in our endoscopy unit or in the operating theater (out of hours). Patients were placed in the left lateral position and a double-channel bleeder scope was used for left-sided obstructions, while a normal colonoscope (with at least a 3.7-mm-diameter working channel) was used for transverse and right-sided lesions. Biopsies of the tumor were obtained in cases of unconfirmed diagnosis.
Uncovered metallic stents were used, with the stent placed over a guide wire, using a combination of direct endoscopic visualization and fluoroscopy (WallFlex™ Colonic Stent—Boston Scientific). Stent deployment only took place after a convincing cologram was obtained (often requiring 100–200 ml of contrast) (Fig. 1). Dilatation was never performed prior to stent placement given the risk of perforation. The length of stent employed (60, 90, or 120 mm) was selected on the basis of the predicted tumor length on the CT images, with 20 mm coverage at each side of lesion. No more than one stent was used in any procedure. After deployment of the stent, correct positioning was confirmed with fluoroscopic imaging.
Data retrieval and outcome of interest
We reviewed a prospectively maintained database of all colorectal cancers managed in our unit since 2002. Institutional medical ethics board approval was granted. Patients who had SEMS placement for acute malignant obstruction was identified. Patient records were reviewed, and the following data was collated: patient demographics, site of obstruction, technical and clinical success of SEMS insertion, details of radiological staging, length of hospital stay, details of subsequent resection, postoperative complications including requirement for further acute interventions, pathological staging, details of adjuvant therapy, recurrence (locoregional recurrence or distant metastases), and survival outcomes.
Primary outcomes were to assess the success of SEMS placement as a bridge to surgery and associated oncological (5-year survival) data. Secondly, we reviewed complications, morbidity, and clinical success of SEMS placement in obstructing colorectal cancers. Clinical success was defined as decompression of the obstructed proximal bowel and restoration of luminal patency, without further interventions during the hospital stay.
Statistical analysis
All continuous variables were described as median and range while categorical variables are expressed as frequency and percentage. Survival data was reported as percentage with analysis performed using STATA software (Version 13, StataCorp LP, USA).
Results
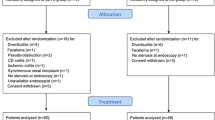
Between 2006 (commencement of SEMS) and February 2018, 103 patients have had SEMS placement in the setting of acute obstruction large bowel obstruction due to malignancy. Of these, 26 (25%) had SEMS insertion as a bridge to surgery, while 77 patients (75%) had insertion with palliative intent. Table 1 depicts the patient treatment strategy and clinical outcomes for all patients who were stented as a bridge to surgery.
The technical success rate for all SEMS was 97% (n = 100) (n = 24/26 and n = 76/77 in the bridge to surgery and palliative group respectively). In two cases, there was a failure to decompress the obstruction and a decision was made to proceed with emergency resection. Across the entire series, there was only one perforation, necessitating an emergency resection. In this case, successful decompression was achieved, with clinical and radiological evidence of resolution. However, day-six post stent placement, the patient developed severe abdominal pain. A CT abdomen/pelvis was obtained demonstrating perforation at the distal end of the stent. This patient underwent a laparotomy, resection of the neoplastic lesion at the splenic flexure and a side to side anastomosis. The final histology demonstrated a moderately differentiated pT4N1bMX adenocarcinoma with 3/37 nodes involved. The patient had a total follow-up of 38 months with no evidence of disease recurrence.
Bridge to surgery cohort
Twenty-six patients underwent SEMS as a bridge to definitive resection. Fifty percent were male and median (range) for this group was 70 years (50–90). The two most common locations for the obstructing tumors were the sigmoid colon and descending colon (43.3% and 23.3% respectively). Technical success was achieved in 92% (n = 24/26) with 7.69% (n = 2/26) having a complication (Table 2). The patient with the sigmoid colon malignancy was found to have node-positive disease (2/12) with 84 months of follow-up demonstrating no evidence of recurrence. The second patient with a malignancy at the splenic flexure also had node-positive disease (1/16) with 37 months follow-up and no evidence of disease recurrence. There was one stent-related perforation as detailed above (3.85%). All other patients proceeded to have an elective resection. The overall rate of laparoscopic resection was 78% in the elective cohort (Table 3).
The median follow-up was 31 months.
Thirteen patients died over the follow-up period (Table 4). Eight deaths were directly attributable to their colorectal cancer diagnosis. Two patients had metastatic disease at time of last follow-up. Five-year overall survival in this cohort was 53.5%.
Palliative cohort
Seventy-seven patients underwent SEMS with palliative intent. Sixty-one percent were male and median (range) for this group was 74 years (34–94). The two most common locations for the obstructing tumors were the sigmoid colon and rectum (50.6% and 17.8% respectively). Technical success was achieved in 94.8% (n = 73/77), with 10% (n = 8/77) having a complication. The complications experienced included stent migration (n = 5) and tumor infiltration of the stent resulting in recurrent obstruction (n = 5). There was one stent-related perforation (1.3%). Interestingly, two patients initially treated with palliative intent went on to have an elective resection following neoadjuvant therapy (Table 5).
Discussion
Emergency surgery for malignant bowel obstruction is challenging and associated with considerable morbidity and mortality [10]. Colonic stenting facilitates the decompression of an acute obstruction and potentially converts an emergency intervention to an elective/scheduled surgery. This has the added advantage of increased laparoscopic resection, with potential of reduced stoma rates [7].
SEMS is already known to be an effective management strategy in large bowel obstruction. A large multi-center series of over 500 patients showed the technical and clinical success to be greater than 90% [16]. Using it as a bridge to surgery provides time for patient stabilization and optimization of biochemical and nutritional status and facilitates full clinical staging of the neoplasm [17]. This strategy has been associated with lower rates of stoma formation, increased rates of primary anastomosis, and less morbidity [18,19,20]. Our study has shown that the technical success of colonic stenting in the acute setting is 97%, with a low rate of major complication. There was no evidence of locoregional recurrence in our cohort of patients who underwent colonic stenting as a bridge to surgery. Furthermore, the patient who experienced the complication of a stent perforation did not suffer adverse oncological outcomes as a consequence.
The immediate benefit of SEMS placement is well established. However, a major concern over SEMS placement as bridge to surgery is the negative impact it may have on oncological outcomes. Some studies report increased tumor progression rates and metastasis in those having SEMS [21, 22]. Proposed reasons for this are the occurrence of micro-perforations at time of stenting that results in tumor seeding and dissemination [19]. As of late 2014, the European Society of Gastrointestinal Endsocopists (ESGE) does not recommend the use of emergency setting as a bridge to surgery as standard practice. They only recommend it in highly selective cases, where the patients are extremely comorbid, which make emergency surgery very high risk for perioperative mortality [21].
A multi-center Dutch randomized controlled trial (RCT) was one of the fundamental papers that observed a high perforation in those having SEMS placement (12.8%), with a higher rate of 30-day mortality, resulting in early closure of the trial [15]. The RCT by Pirlet et al. reported a high incidence of stent-related complications [23]. There has been criticism of both trial designs, in particular heterogeneity regarding the experience and competency of those providing the emergency stenting service, which accounts for the variable success and clinical outcomes [16]. Several other studies have reported increased local recurrence in those patients having SEM-related perforation, but the majority have not translated into inferior oncological outcomes [22, 24]. Erichsen et al. reported a 5-year recurrence rate of 39% after SEMS vs. 30% having emergency surgery (ES) (adjusted risk ratio 1.1 95% CI 0.99–1.28), but no difference in long-term survival [25]. The largest trial to date by Hill and colleagues (CREST Trial) observed a reduced stoma formation in the BTS cohort without a detrimental effect on 1-year survival [7].
A comprehensive meta-analysis by Allievi et al. reviewed seven RCTs between 2009 and 2016 [26]. They observed no difference in mortality rates between SEMS and emergency surgery cohorts (7% each), but noted significant difference in postoperative complication rates (37% SEMS vs. 54% ES), stoma rates (25% SEMS vs. 46% ES), and wound infection rates (8% SEMS vs. 15%) [26]. Alcántara et al. also noted a higher rate of anastomotic leakage in those proceeding directly to emergency surgery [27], while Oistamo et al. noted higher lymph node yields in those having SEMS as a bridge to surgery vs. those having emergency resection [28]. There are only a few studies that have examined long-term overall survival. Neither Arezzo et al. (ESCO Trial) [29] nor Choi et al. observe survival differences [30] in the BTS cohort vs. Emergency Surgery.
Conclusion
Colorectal stenting is an effective management strategy for patients presenting with an acute obstructing colorectal neoplasm. We observed high technical success, reduced numbers of stoma formation, greater rates of laparoscopic surgery with low associated morbidity, and no negative impact to long-term survival. Its selective use should remain part of the armamentarium of treatment options for those presenting with an acute malignant obstruction.
References
Jullumstrø E, Wibe A, Lydersen S, Edna TH (2011) Colon cancer incidence, presentation, treatment and outcomes over 25 years. Colorectal Dis 13(5):512–518
Amelung FJ, Draaisma WA, Consten ECJ, Siersema PD, Ter Borg F (2017) Self-expandable metal stent placement versus emergency resection for malignant proximal colon obstructions. Surg Endosc 31(11):4532–4541
Jeong DS, Kim YH, Kim KJ (2017) Surgical outcomes and risk factors in patients who underwent emergency colorectal surgery. Ann Coloproctol 33(6):239–244
Huang X, Lv B, Zhang S, Meng L (2014) Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg 18(3):584–591
Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA (1994) New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum 37(11):1158–1159
Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX (2012) Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc 26(1):110–119
Hill J, Kay C, Morton D, Magill L, Handley K, Gray RG (2016) CREST: randomised phase III study of stenting as a bridge to surgery in obstructing colorectal cancer—results of the UK coloRectal endoscopic stenting trial (CREST). J Clin Oncol 34(15_suppl):3507–3507
Larkin JO, Moriarity AR, Cooke F, McCormick PH, Mehigan BJ (2014) Self-expanding metal stent insertion by colorectal surgeons in the management of obstructing colorectal cancers: a 6-year experience. Tech Coloproctol 18(5):453–458
Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ (2007) Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 246(1):24–30
Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD (2004) The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 240:76–81
Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De’Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L (2018) 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg 13:36
Kim SJ, Kim HW, Park SB, Kang DH, Choi CW, Song BJ, Hong JB, Kim DJ, Park BS, Son GM (2015) Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg Endosc 29(12):3499–3506
Han S-H, Lee JH (2014) Colonic stent-related complications and their management. Clin Endosc 47(5):415–419
Lee YJ, Yoon JY, Park JJ, Park SJ, Kim JH, Youn YH, Kim TI, Park H, Kim WH, Cheon JH (2018) Clinical outcomes and factors related to colonic perforations in patients receiving self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc 87(6):1548–1557.e1
Van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P, collaborative Dutch Stent-In study group (2011) Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 12(4):344–352
Matsuzawa T, Ishida H, Yoshida S, Isayama H, Kuwai T, Maetani I, Shimada M, Yamada T, Saito S, Tomita M, Koizumi K, Hirata N, Sasaki T, Enomoto T, Saida Y (2015) A Japanese prospective multicenter study of self-expandable metal stent placement for malignant colorectal obstruction: short-term safety and efficacy within 7 days of stent procedure in 513 cases. Gastrointest Endosc 82(4):697–707
Costa Santos MP, Palmela C, Ferreira R, Barjas E, Santos AA, Maio R, Cravo M (2016) Self-expandable metal stents for colorectal cancer: from guidelines to clinical practice. GE Port J Gastroenterol 23(6):293–299
Hong SP, Kim TI (2014) Colorectal stenting: an advanced approach to malignant colorectal obstruction. World J Gastroenterol: WJG 20(43):16020–16028
Tan CJ, Dasari BV, Gardiner K (2012) Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg 99(4):469–476
Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J (2013) Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol 22(1):14–21
van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A, European Society of Gastrointestinal Endoscopy (2014) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 46:990–1053
Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA (2014) Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 101:1751–1757
Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL (2011) Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25(6):1814–1821
Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, Cunningham C, Lindsey I (2013) Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg 100:1805–1809
Erichsen R, Horváth-Puhó E, Jacobsen JB, Nilsson T, Baron JA, Sørensen HT (2015) Long-term mortality and recurrence after colorectal cancer surgery with preoperative stenting: a Danish nationwide cohort study. Endoscopy 47(6):517–524
Allievi N, Ceresoli M, Fugazzola P, Montori G, Coccolini F, Ansaloni L (2017) Endoscopic stenting as bridge to surgery versus emergency resection for left-sided malignant colorectal obstruction: an updated meta-analysis. Int J Surg Oncol 2017:2863272
Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S (2011) Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 35(8):1904–1910
Öistämö E, Hjern F, Blomqvist L, Falkén Y, Pekkari K, Abraham-Nordling M (2016) Emergency management with resection versus proximal stoma or stent treatment and planned resection in malignant left-sided colon obstruction. World J Surg Oncol 14(1):232
Arezzo A, Balague C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Arroyo A, Sola-Vera J, De Paolis P, Bossotti M, Bannone E, Forcignanò E, Bonino MA, Passera R, Morino M (2017) Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc 31(8):3297–3305
Choi JM, Lee C, Han YM, Lee M, Choi YH, Jang DK, Im JP, Kim SG, Kim JS, Jung HC (2014) Long-term oncologic outcomes of endoscopic stenting as a bridge to surgery for malignant colonic obstruction: comparison with emergency surgery. Surg Endosc 28(9):2649–2655
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donlon, N.E., Kelly, M.E., Narouz, F. et al. Colonic stenting as a bridge to surgery in malignant large bowel obstruction: oncological outcomes. Int J Colorectal Dis 34, 613–619 (2019). https://doi.org/10.1007/s00384-019-03239-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03239-9