Abstract
Background
Although many reports have indicated the feasibility of laparoscopic gastrectomy (LG) regarding short-term surgical outcomes, the role of LG remains controversial because studies of long-term outcomes of LG are insufficient. The purpose of this study was to evaluate the long-term oncologic outcomes of patients who have undergone LG.
Methods
Between May 2003 and December 2009, 714 consecutive patients underwent LG for gastric cancer. After excluding operative mortality (n = 4) and a case of Krukenberg tumor that was not identified at the time of surgery (n = 1), a total of 709 patients were analyzed for long-term oncologic outcomes. Gastric cancer cases were analyzed according to the American Joint Committee on Cancer classification (seventh edition). Overall survival and relapse-free survival were estimated by using the Kaplan-Meier method.
Results
Median follow-up was 46.2 months. Postoperative recurrence was observed in 26 patients (3.7%). The instances of recurrence were as follows: seven peritoneal, six locoregional, five hematogenous, four distant lymph nodes, and four mixed recurrence. There were neither port-site nor wound site metastases. The 5-year relapse-free survival rates were: 95.8% in stage I, 83.4% in stage II, and 46.4% in stage III. Five-year overall survival rates were: 96.4% in stage I, 83.1% in stage II, and 50.2% in stage III. The independent risk factors for recurrence were T stage and N stage. For survival, age, T stage, and N stage were statistically independent prognostic factors
Conclusions
Our single-center study of a large patient series revealed that LG for gastric cancer had acceptable long-term oncologic outcomes comparable to those of conventional open surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric cancer is one of the most common causes of cancer-related death in the world [1]. In Korea and Japan, the early detection of gastric cancer has increased because of widespread screening and regular personal check-ups. Because more than 90% of patients with early gastric cancer (EGC) are cured by surgery alone, improving the postoperative quality of life of patients while maintaining long-term survival is a growing concern among surgeons.
The laparoscopic approach is rapidly becoming the preferred method of treatment for patients with EGC due to the many advantages of minimally invasive surgery [2]. Several studies have already demonstrated that surgeons can safely perform laparoscopic gastrectomy (LG) and that these patients had less postoperative pain, faster recovery, shorter hospital stay, and better quality of life than those who underwent conventional open surgery [3–5]. Accordingly, LG has become an attractive treatment option for patients with EGC and a potential treatment option for advanced gastric cancer [6–8]. However, the role of LG remains controversial [9–12], because studies of the long-term outcomes of LG are insufficient [13, 14].
Therefore, the purpose of this study was to evaluate the long-term oncologic outcomes of patients who underwent LG, focusing on postoperative recurrence and survival rates.
Methods
Patients
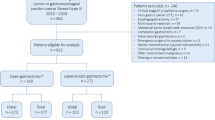
Between May 2003 and December 2009, 714 consecutive patients who underwent LG with lymph node dissection for gastric cancer at Yonsei University Severance Hospital, Seoul, Korea were included in this study (Fig. 1). After excluding instances of operative mortality (n = 4) and one Krukenberg tumor that was not identified at the time of surgery (n = 1), 709 patients were analyzed for long-term oncologic outcomes (Fig. 2).
Patient demographics, comorbidities, and data on surgery, pre-, and postoperative monitoring, including complications and length of hospital stay, were recorded in the database. The data from these patients were prospectively collected and retrospectively analyzed. We used clinical and pathologic staging according to the American Joint Committee on Cancer (seventh edition) TNM classification. This study was approved by the Institutional Review Board of Yonsei University Severance Hospital, and written informed consent for surgery was obtained at the time of operation.
Indications and surgical procedures
Preoperative diagnosis was performed using endoscopy, endoscopic ultrasonography, and computed tomography. Through preoperative or intraoperative findings, when the tumor was confined to the mucosa or submucosa of a gastric wall, it was indicated for surgery in the initial period. As experience accumulated, the indication was expanded to include tumors not involving serosa with lymph node involvement limited to perigastric lymph node based on the preoperative clinical staging workup.
During extracorporeal anastomosis, the location of the tumor was identified by palpation of the clips applied endoscopically the day before the operation. When we perform intracorporeal anastomosis or intraoperative localization is needed, we added a procedure of laparoscopic ultrasound or portable plain radiography to detect the clips applied endoscopically [14, 15].
The surgical procedures were described previously in detail as follows: 1) total or subtotal gastrectomy was performed, according to location; 2) D1 + α (dissection of group 1 and number 7 lymph node), D1 + β (dissection of group 1 and number 7, 8a, and 9 lymph nodes), or D2 lymphadenectomy (dissection of all group 1 and group 2 lymph nodes) was performed according to the rules of the Japanese Research Society for Gastric Cancer [16–20].
Follow-up, categorization of recurrence pattern, and survival
All patients were monitored postoperatively by physical examination and laboratory tests, including those for tumor markers (such as carcinoembryonic antigen and CA 19-9), every 3 months for the first 2 years, every 6 months for the next 3 years, and then annually. In addition, examinations, including chest radiography, abdominopelvic CT, and endoscopy, were performed at least once a year. If necessary, further evaluation, such as positron emission tomography or magnetic resonance imaging, was initiated to better clarify a recurrence. Adjuvant chemotherapy with 5-fluorouracil (5-FU)-based regimens (mostly 5-FU with cisplatin) was recommended to all eligible patients except those with stage Ia and Ib cancer.
Recurrence patterns were classified into five categories at the time of diagnosis: hematogenous, peritoneal, locoregional, distant lymph node, and mixed recurrence. Hematogenous recurrences included tumors in other distant sites, such as liver, lung, bone, and brain. Peritoneal recurrences included peritoneal seedlings or Krukenberg’s tumors. Locoregional recurrences included tumors in adjacent organs, namely, remnant stomach, anastomoses, duodenal stumps, and regional lymph nodes (perigastric, left gastric, common hepatic, celiac, hepatoduodenal, retropancreatic, and mesenteric). Recurrences in distant lymph nodes were defined as extra-abdominal or para-aortic lymph nodes. When patients had more than one other recurrence at the time of diagnosis, they were categorized as having a mixed recurrence.
All patients were observed until death or the last follow-up date of December 31, 2010, whichever occurred first. However, 20 patients were not followed up to that time. The median period of follow-up was 46 (range, 3–92.8) months.
Statistical analysis
Overall survival (OS) and relapse-free survival (RFS) were estimated using the Kaplan-Meier method. OS was calculated from the day of surgery until death or until the end of follow-up. Relapse-free survival was calculated from the day of surgery to the day of recurrence or death. To identify the independent risk factors for recurrence, binary logistic regression was used. Multivariate analyses for survival were conducted using Cox’s proportional hazard model. P < 0.05 (two-sided) was considered statistically significant. All statistics analyses were performed using the Statistical Package for the Social Sciences (SPSS®) version 18.0 for Windows (SPSS, Inc., Chicago, IL).
Results
Perioperative characteristics and pathologic features
Table 1 shows the clinical characteristics and pathologic features of the 714 patients after LG. Systemic lymph node dissection (D1 + β or D2: 98.2%) was mostly performed. The median number of retrieved lymph nodes was 36 (range, 5–97). We performed an R0 resection in 708 patients (99.2%). Most patients had T1 (79.2%), N0 (83.2%), and stage I cancer (84.6%). Approximately 20% of patients had advanced gastric cancer (tumor invasion over the proper muscular layer).
Long-term oncologic outcomes: recurrence and relapse-free survival
Postoperative recurrence occurred in 26 patients (3.7%). The recurrence pattern included peritoneal (7 cases, 27%), locoregional (6 cases, 23%), hematogenous (5 cases, 19%), distant lymph node (4 cases, 15%), and mixed recurrences (4 cases, 15%; Fig. 2). Among locoregional recurrences, five cases were recurrence in remnant stomach, and one case was a peripancreatic lymph node recurrence. The median RFS was 34.9 (range, 2.5–86.6) months. The median RFS among patients who had postoperative recurrence was 14 (range, 3–64.6) months. Most of the recurrences occurred within 36 months (88.5%). According to stage, the 5-year RFS rates were 95.8%, 83.4%, and 46.4% for stage I, stage II, and stage III, respectively (Fig. 3A). The patients who had advanced gastric cancer showed had 5-year RFS rates of: 88.9% in T2, 77.7% in T3, and 66.9% in T4.
Patient’s long-term oncologic outcomes: the cause of death and OS
A total of 40 patients were dead at the time of analysis. The causes of death were 17 postoperative recurrences (42.5%), 20 deaths due to other causes (50%), and 3 deaths due to unknown cause (7.5%). Causes of death other than gastric cancer were: 5 other cancers, 4 neurological disease, 3 pneumonia, 3 old age, 2 strangulation with sepsis, 1 liver cirrhosis, 1 chemotherapy-related complication, and 1 accidental drowning (Fig. 2). The 5-year OS rates were 96.4% in stage I, 83.1% in stage II, and 50.2% in stage III (Fig. 3B). The patients who had advanced gastric cancer had 5-year OSs of 89.6% in T2, 76.9% in T3, and 69.9% in T4.
Multivariate analyses of risk factors for recurrence and survival
Tables 2 and 3 show multivariate analyses of risk factors for recurrence and survival. T stage and N stage were independent risk factors for recurrence. Regarding survival, age, T stage, and N stage were statistically independent prognostic factors.
Discussion
According to our results of the rate of curative resection and the number of retrieved lymph nodes, LG has been performed with sound oncologic principles. This study revealed that long-term outcomes of LG were comparable with those of conventional open gastrectomy. The risk factors for recurrence and survival with LG were not different from those of conventional open surgery.
In particular, three major concerns of the long-term oncologic outcomes of LG exist. The first is the issue of potential peritoneal recurrence or port-site metastasis caused by insufflated gas to form a pneumoperitoneum [9, 11]. The second is the possible locoregional recurrence due to inadequate lymph node dissection [12]. The third is insufficient data on long-term results after LG.
Herein, we addressed these three major concerns with our experience of LG. First, there were neither port-site metastases nor wound recurrences. Peritoneal recurrence occurred in 7 of 709 patients (1%). Even regarding T4a lesions (serosa exposed), peritoneal recurrence occurred in only 2 of 34 patients (5.9%). The peritoneal recurrence rate of LG was similar as that for conventional open gastrectomy, even for advanced gastric cancer [21]. Second, we can support the adequacy of laparoscopic lymph node dissection with the median number of retrieved lymph nodes of 36 per patient, which is comparable to that of open surgeries. Recurrence in regional lymph nodes was noted in only one patient (0.1%).
The recurrences in remnant stomach among locoregional recurrences in our study were high compared with other studies. However, the recurrence rate in remnant stomach was 0.7% (5/714), which is not high compared with other studies [16, 22–24]. We are not sure whether the high proportion of remnant stomach recurrence among locoregional recurrences in our study is related to difficulty in tumor localization or if it comes from less recurrences in regional lymph nodes or in the gastric bed. Actually, all patients (n = 5) who had recurrence in remnant stomach showed the margin of resection was not involved by cancer microscopically and macroscopically. Among them, two recurrences occurred far from anastomotic area detected 2 and 5.4 years after the operation, respectively. Both of these recurrences were early gastric cancer located far from the site of anastomosis. These lesions might have been missed at the time of initial diagnosis or metachronous cancers. The other three recurrences developed in the anastomotic area, although initial proximal margins were microscopically negative. The lengths of resection margin in these patients were 20, 25, and 25 mm from the tumor, respectively. These three recurrences might have been prevented if wider resection margins were obtained at the operation.
Thus, the rate of local failure after LG was satisfactory and comparable to that of open surgery [22–24]. Because the majority of recurrences occur within 3 years of operation [25], the median follow-up duration of this study (46.2 months) is sufficient to evaluate long-term outcomes. Although extended follow-up to determine late recurrence is still needed to detect late recurrence, we found that 88.5% of recurrences were detected within 3 years of operation, which was similar to that for open surgery [25–27].
As the experience of LG for EGC has been accumulated, some centers have attempted to expand the indication of LG to advanced gastric cancer, and a few studies [7, 8, 28, 29] reported comparable results between LG and open gastrectomy regarding advanced gastric cancer. We already demonstrated the acceptable long-term outcomes of LG for lymph node-positive EGC [17]. This study also revealed that the RFS and OS rates after LG were comparable with those after conventional open surgery for advanced gastric cancer [26]. Among 148 patients with AGC, 21% and 40% of patients with AGC were followed up less than 24 months and 36 months, respectively. Thus, more recurrences can be detected if we observe our patients longer especially those who received their operations in recent years. However, as reported in many papers, the majority of recurrences after gastrectomy are observed within 3 years. The median follow-up duration of 39 months for AGC group in our study is adequate to assess recurrence, although it is not enough to detect all recurrences.
Our study has some limitations. These findings are limited by the retrospective nature of the analyses, single-center experience, lack of comparative data with open surgery, and the selection bias of applying LG to preoperatively diagnosed relative early-stage cancer. Despite these limitations, the rate of follow-up loss in this study was only 2.8%, and the pattern of recurrences and the causes of death were thoroughly investigated. Therefore, our study is not only based on a well-followed and accurate database but also is one of the largest series of single-institution experience regarding the long-term oncologic outcomes of LG. However, solid conclusions for long-term outcomes of LG should be drawn from multicenter, randomized, controlled trials, such as the KLASS trial of Korea [2]. Although the KLASS trial already reported satisfactory short-term results of LG for EGC [2], we are awaiting long-term results to indicate the definite role of LG. In addition, a multicenter, prospective, randomized, controlled trial also is needed to confirm the usefulness of laparoscopic surgery as an alternative method to open surgery for advanced gastric cancer (JLSSG0901: Adv.GC-LAP/OPEN, PII/III).
References
Jermal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251:417–420
Kim Y, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae J (2008) Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 248:721–727
Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, Lee HJ, Cho GS, Han SU, Hyung WJ (2008) Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale Korean multicenter study. Ann Surg Oncol 15:2692–2700
Lee JH, Han HS (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 19:168–173
Ziqiang W, Feng Q, Zhimin C, Miao W, Lian Q, Huaxing L, Peiwu Y (2006) Comparison of laparoscopically assisted and open radical distal gastrectomy with extended lymphadenectomy for gastric cancer management. Surg Endosc 20:1738–1743
Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C (2007) Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg 194:839–844
Hur H, Jeon HM, Kim W (2008) Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years’ experience. J Surg Oncol 98:515–519
Volz J, Kster S, Spacek Z, Paweletz N (1999) The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 86:770–774
Fujiwara M, Kodera Y, Kasai Y, Kanyama Y, Hibi K, Ito K, Akiyama S, Nakao A (2003) Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg 196:75–81
Curet MJ (2004) Port site metastases. Am J Surg 187:705–712
Memon MA, Khan S, Yunus RM, Barr R, Memon B (2008) Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc 22:1781–1789
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N, Japanese Laparoscopic Surgery Study G (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245:68–72
Kim HI, Hyung WJ, Lee CR, Lim JS, An JY, Cheong JH, Choi SH, Noh SH (2011) Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for laparoscopic gastrectomy. Surg Endosc 25:958–963
Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, Noh SH (2005) Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc 19:1353–1357
Song J, Lee H, Cho GS, Han S, Kim M, Ryu SW, Kim W, Song KY, Kim H, Hyung WJ (2010) Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol 17:1777–1786
An JY, Heo G, Cheong J, Hyung WJ, Choi SH, Noh SH (2010) Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol 102:77–81
Hyung WJ, Song C, Cheong JH, Choi SH, Noh SH (2007) Factors influencing operation time of laparoscopy-assisted distal subtotal gastrectomy: analysis of consecutive 100 initial cases. Eur J Surg Oncol 33:314–319
Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5:1–5
Japanese Gastric Cancer A (1998) Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1:10–24
Roukos DH, Lorenz M, Karakostas K, Paraschou P, Batsis C, Kappas AM (2001) Pathological serosa and node-based classification accurately predicts gastric cancer recurrence risk and outcome, and determines potential and limitation of a Japanese-style extensive surgery for western patients: a prospective with quality control 10-year follow-up study. Br J Cancer 84:1602–1609
Furusawa M, Notsuka T, Tomoda H (1991) Recurrence of early gastric cancer. Semin Surg Oncol 7:344–350
Lee S, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N (2010) Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 211:33–40
Wu B, Wu D, Wang M, Wang G (2008) Recurrence in patients following curative resection of early gastric carcinoma. J Surg Oncol 98:411–414
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS (2000) Recurrence following curative resection for gastric carcinoma. Br J Surg 87:236–242
Ahn HS, Lee H-J, Seokyung-Hahn, Kim W-H, Lee KU, Sano T, Edge SB, Yang H-K (2010) Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. E-pub
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240:808–816
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Tanimura S, Higashino M, Fukunaga Y, Kishida S, Nishikawa M, Ogata A, Osugi H (2005) Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc 19:1177–1181
Acknowledgment
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1020410).
Disclosures
Drs. Kyung Ho Pak, Woo Jin Hyung, Taeil Son, Kazutaka Obama, Yanghee Woo, Hyoung-Il Kim, Ji Yeong An, Jong Won Kim, Jae-Ho Cheong, Seung Ho Choi, and Sung Hoon Noh have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pak, K.H., Hyung, W.J., Son, T. et al. Long-term oncologic outcomes of 714 consecutive laparoscopic gastrectomies for gastric cancer: results from the 7-year experience of a single institute. Surg Endosc 26, 130–136 (2012). https://doi.org/10.1007/s00464-011-1838-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-1838-3