Abstract
Background
Fat volume and large abdominal shape are known to disrupt the procedures of lymph node retrieval used in gastric cancer surgery. The present study examined the effect of increasing fat content on surgical outcomes, including estimated blood loss and the number of lymph nodes retrieved during gastrectomy.
Methods
Of 154 patients, 50 underwent the conventional open procedure (OPEN) and 104 underwent laparoscopy-assisted distal gastrectomy (LADG). The BMI-related factors of total fat, subcutaneous fat, and visceral fat area, as well as the peritoneum–celiac axis distance were calculated by computed tomography. Regression analysis was used to determine the effects of BMI-related factors that obstruct the surgical procedures on the specific outcomes of estimated blood loss and the number of lymph nodes retrieved.
Results
In the OPEN, but not in the LADG, increases in all BMI-related factors were related to increases in estimated blood loss. The increases in BMI, subcutaneous fat, and the peritoneum-celiac axis distances were related to decreased numbers of retrieved lymph nodes only in the OPEN. Only the factor of visceral fat at the celiac level was modestly associated with a decreased number of dissected lymph node in both groups.
Conclusions
The present study demonstrated that increased fat content and large body shape have little effect on the number of lymph nodes retrieved and blood loss in LADG. However, for patients undergoing conventional open distal gastrectomy, increased fat content and large body shape do impact on the amount of blood lost and the number of lymph nodes retrieved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The technical difficulties associated with conventional open distal gastrectomy with D2 lymph node dissection of gastric cancer are increased in patients with high body mass index (BMI) values, since the N2 regional lymph nodes lie deep within the fatty tissues around the major abdominal vessels, which may be associated with hemorrhage.1 Multivariate logistic regression analysis of a large number of patients who underwent gastrectomy with D2 and D3 lymph node dissection has revealed that obesity is one of major predictors of serious surgical complications,2,3 and several studies have demonstrated increased postoperative morbidity and mortality after gastrectomy in obese patients.4–7,2
A person who is twice the ideal weight or who has a BMI value >40 kg/m2 is considered to be obese.4 The excess intraperitoneal fat tissue in obese patients often results in a reduced field of view and difficulty in controlling intraoperative blood loss during surgery. In addition, both the fat volume and abdominal shape of gastric cancer patients influence their short-term surgical outcomes after distal gastrectomy with D2 lymph node dissection.8 However, little is known about the individual factors (e.g., intra-abdominal fat, subcutaneous fat, and abdominal shape) that make lymph node dissection difficult.
Laparoscopy-assisted distal gastrectomy (LADG), which is increasingly used for gastric cancer surgery,9,10 is a safe and useful technique when performed by a skilled surgeon.11 Several studies have reported lower intraoperative blood loss9,12–14 and similar accuracy of lymph node dissection15,14 for LADG compared with conventional open distal gastrectomy. Accurate lymph node dissection requires a clear operative field without massive bleeding. Laparoscopy provides a good field of vision even in the depths of the abdominal cavity, which facilitates lymph node dissection. However, few studies have examined the feasibility of LADG in obese patients.16,17
In the present study, we evaluate the influence of fat volume such as subcutaneous fat, and visceral fat as the respective fatty areas, and body size assessed by the distance between the peritoneum and root of celiac axis measured by multidetector row computed tomography (MDCT) when performing a distal gastrectomy for gastric cancer. Additionally, we examine these factors when the operation is laparoscopy assisted (LADG).
Materials and Methods
Patient Characteristics
Between March 2005 and June 2006, 154 patients with early gastric cancer underwent distal gastrectomy with modified D2 lymph node dissection at the Department of Gastrointestinal Surgery of the Cancer Institute, Tokyo, Japan. The indication for LADG is limited to clinically diagnosed early gastric cancer, which is an extra-indication for endoscopic submucosal dissection (ESD). Of these patients, 50 underwent distal gastrectomy with the conventional open procedure (OPEN) and 104 underwent LADG (LADG). Although the number of LADG procedures performed in Japan is increasing gradually, LADG is not standard therapy for early gastric cancer. Further, the Japanese Research Society for Gastric Cancer (JRSGC) has defined LADG as a therapeutic approach for use in clinical trials.19 Therefore, we ask patients preoperatively whether they are willing to undergo LADG or would prefer the conventional open method. In the present study, the greater number of LADG procedures was due to patient requests. In addition, the two gastric cancer specialists recruited to the study have extensive experience with LADG, having performed more than 300 such procedures. All data were collected retrospectively and the collection of patients’ individual data was approved by an institutional review board. The patients’ backgrounds and clinicopathologic characteristics were analyzed retrospectively.
Histologically, all of the tumors were classified as adenocarcinomas that had invaded the mucosa or submucosa of the stomach without lymph node metastasis (cT1, cN0). Clinical classification of tumor depth (cT) and nodal involvement (cN) was evaluated preoperatively and intraoperatively by barium radiography, upper gastrointestinal tract endoscopy, abdominal ultrasonography, computed tomography (CT), and endoscopic ultrasonography. The indication for these surgical procedures was intramucosal or submucosal carcinoma without lymph node metastasis (cT1, cN0). Gender, age, BMI, preoperative complications, and clinical staging were documented for all the patients.
Exclusion Criteria
Patients were excluded if they had cardiac (higher than grade II in the New York Heart Association scale), pulmonary (higher than grade II in the Hugh–Jones scale), hepatic (Child classes B and C) or renal insufficiency.
Lymphadenectomy for Gastric Cancer
For patients in the OPEN and the LADG groups, the scope of lymph node dissection was as described previously.18 The lymph node stations correspond to the specific lymph node tiers designated by the Japanese Research Society for Gastric Cancer (JRSGC).19 Lymphadenectomy of the modified D2 dissection (D1+beta) was performed for all patients who were diagnosed preoperatively as having mucosal or submucosal gastric cancer. We used the recently revised definition of second-tier nodes by the JRSGC,19 which includes the hepatoduodenal ligament (station 12a) and the root of the superior mesenteric vein (station 14 v), in addition to the criteria of the American Joint Committee on Cancer (AJCC; 1987).20 Complete D2 dissection is defined by the JRSGC as including all of the above stations. The dissection of first-tier nodes as well as preferential lymph nodes along the left gastric (station 7), common hepatic (station 8a) and celiac (station 9) arteries is defined as a modified D2 dissection, and these four stations are defined as selective second-tier stations.
Reconstruction
Pylorus-preserving gastrectomy (PPG) was indicated if the cancer was located in the distal stomach, at least 5 cm proximal to the pyloric ring. The application of PPG was restricted to patients with cancer in the gastric body, so as to maintain a safe distal margin (2 cm) from the lesion. The distal part of the stomach was resected while retaining a 3-cm pyloric cuff. LADG with Billroth I (B-I) anastomosis was indicated if the cancer was located in the distal stomach less than 5 cm proximal to the pyloric ring. In this instance, B-I reconstruction was performed using end-to-end anastomosis (EEA) with a mechanical stapling device (Tyco Healthcare, Japan). All anastomotic procedures were established extracorporealy; therefore, this operation is not purely laparoscopic distal gastrectomy but laparoscopic-assisted distal gastrectomy.
Clinical Data
The following parameters were recorded: operation time, estimated blood loss, degree of lymph node dissection, and intraoperative complications. All resected stomachs were opened immediately after surgery, and the dissected lymph nodes were categorized and counted by pathologist according to the anatomic distribution and numbering of the regional lymph nodes, based on the JRSGC classification system.19 Lymph nodes were retained for comparison of the procedures with respect to the quality of the lymph node dissection. Sections cut from formalin-fixed specimens were stained with hematoxylin–eosin. Histologic determinations were made of the depth of wall invasion, number of harvested lymph nodes, and presence or absence of lymph node metastasis.
The following postoperative data were recorded: gastric fullness (for cases of upper abdominal distention, remnant stomach fullness on X-ray, and starvation longer than 24 h), anastomotic problems (leakage, stenosis, bleeding ulcer), ileus, early-dumping syndrome, pancreatitis and pancreatic juice leakage, total amount of analgesic drugs up to and including postoperative Day 3, time to first flatus, time to first oral intake, and postoperative hospital stay.
Evaluation of Factors Related to High BMI
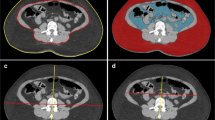
To estimate the BMI-related factors, subcutaneous fat, and visceral fat were calculated from the respective fat area at the celiac axis using MDCT with a fat area evaluation program (Slim Vision; KGT Inc., Japan). In addition, the distance between the peritoneum and root of the celiac axis were measured using the Slim Vision software.
All the CT scans were performed in the 4-week period before surgery. CT scans were performed using a four-channel MDCT (LightSpeed QX/i; GEYMS, Japan) using the following parameters: 120 kVp, 200 mAs, 10.0-mm beam collimation, 0.75 beam pitch, 2.5-mm slice thickness, prone position for 60 s after contrast medium (Iopamiron-370 syringe or iopamidol; Nihon Schering, Japan) injection.
Influences of Fat Volume and Body Shape on Operation Time, Estimated Blood Loss, and Numbers of Excised Lymph Nodes
To evaluate the influences of fat volume and abdominal shape on operation time, estimated blood loss, and numbers of excised lymph nodes, we fitted separate regression models with different slopes and different intercepts corresponding to OPEN and LADG. The individual influences of OPEN and LADG were tested by the t test for the regression coefficients of the slopes. The differences between the influences of OPEN and LADG were tested by the F test for the comparison of the two regression coefficients of the slopes.
Statistical Analysis
All data are presented as means ± SE. The results were compared for patients undergoing OPEN and LADG. Statistical analysis was performed using Welch’s t test to examine the differences between the means of variables, and the Fisher’s exact test was used to test the level of independence between the two groups. The hypotheses were tested with a significance level (P value) of 0.05.
Results
Clinicopathologic Characteristics of the Patients
The clinical histories were similar for all the patients (Table 1), as were their concurrent illnesses. There were no significant differences between the groups in terms of age, BMI or clinical staging, although the number of females was significantly higher in the LADG group.
The mean operation time for the OPEN procedure was more than 45 min shorter than that for the LADG procedure (176 ± 6 min vs. 227 ± 5 min; P < 0.001).The mean estimated blood loss volume for the OPEN procedure was more than four times greater than that for the LADG procedure (167 ± 15 mL vs. 38 ± 3 mL; P < 0.001). The mean number of dissected lymph nodes in the LADG group was higher than that in the OPEN group (34 ± 1 vs. 29 ± 1; P < 0.002).
Comparison of BMI Values, Fat Volumes, and Abdominal Shapes between LADG and OPEN
The BMI values and BMI-related factors are summarized in Table 2. There were no significant differences between the groups in terms of BMI and subcutaneous fat area. The visceral fat areas at the celiac axis (P = 0.020) were significantly larger in the OPEN group, and the peritoneum to root of the celiac axis distance was significantly longer in the OPEN group (P = 0.015).
Influences of Fat Volume and Abdominal Shape on Operation Times of LADG and OPEN
The influences of fat volume and abdominal shape on operation time were evaluated as regression coefficients. No significant positive regression coefficients for operation time were found for either the OPEN or LADG procedure with respect to BMI, subcutaneous fat at the celiac level, visceral fat at celiac level, and distance between the peritoneum and celiac axis. A comparison of the two regression coefficients of operation time between the LADG and OPEN procedures did not show any significant differences for any of the factors.
Influences of Fat Volume and Abdominal Shape on Estimated Blood Losses during LADG and OPEN
For the OPEN procedure, significant increased regression coefficients were found for all factors related to fat volume and abdominal shape, including BMI (P < 0.001; Fig. 1A), subcutaneous fat at the celiac level (P = 0.029; Fig. 1B), visceral fat at the celiac level (P < 0.001; Fig. 1C) and distance between the peritoneum and celiac axis (P < 0.001; Fig. 1D). The increases in all these factors were significantly related to increases in estimated blood loss in the OPEN procedure. On the other hand, no significant increases in the regression coefficients for factors related to fat volume and abdominal shape, including BMI (P = 0.167; Fig. 1A), subcutaneous fat at the celiac level (P = 0.573; Fig. 1B), visceral fat at the celiac level (P = 0.068; Fig. 1C), and distance between the peritoneum and celiac axis (P = 0.122; Fig. 1D), were found for estimated blood loss in the LADG procedure. Comparison of the regression coefficients for estimated blood loss between the LADG and OPEN procedures showed significant differences, albeit not for the factor of subcutaneous fat (P = 0.125).
Influence of fat volume and abdominal shape on estimated blood loss. To evaluate the influences of fat volume and abdominal shape on estimated blood loss, separate regression models were fitted with different slopes and different intercepts corresponding to OPEN and LADG. Data are presented as the regression coefficients and corresponding P values. The individual influences of OPEN and LADG are tested by the t test for their regression coefficients of the slopes. The differences in the influences of OPEN and LADG are tested by the F test for the comparison of two regression coefficients of the slopes. BMI Body weight/height2. P values < 0.05 are considered to indicate statistical significance. Effects on estimated blood loss of: a BMI, b subcutaneous fat volume at the celiac level, c visceral fat volume at the celiac level; and d distance between the peritoneum and celiac axis on estimated blood loss.
Influences of Fat Volume and Abdominal Shape on Numbers of Dissected Lymph Nodes of LADG and OPEN
For the OPEN procedure, negative regression coefficients for the numbers of dissected lymph nodes were found for BMI (P = 0.010; Fig. 2A), subcutaneous fat at the celiac level (P = 0.018; Fig. 2B), and distance between the peritoneum and celiac axis (P < 0.001; Fig. 2D). The factor of visceral fat at the celiac level (P = 0.133; Fig. 2C) showed only modest influence on the numbers of dissected lymph nodes for the OPEN procedure. In contrast to the data obtained for the OPEN procedure, no negative regression coefficients were found for any of the factors in the LADG group. However, the factor of visceral fat at the celiac level (P = 0.142; Fig. 2C) showed modest influence on the numbers of dissected lymph nodes. Comparison of the regression coefficients for numbers of dissected lymph nodes between the LADG and OPEN procedures showed significant differences for BMI (P = 0.050), subcutaneous fat at the celiac level (P = 0.049), and distance between the peritoneum and celiac axis (P < 0.001).
Influence of fat volume and abdominal shape on numbers of excised lymph nodes. To evaluate the influences of fat volume and abdominal shapes on the numbers of excised lymph nodes, separate regression models were fitted with the different slopes and different intercepts corresponding to OPEN and LADG. Data are presented as the regression coefficients and corresponding P values. The individual influences of OPEN and LADG are tested by the t test for their regression coefficients of the slopes. The differences in the influences of OPEN and LADG are tested by the F test for the comparison of two regression coefficients of the slopes. BMI weight/height2. P values < 0.05 are considered to indicate statistical significance. Effects on numbers of excised lymph nodes of: a BMI, b subcutaneous fat volume at the celiac level, c visceral fat volume at the celiac level, and d distance between the peritoneum and celiac axis.
Discussion
Since 1996, the use of LADG has increased rapidly in Japan,21 and many retrospective22–24,13 and prospective9,25 studies have shown the safety, efficacy, and feasibility of laparoscopy-assisted gastrectomy. However, LADG with extended lymph node dissection for gastric cancer is generally considered to be more complicated than the conventional open procedure, owing to the complexity of lymph node dissection.26,25 Additional technical difficulties, including high conversion or extension of incisions and prolonged operation time, have been noted for LADG in heavier patients.16 Open gastric surgery also shows higher rates of postoperative complications, longer operation times, greater estimated blood loss volumes, and lower numbers of dissected lymph nodes in obese patients than in non-obese patients.5–7 However, in several studies of laparoscopic cholecystectomy27,28 and LADG,17 no significant differences have been found between obese and non-obese patients in terms of operating time, conversion rate to open surgery, postoperative complication rate or length of hospital stay. Therefore, we propose that LADG can be used to treat obese patients if the surgeon is skilled.
Early operative outcomes are assessed using the parameters of operation time, estimated blood loss volume, and number of dissected lymph nodes.15,12 Although some studies have demonstrated significantly higher numbers of dissected lymph node in OPEN procedures compared to LADG procedures,14,12,29 we have previously demonstrated that the quality of lymph node dissection in LADG is comparable to that in OPEN procedures15 if the surgeon is skilled and experienced. In the present study, there was less blood loss and more lymph nodes dissected in the LADG group compared with the OPEN group. Both of these outcomes can be explained by the better accessibility of the laparoscopic view in deep lesions in the abdominal cavity afforded by LADG and better control of bleeding because of the improved view, even in abundant adipose tissues. The proportion of males was higher in the OPEN group than in the LADG group. Visceral fat and larger abdominal shape are recognized more often in male patients than in female patients with same BMI. The analysis of BMI-related factors revealed different body compositions, such as significantly more visceral fat and longer peritoneum to celiac axis distance, in the OPEN group. These factors might disrupt the operative procedures, resulting in poor surgical outcomes in the OPEN group, such as greater blood loss and fewer dissected lymph nodes.
To reveal the BMI-related factors that disrupt the operative procedure and give poorer operative outcomes in the OPEN group, regression analysis was performed to evaluate the influences of these BMI-related factors on the operative outcomes as continuous variables. The operative time in both groups was not extended without affecting the degree of obesity and large abdominal shape. The effect on estimated blood loss was significantly different between the two procedures. Although BMI-related factors affected blood loss in the OPEN group, none of the BMI-related factors were associated with blood loss in the LADG group. Further multivariate analysis identified a strong association between peritoneum to celiac distance and increased estimated blood loss during the OPEN procedure (data not shown). Among all the BMI-related factors, factors related to abdominal shape were the most disruptive for the OPEN procedure. The advantages of LADG for obese and large-bodied patients, i.e., ensuring accessibility and reducing bleeding, may account for the reduced blood loss during LADG, as compared to the OPEN procedure.
For the OPEN procedure, BMI, subcutaneous fat, and distance between the peritoneum and celiac axis were associated with a decreased number of dissected lymph nodes. On the other hand, there were no influences of these BMI-related factors on lymph node dissection in the LADG procedure. Only the factor of visceral fat at the celiac level was modestly associated with a decreased number of dissected lymph node in both groups. These data suggest that lymph node dissection in obese and large-bodied patients is more disrupted by the OPEN procedure than by the LADG procedure. However, it should be noted that intra-abdominal obesity may disturb lymph node dissection even in the LADG.
Several studies have pointed out that for patients who are undergoing OPEN gastrectomy, being overweight increases the risk of surgical complications.2,3,5,6,8 In the present study, no influence of BMI-related factors on complication rate was detected for either group (data not shown). As this was a single institutional trial with a limited number of patients, the overall complication rate was not as high as that seen in other studies with high numbers of patients.
In conclusion, the present study clearly demonstrates that increasing fat content and body size have little effect on bleeding and lymph nodes retrieved in LADG, whereas increasing fat content and body size disturbs the precise lymph nodes dissection and increase the blood loss in purely conventional open distal gastrectomy probably due to the high level of accessibility and clear view of the operative field conferred by the laparoscope and laparoscopic grasper forceps.
References
Barry JD, Blackshaw GR, Edwards P, Lewis WG, Murphy P, Hodzovic I, Thompson IW, Allison MC. Western body mass indices need not compromise outcomes after modified D2 gastrectomy for carcinoma. Gastric Cancer 2003;6:80–85.
Kodera Y, Sasako M, Yamamoto S, Sano T, Nashimoto A, Kurita A. Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg 2005;92:1103–1109.
Tsujinaka T, Sasako M, Yamamoto S, Sano T, Kurokawa Y, Nashimoto A, Kurita A, Katai H, Shimizu T, Furukawa H, Inoue S, Hiratsuka M, Kinoshita T, Arai K, Yamamura Y. Influence of overweight on surgical complications for gastric cancer: results from a randomized control trial comparing D2 and extended para-aortic D3 lymphadenectomy (JCOG9501). Ann Surg Oncol 2007;14:355–361.
Moriwaki Y, Kunisaki C, Kobayashi S, Harada H, Imai S, Kasaoka C. Does body mass index (BMI) influence morbidity and long-term survival in gastric cancer patients after gastrectomy? Hepatogastroenterology 2003;50:284–288.
Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, Kohno H, Nagasue N. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology 2000;59:18–23.
Kodera Y, Ito S, Yamamura Y, Mochizuki Y, Fujiwara M, Hibi K, Ito K, Akiyama S, Nakao A. Obesity and outcome of distal gastrectomy with D2 lymphadenectomy for carcinoma. Hepatogastroenterology 2004;51:1225–1228.
Inagawa S, Adachi S, Oda T, Kawamoto T, Koike N, Fukao K. Effect of fat volume on postoperative complications and survival rate after D2 dissection for gastric cancer. Gastric Cancer 2000;3:141–144.
Lee JH, Paik YH, Lee JS, Ryu KW, Kim CG, Park SR, Kim YW, Kook MC, Nam BH, Bae JM. Abdominal shape of gastric cancer patients influences short-term surgical outcomes. Ann Surg Oncol 2007;14:1288–1294.
Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306–S311. doi:10.1067/msy.2002.120115.
Adachi Y, Suematsu T, Shiraishi N, Katsuta T, Morimoto A, Kitano S, Akazawa K. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg 1999;229:49–54.
Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg 2000;135:806–810.
Lee SI, Choi YS, Park do J, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg 2006;202:874–880.
Noshiro H, Nagai E, Shimizu S, Uchiyama A, Tanaka M. Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc 2005;19:1592–1596.
Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H. Laparoscopic assisted distal gastrectomy for early gastric cancer: five years' experience. Surgery 2005;137:317–322.
Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol 2005;11:7508–7511.
Noshiro H, Shimizu S, Nagai E, Ohuchida K, Tanaka M. Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann Surg 2003;238:680–685.
Yasuda K, Inomata M, Shiraishi N, Izumi K, Ishikawa K, Kitano S. Laparoscopy-assisted distal gastrectomy for early gastric cancer in obese and nonobese patients. Surg Endosc 2004;18:1253–1256.
Shimoyama S, Kaminishi M, Joujima Y, Oohara T, Hamada C, Teshigawara W. Lymph node involvement correlation with survival in advanced gastric carcinoma: univariate and multivariate analyses. J Surg Oncol 1994;57:164–170.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1998;1:10–24.
Beahrs OH, Henson DE, Hutter RVP, Myers MH. American Joint Committee on Cancer, eds. Manual for Staging of Cancer 3rd ed Philadelphia, Pa: J B Lippincott 1988;1988.
Kitano S, Shiraishi N. Current status of laparoscopic gastrectomy for cancer in. Japan Surg Endosc 2004;18:182–185.
Mochiki E, Nakabayashi T, Kamimura H, Haga N, Asao T, Kuwano H. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg 2002;26:1145–1149.
Asao T, Kuwano H, Mochiki E. Laparoscopic surgery update for gastrointestinal malignancy. J Gastroenterol 2004;39:309–318.
Yano H, Monden T, Kinuta M, Nakano Y, Tono T, Matsui S, Iwazawa T, Kanoh T, Katsushima S. The usefulness of laparoscopy-assisted distal gastrectomy in comparison with that of open distal gastrectomy for early gastric cancer. Gastric Cancer 2001;4:93–97.
Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172–1176.
Rosin D, Brasesco O, Rosenthal RJ. Laparoscopy for gastric tumors. Surg Oncol Clin N Am 2001;10:511–529.
Angrisani L, Lorenzo M, De Palma G, Sivero L, Catanzano C, Tesauro B, Persico G. Laparoscopic cholecystectomy in obese patients compared with nonobese patients. Surg Laparosc Endosc 1995;5:197–201.
Collet D, Edye M, Magne E, Perissat J. Laparoscopic cholecystectomy in the obese patient. Surg Endosc 1992;6:186–188.
Fujiwara M, Kodera Y, Kasai Y, Kanyama Y, Hibi K, Ito K, Akiyama S, Nakao A. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg 2003;196:75–81.
Acknowledgments
We are deeply indebted to Mr. Tomohiro Tachikawa and Mr. Yoshiko Nishimura for technical help with CT scanning for the measurement of BMI-related factors. We are grateful to members of the KGT Inc., Japan for the software package used to evaluate the fat areas. Finally, we thank Ms. Noriko Okita for English correction of the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiki, N., Fukunaga, T., Yamaguchi, T. et al. Increased Fat Content and Body Shape Have Little Effect on the Accuracy of Lymph Node Retrieval and Blood Loss in Laparoscopic Distal Gastrectomy for Gastric Cancer. J Gastrointest Surg 13, 626–633 (2009). https://doi.org/10.1007/s11605-008-0768-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0768-4