Abstract
Background
Natural orifice translumenal endoscopic surgery (NOTES) is surgically challenging. Current endoscopic tools provide an insufficient platform for visualization and manipulation of the surgical target. This study demonstrates the feasibility of using a miniature in vivo robot to enhance visualization and provide off-axis dexterous manipulation capabilities for NOTES.
Methods
The authors developed a dexterous, miniature robot with six degrees of freedom capable of applying significant force throughout its workspace. The robot, introduced through the esophagus, completely enters the peritoneal cavity through a transgastric insertion. The robot design consists of a central “body” and two “arms” fitted respectively with cautery and forceps end-effectors. The arms of the robot unfold, allowing the robot to flex freely for entry through the esophagus. Once in the peritoneal cavity, the arms refold, and the robot is attached to the abdominal wall using the interaction of magnets housed in the robot body with magnets in an external magnetic handle. Video feedback from the on-board cameras is provided to the surgeon throughout a procedure.
Results
The efficacy of this robot was demonstrated in three nonsurvivable procedures in a porcine model, namely, abdominal exploration, bowel manipulation, and cholecystectomy. After insertion, the robot was attached to the interior abdominal wall. The robot was repositioned throughout the procedure to provide optimal orientations for visualization and tissue manipulation. The surgeon remotely controlled the actuation of the robot using an external console to assist in the procedures.
Conclusion
This study has shown that a dexterous miniature in vivo robot can apply significant forces in arbitrary directions and improve visualization to overcome many of the limitations of current endoscopic tools for performing NOTES procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopy revolutionized general surgery beginning in the 1990s, and many procedures that previously were performed through a large open incision currently are performed using minimally invasive techniques. These techniques generally have proved to be safer, with improved patient outcomes.
Natural orifice translumenal endoscopic surgery (NOTES) is a new alternative to abdominal surgery that uses endoscopic techniques to attempt complete elimination of external incisions. Theoretically, NOTES offers significant patient advantages by eliminating complications associated with external incisions including wound infections, pain, and hernia formation, and by reducing adhesions, improving cosmetics, and shortening recovery times [1, 2].
The first study in an animal model to demonstrate the safety and feasibility of a peroral transgastric endoscopic approach to the peritoneal cavity was reported by Kalloo et al. [3] in 2004. This study consisted of 12 acute and 5 survival procedures, including examination of the peritoneal cavity biopsy of the liver. Subsequent survival studies using transgastric access include ligation of fallopian tubes [4], peritoneal exploration with organ resection [5], gastrojejunal anastomosis [6, 7] partial hysterectomy [8], lymphadenectomy [9], and oophorectomy and tubectomy [10]. Alternative methods for accessing the peritoneal cavity also have been evaluated including the transvesical and transcolonic approaches [10, 11].
Multiple transvaginal and transgastric NOTES procedures also have been performed in humans successfully. Rao et al. [12] have successfully attempted the translumenal approach for 17 cases including appendectomy, liver biopsy, and tubal ligation. Additional case reports include hybrid transvaginal laparoscopically assisted cholecystectomy [13, 14], transvaginal cholecystectomy [15, 16], transgastric cholecystectomy [17], and percutaneous endoscopic gastrotomy (PEG) rescue [18]. Feasibility studies include flexible transgastric peritoneoscopy with liver biopsy during laparoscopic gastric bypass surgery [19] and endoscopic peritoneoscopy [20].
Although these studies have demonstrated the feasibility of a NOTES approach, significant constraints also have been identified with the use of a flexible endoscopy platform, including a relative inability to apply off-axis forces, inadequate triangulation, and limitations in passing multiple instruments simultaneously into the peritoneal cavity [21].
Much work toward addressing these constraints currently is focused on further refinement of the flexible endoscopy platform. For example, the ViaCath System currently is being developed as an endoluminal NOTES robotic system [22]. The first-generation system consists of a master console with haptic interfaces, slave drive mechanisms, and flexible instruments located alongside a standard gastroscope or endoscope. A second-generation system with a shoulder–elbow configuration similar to the human arm currently is under development at Purdue University.
Furthermore, the TransPort EndoSurgical Operating Platform (USGI Medical, San Clemente, CA, USA) is four-channel platform scope based on the Shapelock locking technology (USGI Medical, San Clemente, CA) [23]. The TransPort has a flexible state allowing for insertion of the device through the gastrointestinal tract. Once positioned, the base of the endoscope can be locked into position while allowing the distal operating tip to be steered freely. The TransPort contains four operating channels that accommodate two 6-mm and two 4-mm instruments. The EndoSurgical Operating System, including the TransPort operating platform, is available commercially.
An alternative approach to addressing the limitations of the flexible endoscopy platform is the use of in vivo robots that can be inserted through the natural lumen of the intestinal tract. These devices can access the peritoneal cavity through a transgastric, transcolonic, or transvaginal incision. Once fully inserted, these robots are no longer constrained by the entrance incision or the geometry of the lumen. This enables the surgeon to position the robots within each quadrant of the peritoneal cavity for visualization and task assistance. Furthermore, multiple devices can be inserted into the peritoneal cavity through the same access point.
For example, the Magnetic Anchoring and Guidance System (MAGS) uses multiple instruments, including tissue retractors and cautery dissectors, deployed through a single access port [24]. Each instrument is attached to and positioned along the interior abdominal wall using magnetic coupling of magnets embedded in the instruments with an external handheld magnet. Once each instrument is positioned, the handheld magnet can be replaced with an 18-gauge percutaneous, threaded needle anchor. The MAGS together with an endoscope has been used successfully for transgastric, transcolonic, and transvaginal nonsurvivable cholecystectomies in porcine models.
Similarly, a preliminary procedure using three miniature in vivo robots including a peritoneum-mounted imaging robot, a lighting robot, and a retraction robot has demonstrated the feasibility of developing a robotic platform using cooperative robots with sufficient functionality for performing NOTES procedures [25].
Furthermore, mobile in vivo robots provide a remotely controlled, maneuverable platform for vision and surgical task assistance. The basic design of a mobile robot consists of two independently driven helical-profiled wheels providing forward, reverse, and turning maneuverability, and a tail to prevent counter-rotation. This basic platform with visualization and task assistance capabilities has been demonstrated in multiple animal model laparoscopic procedures. A mobile robot also has successfully demonstrated transgastric access to the peritoneum with sufficient traverse mobility in the gastric and peritoneal cavities [26].
Materials and methods
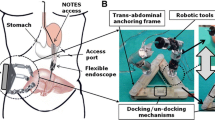
The robotic platform for NOTES, shown in Fig. 1, consists of a miniature in vivo robot inserted fully into the peritoneal cavity through a natural orifice, a surgeon control console, and an external magnetic handle.
The basic design of the dexterous miniature robot for NOTES, shown in Figs. 2 and 3, consists of two “arms” connected to a central “body” by a rotational “shoulder” joint. The body of the robot contains a stereovision pair for providing visualization of the surgical field. Each arm is composed of upper and lower arms, with the lower arm extending and retracting from the upper arm. The cautery end effector can be retracted fully into the upper arm for insertion. With the second-generation robot, the lower arm also rotates with respect to the upper arm. The right arm is fitted with a cautery and the left lower arm with a grasper end effector. The mass of the robot is approximately 110 g.
The robot has two configurations enabling flexibility for natural orifice insertion and rigidity for tissue manipulation. In the articulation configuration, a linkage connecting the body and the upper arm is used to rotate the shoulder joint. For insertion, this linkage is decoupled magnetically from the upper arm, allowing each arm to rotate freely at the shoulder joint. Once the robot is fully inserted into the peritoneal cavity, the linkages are reconnected with the assistance of endoscopic tools. In future designs, the robot will self-assemble for articulation and disassemble for robot removal.
The body of the robot contains embedded magnets that couple with magnets in an external handle or the surgeon console for attachment of the robot to the interior abdominal wall. The exterior magnet handle can be moved along the outer surface of the abdomen for gross repositioning of the robot throughout the procedure. The handle also can be used to deform the abdominal wall, allowing for an additional degree of freedom. This attachment method enables the surgeon to position the robot to obtain views and workspaces within each quadrant of the peritoneal cavity without necessitating an additional endoscope or retroflexed position. The body of the robot also contains three embedded eyelets that allow the surgeon to suture the robot to the abdominal wall.
The surgeon console, shown in Fig. 4, consists of two analog joysticks, each with three degrees of freedom, for controlling the manipulation of the robot arms. The gripper joystick also has two pushbutton controls for opening and closing the grasper jaws. An 8-in. TFT color LCD monitor is located between the two joysticks to display the video from the robot cameras. A foot pedal is used to activate the cautery capability. The design of the surgeon console together with the robot provides essentially a laparoscopic platform for performing NOTES procedures.
Iterations of the dexterous miniature robots for NOTES have been prototyped and tested in three nonsurvivable animal model studies. The procedures were performed at the University of Nebraska Medical Center with experimental protocols approved by the institutional review committee. The weight of the pigs varied from 60 to 80 lb. For each procedure, the pig was fed Gatorade and water for 36 h before the procedure.
The basic procedure was initiated with the creation of a standard natural orifice gastrotomy using a needle knife. An overtube with an external diameter of 27 mm then was inserted through a transesophageal incision and advanced up to the gastrotomy. The robot was configured for insertion and advanced into the peritoneal cavity using a standard therapeutic endoscope. The endoscope was used throughout the procedure to provide retraction and supplementary visualization. Two of the procedures focused on evaluating the functionality of the robot. For these procedures, the robot was inserted into the peritoneal cavity through a transabdominal incision, with gross tissue retraction provided by an articulating fan retractor and supplementary visualization by a standard laparoscope.
In the first procedure, the robot was lifted from the floor of the peritoneal cavity using the external magnetic handle composed of two magnets, each with a surface field of 6,645 Gauss. The surgeon then maneuvered the handle to explore each quadrant of the peritoneal cavity using the on-board cameras and to identify a small bowel target for tissue manipulation. The robot then was positioned for dissection, and the grasper arm was extended to grasp the tissue. The grasper arm retracted the tissue to allow access for the cautery arm. The shoulder of the cautery arm then was rotated, and the cautery was extended for cauterization of the small bowel. The lower arm of this robot could not rotate, thus limiting the surgeon’s dexterity for performing cholecystectomy. At the end of the procedure, the robot could be retrieved by pulling its tether through the hole initially used for its insertion.
In subsequent procedures, the surgeon used the dexterous robot to attempt cholecystectomy. Again, the surgeon used magnetic coupling to attach the robot to the interior abdominal wall and manipulated the exterior magnetic handle to explore each quadrant of the peritoneal cavity. The robot then was positioned for the cholecystectomy using the external magnetic handle, as shown in Fig. 5. Next, the grasper end effector was oriented and extended to grasp the cystic duct. The cautery end effector then was moved into position for dissection and division of the cystic duct. Dissection continued through iterations of the stretch and dissection task, with the gallbladder being removed from its hepatic attachments. Views from the robot cameras of the gallbladder dissection for the second and third robot procedures are shown in Figs. 6 and 7, respectively.
Results
These nonsurvivable animal model studies using a dexterous in vivo robot platform successfully demonstrated the feasibility of performing a NOTES cholecystectomy from essentially a laparoscopic platform. The two-configuration design of the robot enabled sufficient flexibility for transgastric insertion while providing a stable platform for visualization and tissue manipulation. The placement of the cameras between the two robot arms improved triangulation compared with a flexible endoscopy platform. Furthermore, the robot design allowed the application of sufficient off-axis forces for tissue retraction and dissection. The addition of an ultrabright LED to the robot body in the third animal study greatly improved visualization of the surgical environment.
Although these studies successfully demonstrated the feasibility of performing an in vivo robot-assisted NOTES cholecystectomy, problems were encountered. The second study was converted to an open procedure after dissection of the cystic duct due to insufficient magnetic coupling for attaching the robot to the interior abdominal wall. The dissection of the gallbladder from its hepatic attachments was performed with the robot supported externally. The third study demonstrated improved visualization with sufficient magnetic coupling, but ended prematurely due to mechanical failure of the shoulder joint. These issues have been addressed in continuing iterations of the robot design without limiting robot functionality.
Discussion
Studies demonstrating the feasibility of NOTES procedures have identified significant challenges associated with using a flexible endoscopy platform to perform NOTES procedures. These devices are designed to be used with procedures in which the surgical target is in line with the light source and camera. These are conditions different from what exists for procedures in the peritoneal cavity, creating challenges in image stability, triangulation, force application, and multitasking capabilities. For NOTES to be widely adopted as an alternative to laparoscopic surgery, these challenges must be addressed.
One potential approach for addressing the limitations of the flexible endoscopy platform is to use miniature in vivo robots. An in vivo robot can be advanced into the peritoneal cavity using the upper approach. Once fully inserted, the robot is no longer constrained by the entrance incision, allowing the platform to provide stable visualization and sufficient force application from multiple orientations and workspaces within the peritoneal cavity.
The three animal model procedures using a dexterous miniature robot described in this report together demonstrate the feasibility of using an in vivo robot platform for performing NOTES procedures in the peritoneal cavity. Continuing improvements to the robotic platform are being pursued including an additional degree of freedom at the shoulder joint to improve cholecystectomy dissection and reduced size to enable peroral insertion. Also, cooperative robots are being developed that provide additional capabilities, including gross tissue retraction, to provide functionality sufficient for performing a NOTES cholecystectomy using only in vivo robots.
References
Kalloo AN, Rattner D, Brugge W, Gostout CJ, Hawes RH, Kantsevoy SV, Marohn M, Pasricha J, Ponsky J, Richards W, Rothstein R, Soper N, Swanstrom L, Thompson C (2005) ASGE/SAGES working group on natural orifice translumenal endoscopic surgery white paper. Gastrointest Endosc 62:199–203
Ko C-W, Kalloo AN (2006) Peroral transgastric abdominal surgery. Chin J Dig Dis 7:67–70
Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV (2004) Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc 60:114–117
Jagannath SB, Kantsevoy SV, Vaughn CA, Chung SSC, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Scorpio DG, Magee CA, Pipitone LJ, Kalloo AN (2005) Peroral transgastric endoscopic ligation of fallopian tubes with long-term survival in a porcine model. Gastrointest Endosc 61:449–453
Wagh M, Merrifield B, Thompson C (2005) Endoscopic transgastric abdominal exploration and organ resection: initial experience in a porcine model. Clin Gastroenterol Hepatol 3:892–896
Kantsevoy SV, Jagannath SB, Niiyama H, Chung SSC, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Vaughn CA, Barlow D, Shimonaka H, Kalloo AN (2005) Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc 62:287–292
Bergstrom M, Ikeda K, Swain P, Park PO (2006) Transgastric anastomosis by using flexible endoscopy in a porcine model (with video). Gastrointest Endosc 63:307–312
Merrifield BF, Wagh MS, Thompson CC (2006) Peroral transgastric organ resection: a feasibility study in pigs. Gastrointest Endosc 63:693–697
Fritscher-Ravens A, Mosse C, Ikeda K, Swain P (2006) Endoscopic transgastric lymphadenectomy by using EUS for selection and guidance. Gastrointest Endosc 63:302–306
Wagh MS, Merrifield BF, Thompson CC (2006) Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointest Endosc 3:473–478
Lima E, Henriques-Coelho T, Rolanda C, Pego JM, Silva D, Carvalho JL (2007) Transvesical thoracoscopy: a natural orifice translumenal endoscopic approach for thoracic surgery. Surg Endosc 21:854–858
Rao GV, Reddy DN, Banerjee R (2008) NOTES: human experience. Gastrointest Endosc Clin N Am 18:361–370
Bessler M, Stevens PD, Milone L, Parikh M, Fowler D (2007) Transvaginal laparoscopically assisted endoscopic cholecystectomy: a hybrid approach to natural orifice surgery. Gastrointest Endosc 6:1243–1245
Zornig C, Emmermann A, von Waldenfels HA, Mofid H (2007) Laparoscopic cholecystectomy without visible scar: combined transvaginal and transumbilical appraoch. Endoscopy 39:913–915
Marescaux J, Dallemagne B, Perretta S, Wattiez A, Mutter D, Coumaros D (2007) Surgery without scars: report of transluminal cholecystectomy in a human being. Arch Surg 142:823–826
Zorron R, Filgueiras M, Maggioni LC, Pombo L, Carvalho GL, Oliveira AL (2007) NOTES transvaginal cholecystectomy: report of the first case. Surg Innov 14:279–283
Medical U (2007) USGI announces first NOTES transgastric cholecystectomy procedures, using the USGI Endosurgical Operating System, performed by Dr. Lee Swanstrom at Legacy Hospital in Portland, OR. Updated 2007. http://www.usgimedical.com/news/releases/062507.htm. Retrieved 17 Sep 2007
Marks JM, Ponsky JL, Pearl JP, McGee MF (2007) PEG “rescue”: a practical NOTES technique. Surg Endosc 21:816–819
Steele K, Schweitzer MA, Lyn-Sue J, Kantsevoy SV (2008) Flexible transgastric peritoneoscopy and liver biopsy: a feasibility study in human beings (with videos). Gastrointest Endosc [Epub ahead of print]
Hazey JW, Narula VK, Renton DB, Reavis KM, Paul CM, Hinshaw KE, Muscarella P, Ellison EC, Melvin WS (2008) Natural orifice transgastric endoscopic peritoneoscopy in humans: initial clinical trial. Surg Endosc 22:16–22
Mummadi RR, Pasricha PJ (2008) The eagle or the snake: platforms for NOTES and radical endoscopic therapy. Gastrointest Endosc Clin N Am 18:279–289
Abbott DJ, Becke C, Rothstein RI, Peine WJ (2007) Design of an endoluminal NOTES robotic system. 2007 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Deigo, CA, 29 October–2 November 2007
Swanstrom LL, Whiteford M, Khajanchee Y (2008) Developing essential tools to enable transgastric surgery. Surg Endosc 22:600–604
Scott DJ, Tang S-J, Fernandex R, Bergs R, Goova MT, Zeltser I, Kehdy FJ, Cadeddu JA (2007) Completely transvaginal NOTES cholecystectomy using magnetically anchored instruments. Surg Endosc 21:2308–2316
Lehman AC, Berg KA, Dumpert J, Wood NA, Visty AQ, Rentschler ME, Platt SR, Farritor SM, Oleynikov D (2008) Surgery with cooperative robots. Comput Aided Surg 13:95–105
Rentschler ME, Dumpert J, Platt SR, Farritor SM, Oleynikov D (2006) Natural orifice surgery with an endoluminal mobile robot. Surg Endosc 21:1212–1215
Acknowledgements
This material is based in part on work supported under a National Science Foundation Graduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 2008 Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Meeting, Philadelphia, Pennsylvania, April 9–12, 2008.
Rights and permissions
About this article
Cite this article
Lehman, A.C., Dumpert, J., Wood, N.A. et al. Natural orifice cholecystectomy using a miniature robot. Surg Endosc 23, 260–266 (2009). https://doi.org/10.1007/s00464-008-0195-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0195-3