Abstract
Introduction
Natural orifice translumenal endoscopic surgery (NOTES) is an evolving field and suitable instruments are lacking. The purpose of this study was to perform transvaginal cholecystectomies using instruments incorporated into a magnetic anchoring and guidance system (MAGS).
Methods
Non-survival procedures were conducted in pigs (n = 4). Through a vaginotomy created under direct vision, a rigid access port was inserted into the peritoneal cavity and used to maintain a CO2 pneumoperitoneum. MAGS instruments were deployed through the port and held in place on the peritoneal surface using magnetic coupling via an external handheld magnet which was optionally exchanged for an 18ga percutaneous threaded needle anchor; instruments included a tissue retractor (a clip-fixated magnet or flexible graspers) and a cautery dissector. A gastroscope was used for visualization.
Results
The first two procedures ended prematurely due to instrumentation shortcomings and inadvertent magnetic coupling between instruments; one case required a laparoscopic rescue. Three new forms of instrumentation were developed: (1) a longer access port (50 cm) which provided easier deployment of instruments and suitable reach, (2) a more robust cauterizer with a longer, more rigid, pneumatically deployed tip with better reach and sufficient torque to allow blunt dissection, and (3) a more versatile tissue retractor with bidirectional dual flexible graspers which provided excellent cephalad fundus retraction and inferiolateral infundibulum retraction. With these modifications, 100% of the cholecystectomy was completed in the third and fourth animals using only a NOTES/MAGS approach. Retrieval of the tissue retractor resulted in a rectal injury in the third animal but further procedural modifications resulted in a successful procedure in the fourth animal with no complications.
Conclusions
While still under development with more refinements needed, completely transvaginal cholecystectomy using MAGS instruments is feasible. By offering triangulation and rigidity, MAGS may facilitate a NOTES approach while alleviating shortcomings of a flexible platform.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Natural orifice translumenal endoscopic surgery is a novel and rapidly evolving field. The first published report was in the field of urology by Gettman, et al., who performed a transvaginal nephrectomy on a porcine model [1]. More recently, numerous reports have appeared in the literature with a wide array of gastrointestinal and other abdominal operations being performed via transgastric, transcolonic, and transurethral approaches [2–13]. Tremendous enthusiasm for NOTES has grown, in an effort to realize potential benefits for patients in terms of less pain, decreased scarring, and a faster recovery. These benefits have not yet been proven, and much of the ongoing research focuses on feasibility and safety [14–16].
Central to this innovative field is the development of novel instrumentation that will overcome the shortcomings associated with conventional flexible endoscopic platforms. Namely, the flexible endoscope does not afford suitable stability and torque, provides inadequate maneuverability and reach, makes tissue triangulation difficult, limits the use of multiple instruments simultaneously, and is often associated with visual disorientation. Additionally, there is an obvious lack of surgical instruments with only prototype suturing and stapling devices available [3, 5, 7, 10, 11, 17]. While some investigators have explored the use of ShapelockTM (USGI, San Clemente, California) technology, such instrumentation has met with limited success [9]. Our research team has pursued an alternative approach and developed a magnetic anchoring and guidance system (MAGS) [18]. While this platform was initially designed to decrease the number of trocars used for laparoscopic operations [19, 20], subsequent work has proved its utility for NOTES [21, 22]. This system allows numerous instruments to be deployed through a single access port with magnetic coupling of internal components within the abdominal cavity via external magnets. Indeed, this platform has facilitated laparoscopic nephrectomy in a porcine model with only one [20] or two [19] trocars.
While we have successfully used the MAGS platform for transgastric, transcolonic, and transvaginal cholecystectomy [21, 22], we currently favor the transvaginal approach as this route accommodates a large-diameter rigid access port with excellent reach to the upper abdomen using a conventional endoscope, facilitates access and closure using well-accepted surgical techniques and standard open instrumentation, and eliminates the concerns for potential gastrointestinal leakage. However, our techniques and instruments have not fully evolved, as laparoscopic assistance has been required in our previous studies. The purpose of this study was to perform NOTES cholecystectomy without laparoscopic assistance using MAGS and flexible endoscopic equipment.
Materials and methods
Animals
This study was conducted in the animal endosuite at the Southwestern Center for Minimally Invasive Surgery, University of Texas in Dallas, Texas and the protocol was approved by the institutional animal care and use committee. Transvaginal NOTES cholecystectomy was attempted in four non-survival experiments on pigs (23–57 kg) with successive refinement of the instrumentation and procedural technique (Table 1). Rapid prototyping for access ports and MAGS instruments was performed by engineers at the Texas Manufacturing Assistance Center, Automation and Robotics Research Institute, University of Texas at Arlington.
Transvaginal Access Ports
Access ports (Fig. 1) included a commercially available flexible overtube (19.5 mm OD × 50 cm length, GuardusTM Overtube, US Endoscopy, Mentor, OH) and prototype rigid access ports. The tubes for prototypes (21 mm OD × 30 cm length, 26 mm OD × 30 cm length, and 26 mm OD × 50 cm length) were constructed from polyvinyl chloride (PVC) pipe, threaded, and mated to a receptacle which accommodated the seal and insufflation connection from a conventional laparoscopic trocar (US Surgical Corporation, Norwalk, CT). Instrument tethers (electric, mechanical, and pneumatic) were fixed to the outer surface of the 26 mm OD tubes using silicone sealant (pigs 1–3) or simply held externally by hand during insertion (pig 4).
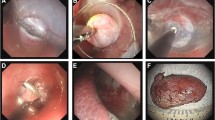
(A) a flexible overtube over the endoscope was used for visualized transvaginal entry into the peritoneal cavity in pig 4. (B) a 26 mm × 30 cm prototype rigid access port integrated tethers for the magnetic detent cauterizer. (C) a 26 mm × 50 cm rigid port incorporated tethers for the flexible grasper cradle and the generation 2 pneumatic cauterizer. (D) the transvaginally placed rigid access port allowed maintenance of the pneumoperitoneum and insertion of the MAGS instruments, which were maneuvered using the external handheld magnet (midepigastric area) and optionally held with the needle anchor (right-upper quadrant)
MAGS Instruments
As previously described [18–22], MAGS instruments (Figs. 1–5) are designed to allow maneuvering of intra-abdominal instruments by use of an external handheld magnet, whereby magnetic attraction forces allow coupling of the internal and external components across the abdominal wall without the need for incisions or trocars. Permanent magnets made from neodymium-iron-boron (NdFeB) are focused and shielded to optimize strength and generate sufficient coupling forces to control the 25–45 gm instruments. Once the internal component is positioned at a desired location, the external component can be optionally exchanged for an 18 gauge percutaneous, threaded needle anchor, which provides secure, rigid, hands-free fixation (Fig. 3).
Gallbladder retraction was afforded via MAGS tissue retractors in two configurations: an attachable magnet (Fig. 2) and a flexible grasper cradle (Fig. 3). The attachable retractor included a permanent magnet that was positioned next to the gallbladder using the external magnet. The internal component was housed in a 16 × 20 mm plastic casing, with an attached suture loop, which was fixated to the gallbladder using endoscopically-deployed endoclips (QuickClip2TM long rotatable clip fixing device, Olympus, Tokyo, Japan). The fundus of the gallbladder could then be retracted above the costal margin by coupling the internal component to an external magnet; this retractor was passed through the lumen (20 mm) of the 26 mm access port for deployment. The flexible grasper included an 18 × 63 mm internal baseplate containing magnets and a cradle configuration that allowed suspension and passage of one or two standard endoscopic biopsy forceps. For insertion into the abdomen, the flexible graspers were back-loaded into the 26 mm access port, with the cradle held inside the tube lumen and the tethers held externally, and deployed once the tube was positioned within the abdomen. After the cradle was maneuvered magnetically into the right lateral subcostal area, the needle anchor was used for stabilization and the external magnet was removed. One flexible grasper was used to provide cephalad fundus retraction by pushing externally on the cable and the second grasper was used for lateral, inferior infundibulum retraction by pulling externally on the cable. The graspers were positioned on the gallbladder with assistance from the endoscope equipped with a biopsy forceps and by pivoting the cradle using the needle anchor.
(A) a flexible grasper cradle used two biopsy forceps supported by a magnetic baseplate that could be held securely by an 18 ga needle anchor placed percutaneously. (B) After the internal component was maneuvered into position, the external handheld magnet was exchanged for the needle anchor. (C) The tip of the needle anchor was threaded into a receptacle on the baseplate of the grasper cradle. (D) the two graspers provided suitable retraction of the gallbladder fundus and infundibulum
Dissection was afforded via MAGS cautery dissectors in three configurations (Figs. 1 and 4). Previous spring-loaded designs had proven efficacious in similar applications [21, 22], but were prone to lodging on surrounding tissues during insertion and removal. We therefore engineered designs that would allow the instruments to be deployed in a collapsed configuration with deployment of the cautery arm into a 45° position once coupled to the abdominal wall. We also used a non-stick silicone-coated blade electrode (Valleylab, Boulder, CO) to avoid charring problems that we had previously experienced using uncoated electrodes. The first design used a detent mechanism that held the cautery arm in a closed position using a magnet; biopsy forceps controlled via the endoscope were used for intracorporeal arm deployment. This iteration used a 20 cm long tether (tip of port to cautery instrument), a 15 mm diameter baseplate, and a fully exposed blade; tip reach (peritoneal surface to cautery tip) was 8.5 cm. The second design used a pneumatic piston to activate the arm via compressed air. For insertion into the abdomen, the electric and pneumatic tethers were back-loaded into the 26 mm access port, with the cautery instrument passed into the tube lumen. Generation 1 of the pneumatic device used a 20 cm long tether, a 15 mm diameter baseplate, and an insulated blade with only the electrode tip exposed; a rearward set hinge allowed the cautery arm to be fully recessed when collapsed within the baseplate and the tip reach was 5.2 cm. Generation 2 of the pneumatic device used a 40 cm long tether, a 17 mm diameter baseplate with additional magnets, and an insulated blade with only the electrode tip exposed; a forward set hinge facilitated a tip reach of 6.2 cm. All cauterizers were maneuvered using an external magnet and deformation of the abdominal wall allowed angulations during dissection.
Endoscopic Equipment
A diagnostic gastroscope with a single working channel (Olympus GIF 160, Tokyo, Japan) was used for visualization and assistance during all procedures. Standard endoscopic accessories included biopsy forceps (Radial JawTM large-capacity forceps, Boston Scientific, Natick, MA), polypectomy snares (SensationTM, Boston Scientific, Natick, MA), Roth nets (Roth NetTM, US Endoscopy, Mentor, OH), and endoclips (QuickClip2TM long rotatable clip fixing device, Olympus, Tokyo, Japan).
Operative Procedure
Animals were premedicated with Telazol 4.0 mg/kg (IM) and Atropine 0.04 mg/kg (IM) and general endotracheal anesthesia was maintained with 2–5% isoflurane. With the pig in a lithotomy position, the urinary bladder was decompressed with a catheter and handheld retractors (speculum, army-navy, and malleable) were used to visualize the posterior fornix of the vagina. Three stay sutures were placed in the posterior vaginal wall and held on traction to facilitate the creation of a 2 cm culpotomy (vaginotomy) using electrocautery. Blunt dissection was used to complete the culpotomy and to develop the rectovaginal space in a cephalad direction. An endoscope was inserted through the culpotomy and biopsy forceps were used to create an opening in the peritoneum under endoscopic visualization. The endoscope was then inserted into the cul-de-sac of Douglas (anterior to the rectum) within the peritoneal cavity under endoscopic guidance. Air from the endoscope was used to initially establish a partial pneumoperitoneum and the peritoneal cavity was explored. The endoscope was withdrawn and replaced with a rigid 21 mm access port (pigs 1–3), or a flexible overtube (pig 4) that allowed dilation of the tract and further exploration (Fig. 1). Tubes were lubricated with a water-soluble lubricant. The initial tube was withdrawn and replaced with the 26 mm rigid access port, which allowed maintenance of a pneumoperitoneum (15 mmHg limit) using a high-flow laparoscopic CO2 insufflator (Karl Storz Endoscopy, Culver City, CA). In pig four, the animal was repositioned to a supine position prior to insertion of the 26 mm port. The operation was then performed using various iterations of the instrumentation described above (Table 1).
In pigs 1 and 2, the cautery dissector was first deployed followed by the gallbladder retractor(s). In pigs 3 and 4, the order of deployment was reversed; during deployment of the flexible grasper, prior to deployment of the cauterizer, a standard 15 mm laparoscopic port was temporarily inserted of into the end of the access port and held with tape to maintain pneumoperitoneum. During this interval, the cautery tethers were still back-loaded into the tube and, being held externally, the tethers precluded sealing off the access port using the threaded adapter. Once the flexible grasper was anchored into position, the cauterizer was inserted and coupled.
The cystic duct and artery were circumferentially dissected using the cauterizer, ligated with endoclips placed endoscopically, and divided with cautery (Fig. 5). The gallbladder was then removed from its hepatic attachments using the cauterizer; retraction was adjusted as needed to maintain suitable tissue tension. Using an endoscopic Roth net or snare, the gallbladder was retrieved into the access port and removed from the abdomen. The cauterizer was removed by maneuvering it into the access port using magnetic coupling. The flexible grasper retractor was held at the access port tip and removed simultaneously with the port (pig 3) or dismantled in vivo in a piecemeal fashion by first removing the forceps by pulling the cables externally and then by maneuvering the empty cradle into the access port using magnetic coupling. The culpotomy was closed with running suture using conventional open surgical instruments. Autopsy was performed immediately following the procedure and outcomes were recorded.
Results
In pig 1 (57 kg), transvaginal access was achieved using the 21 mm and 26 mm access ports without difficulty (Table 1), but the 30 cm length did not allow the tip to fully extend beyond the lower abdominal viscera; this resulted in a ball-valve phenomenon, whereby the small intestine fell into the port’s distal opening and occluded CO2 passage. When the occluded port pressure fell below 15mmHg, the insufflator sensed a low pressure, attempted to re-insufflate, and generated high pressures within the abdomen. The magnetic detent cauterizer was successfully deployed to its operating position but had poor control due to its short 20 cm tether. The attachable retractor was successfully deployed and fixated to the gallbladder with two of two attempted clips, provided good coupling strength, and allowed good cephalad retraction of the fundus. Lateral retraction of the infundibulum was poor due to the instability of the flexible scope; additionally, when retraction was attempted, an adequate view of the operative field was lost. The combination of suboptimal retraction and poor cauterizer mobility resulted in a gallbladder perforation early in the case. Inadvertent magnetic coupling occurred between the attachable retractor and the cauterizer and was uncorrectable using endoscopic assistance; the case was abandoned after 2 hours 45 mins with less than 10% of the dissection completed. At autopsy, there were no inadvertent organ injuries.
In pig 2 (51 kg), there were no access problems but again the ball-valve phenomenon occurred. The generation 1 the pneumatic cauterizer was successfully deployed and activated, but had poor control due to its short 20 cm tether and relatively weak coupling strength (fewer magnets were included in the baseplate to accommodate the pneumatic piston). The single grasper cradle retractor was deployed but immediately coupled to the cauterizer; a rescue using two 5 mm laparoscopic instruments was required to effect separation. The grasper retractor was left in the lower abdomen but not used; instead, the attachable retractor was successfully clipped to the gallbladder on two of two attempts and provided excellent retraction. The dissection was severely compromised by the cauterizer’s rearward hinge location, which resulted in the front of the baseplate colliding with tissue and preventing adequate reach of the tip. Gallbladder perforation occurred early in the case and the case was abandoned after 2 hours 10 mins with less than 5% of the dissection completed. At autopsy, there were no inadvertent organ injuries.
In pig 3, a smaller animal (32 kg), a longer (50 cm) access port was used to successfully overcome the ball-valve insufflation problem. Deployment of the double flexible grasper cradle required two attempts. The first time was unsuccessful due to the grasper becoming lost within the abdomen; after removal of the access port and the attached device, the port was reinserted with suitable control after promptly coupling the device as it emerged from the port. Subsequent magnetic positioning and needle anchoring worked well. The graspers were initially placed on the gallbladder and repositioned three times during the procedure using endoscopic assistance. The graspers provided suitable fundus and infundibulum retraction (Fig. 3). A manageable gas leakage occurred using the temporary 15 mm trocar placed in the access port’s lumen prior to deployment of the cauterizer. The generation 2 cauterizer was successfully deployed and activated, with good mobility provided by the longer 40 cm tether, excellent coupling strength provided by increased magnets in the larger baseplate, and adequate reach provided by the forward hinge location and a longer 6.2 cm arm. Two of two attempted endoclips were successfully placed on the cystic duct and artery; 80% of the dissection was completed prior to a gallbladder perforation, and the specimen was retrieved using a Roth net. The cauterizer was removed within the access port but the torque on the grasper cables prevented insertion of the grasper cradle into the port; the grasper was held at the distal tip of the port and they were removed together. The culpotomy was closed under direct vision and the procedure lasted 4 hours. At autopsy, a complete transection injury was noted at the recto-sigmoid junction (Fig. 6).
In pig 4 (23 kg), visualized initial access port placement was afforded using the flexible overtube. With the obturator in place, pneumoperitoneum was maintained as the endoscope fit snuggly within the obturator’s conical tip opening. The clear tubing material afforded careful visual inspection of the access pathway during both insertion and withdrawal with verification of safe entry. Repositioning the animal into a supine position prior to insertion of the rigid access port allowed a better angle of insertion with improved clearance of the sacral promontory; having no silicone sealant holding the tethers made the outer surface of the tube smoother and made insertion easier. With a small animal and a 50 cm long port, there were no insufflation problems. Deployment of the double flexible grasper cradle was successful on the first attempt and the magnetic positioning and needle anchoring worked well. The graspers were initially placed on the gallbladder using endoscopic assistance and provided suitable retraction without further repositioning. Similar to pig 3, use of the temporary 15 mm trocar placed in the access port’s lumen resulted in a manageable gas leak. The generation 2 cauterizer was successfully deployed, activated, and used for dissection. Two of three attempted endoscopic clips were successfully placed on the cystic duct and artery (one achieved only partial ductal ligation). No gallbladder perforation occurred (Fig. 7) and the specimen was retrieved using a snare, as it was too large for Roth net retrieval. The cauterizer and the grasper baseplate were removed within the access port, after the grasper forceps were withdrawn separately by pulling the cables externally. With the endoscope placed in the distal lumen, the port was slowly withdrawn with visualization of adjacent organs and verification of no injuries. The culpotomy was closed under direct vision and the procedure lasted 3 hours 30 mins. At autopsy, there were no injuries, bleeding, or bile leaks (Fig. 7).
During all procedures the flexible endoscope provided adequate visualization. For unclear reasons, lighting was poor in pig 1, but was excellent in pigs 2–4 using the same equipment and settings. Maintaining correct horizontal orientation was readily attainable during routine viewing of the operative field, as the rigid access port and straight caudal–cephalad scope direction allowed direct control of the scope’s axial rotation. Horizontal orientation was not well maintained during operative endoscopic maneuvers, such as endoscopic clip placement and positioning of flexible graspers, but did not significantly hinder the operation. In all pigs, the 26 mm access port with the threaded seal configuration and external instrument tethers maintained adequate pneumoperitoneum without major leakage.
Discussion
A close working relationship of our multidisciplinary team, consisting of experts in surgery, gastroenterology, and engineering, allowed considerable refinement of this novel procedure over the course of four experiments. We progressively improved our instrumentation and altered our surgical technique to overcome technical hurdles. By the final experiment, we were able to complete the entire procedure using solely a transvaginal approach with no technical problems or complications.
The MAGS platform allowed the use of multiple surgical instruments, deployed in a spatial orientation that facilitated tissue triangulation, and provided a stable, rigid platform with good intra-abdominal range of motion. The use of standard flexible endoscopic instrumentation was possible, and the versatility of using both of these systems simultaneously cannot be overemphasized. The flexible endoscope was quite useful in providing on-the-fly instrumentation assistance, suctioning and irrigating, and overall excellent visualization.
As evidenced by the problems encountered during the first two cases and the rectal injury in the third, there is a substantial learning curve for this complex procedure. By using the needle anchor and altering our instrument deployment sequence, we readily overcame problems with inadvertent magnetic coupling between instruments. The needle anchor diminished the size of the overall magnetic field by eliminating the external handheld magnet at this location and allowed maintenance of a suitable distance between instruments; both of these conditions decreased the likelihood of unintended instrument interactions. It is unclear whether access port insertion in the lithotomy position or intact removal of the flexible grasper cradle was the etiology of the large rectal injury in pig 3. The protective measures we undertook in pig 4, which included visualized initial entry, animal repositioning, and piecemeal instrument removal, seemed effective in eliminating this devastating complication and ensuring safety. While an operative time of 3–4 hours is prolonged, operation efficiency is expected to improve with further practice and technical modifications.
Additional instrumentation refinements are needed. The cautery dissector prototype is relatively mature and affords both blunt and sharp dissection, but tip configurations could be improved. More-robust graspers with increased rigidity, bigger jaws, and better control over positioning would be helpful, especially in the clinical settings of thickened or diseased tissues. While we have previously developed a MAGS video camera [19–21], the current prototype lacks the image quality of conventional laparoscopes and flexible endoscopes, and was not used for this study. With additional improvements, a mobile deployable camera on the MAGS platform would be very useful to allow passage of additional instrumentation through the main lumen of the access port and obviate the need for endoscopic visualization. This may well allow performance of more complex procedures that require additional instruments such as staplers or suturing devices. More integration of robotic control systems, as we have employed in previous [20] and current iterations of our cautery dissector, will likely facilitate additional functionality of the MAGS instruments.
We had very few difficulties with the coupling strength of the MAGS instruments in the porcine model using current designs based on focused and shielded permanent magnet configurations. However, abdominal wall thickness in these specimens was 2.5 cm or less. Magnetic attraction forces diminish exponentially over distance [19] and stronger magnets may be needed to maintain adequate instrument control in humans with thicker abdominal walls.
There are also implications for women’s health, which must be addressed. While a wealth of data is available from gynecologic literature suggesting the safety of a transvaginal approach for hysterectomy [23, 24] or culdoscopy for treatment of infertility [25, 26], long-term outcomes will be necessary to document the safety of gastrointestinal and other major abdominal surgery via this route. Outcomes in terms of dyspareunia, fertility, and pelvic prolapse will be needed in clinical trials. Nonetheless, the transvaginal approach seems advantageous for the reasons previously mentioned. For men, the techniques we described in this study should be readily applicable to a transcolonic approach and will be clinically relevant when suitable decontamination and closure methods are available.
In conclusion, this study clearly documents the feasibility of transvaginal NOTES cholecystectomy using a combination of MAGS and endoscopic instrumentation. The MAGS platform is advantageous in providing a stable surgical platform. With the careful development of effective operative techniques, our preliminary experience using this non-survival animal model suggests that a safe procedure can be performed. Additional investigations are warranted to document outcomes in survival models in animals, and ultimately safety and efficacy in humans.
References
Gettman MT, Lotan Y, Napper CA, Cadeddu JA (2002) Transvaginal laparoscopic nephrectomy: development and feasibility in the porcine model. Urology 59:446–450
Kalloo AN, Singh VK, Jagannath SB, Niiyama H, Hill SL, Vaughn CA, Magee CA, Kantsevoy SV (2004) Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc 60:114–117
Park PO, Bergstrom M, Ikeda K, Fritscher-Ravens A, Swain P (2005) Experimental studies of transgastric gallbladder surgery: cholecystectomy and cholecystogastric anastomosis. Gastrointest Endosc 61:601–606
Jagannath SB, Kantsevoy SV, Vaughn CA, Chung SSC, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Scorpio DG, Magee CA, Pipitone LJ, Kalloo AN (2005) Peroral transgastric endoscopic ligation of fallopian tubes with long-term survival in a porcine model. Gastrointest Endosc 61:449–453
Kantsevoy SV, Jagannath SB, Niiyama H, Vaughn CA, Chung SSC, Cotton PB, Gostout CJ, Hawes RH, Pasricha PJ, Magee CA, Barlow D, Shimonaka H, Kalloo AN (2005) Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc 62:287–92
Kantsevoy SV, Hu B, Jagannath SB, Vaughn CA, Beitler DM, Chung SCC, Cotton PB, Gostout CJ, Hawes RJ, Pasricha PJ, Magee CA, Pipitone LJ, Talamini MA, Kalloo AN (2006) Per-oral transgastric endoscopic splenectomy: Is it possible? Surg Endosc 20:522–525
Ikeda K, Fritscher-Ravens A, Mosse CA, Mills T, Tajiri H, Swain CP (2005) Endoscopic full-thickness resection with sutured closure in a porcine model. Gastrointest Endosc 62:122–129
Wagh MS, Merrifield BF, Thompson CC (2006) Survival studies after endoscopic transgastric oophorectomy and tubectomy in a porcine model. Gastrointest Endosc 63:473–478
Swanstrom LL, Kozarek R, Pasricha PJ, Gross S, Birkett D, Park PD, Saadat V, Ewers R, Swain P (2005) Development of a new access device for transgastric surgery. J Gastrointest Surg 9:1129–1137
Fritscher-Ravens A, Mosse CA, Mukherjee D, Yazaki E, Park PO, Mills T, Swain P (2004) Transgastric gastropexy and hiatal hernia repair for GERD under EUS control: a porcine model. Gastrointest Endosc 59:89–95
Bergstrom M, Ikeda K, Swain P, Park P (2006) Transgastric anastomosis by using flexible endoscopy in a porcine model. Gastrointest Endosc 63:307–312
Pai RD, MD, Fong DG, Bundga ME, Odze RD, Rattner DW, Thompson CC (2006) Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model. Gastrointest Endosc 64:428–434
Lima E, Rolanda C, Pego JM, Henriques-Coelho T, Silva D, Carvalho JL, Correia-Pinto J (2006) Transvesical endoscopic peritoneoscopy: a novel 5 mm port for intra-abdominal scarless surgery. J Urology 176:802–805
Rattner D, Kalloo A (2006) SAGES/ASGE Working group on natural orifice translumenal endoscopic surgery. Surg Endosc 20:329–333
Malik A, Mellinger JD, Hazey JW, Dunkin BJ, MacFadyen BV Jr (2006) Endoluminal and transluminal surgery: current status and future possibilities. Surg Endosc 20:1179–1192
McGee MF, Rosen MD, Marks J, Onders RP, Chak A, Faulx A, Chen VK, Ponsky J (2006) A primer on natural orifice transluminal endoscopic surgery: building a new paradigm. Surg Innov 13:86–93
Kaehler G, Grobholz R, Langner C, Suchan K, Post S (2006) A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy 38:86–89
Cadeddu JA, Eberhart R, Fernandez R, Bergs R (2005) Transabdominal magnetic anchoring system for trocar-less laparoscopic surgery. J Urology 167:4 (abstract)
Park S, Bergs R, Eberhart R, Baker L, Fernandez R, Cadeddu JA (2007) Trocar-less laparoscopy: magnetic positioning of intra-abdominal camera and retractor. Ann Surg 245:379–384
Zeltser IS, Bergs R, Fernandez R, Baker L, Eberhart R, Cadeddu JA (2007) Single trocar laparoscopic nephrectomy using magnetic anchoring and guidance system in the porcine model. J Urology 178:288–291
Scott DJ, Tang SJ, Bergs R, Fernandez R (2006) Magnetically-Anchored Instruments for Transgastric Endoscopic Surgery, Presented at the SAGES Emerging Technologies Session, SAGES Annual Meeting, Dallas, TX April 29, 2006
Scott DJ, Tang SJ, Fernandez R, Bergs R, Cadeddu JA (2007) Transgastric, transcolonic, and transvaginal cholecystectomy using magnetically anchored instruments. Surg Endosc 21(Suppl):S474
Clayton RD (2006) Hysterectomy. Best Pract Res Clin Obstet Gynaecol 20:73–87
Kovac SR (2000) Hysterectomy outcomes in patients with similar indications. Obstet Gynecol 95:787–793
Khouri A, Magos A (2005) The cost of out-patient culdoscopy compared to in-patient laparoscopy in women with infertility. J Obstet Gynaecol 25:160–165
Paulson JD, Ross JW, El-Sahwi S, Paulson JD, Ross JW, El-Sahwi S (1999) Development of flexible culdoscopy. J Am Assoc Gynecol Laparosc 6:487–490
Acknowledgements
We gratefully acknowledge the equipment support provided by Olympus, Karl Storz Endoscopy, the US Surgical Corporation, and Valleylab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scott, D.J., Tang, Sj., Fernandez, R. et al. Completely transvaginal NOTES cholecystectomy using magnetically anchored instruments. Surg Endosc 21, 2308–2316 (2007). https://doi.org/10.1007/s00464-007-9498-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9498-z