Abstract
Muscle aging such as sarcopenia adversely affects motor activities. However, few studies have elucidated the aging physiological mechanism of tongue concerted with the changes muscle composition. The present study aimed to examine the tongue composition changes to detect the effect of tongue fat mass on tongue pressure and swallowing function with aging. Twenty community-dwelling elderly without head and neck cancer, stroke, or neuromuscular disease and 20 healthy young were included. Tongue volume, tongue fat mass, tongue lean muscle mass, and tongue fat percentage were evaluated with 3D magnetic resonance imaging (MRI) and Dixon MRI. Tongue pressure was also measured. Swallowing function among elderly individuals was assessed via videofluorography, which was evaluated using the penetration–aspiration scale (PAS) and normalized residue ratio scale (NRRS). Tongue fat mass and tongue fat percentage significantly increased with aging. The tongue fat percentage of elderly participants was 20%, which was two times greater than that of young participants. No significant difference was observed in tongue volume and tongue lean muscle mass. A significantly negative correlation was observed between tongue fat mass and tongue fat percentage as well as tongue pressure. Conversely, tongue volume was not significantly correlated with tongue pressure. Tongue muscle composition exhibited no effect in the PAS and NRRS. Increase of fat mass is a major change in tongue composition with aging, which is associated with low tongue pressure. Thus, attention must be paid not only to tongue quantity but also to the quality of tongue muscles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcopenia refers to the loss of striated muscle mass, strength, and function associated with aging [1]. Based on recent studies on whole-body sarcopenia, [2,3,4] an increase in intramuscular fatty infiltration, which leads to loss of muscle strength, is correlated with changes in muscle quality. The European Working Group on Sarcopenia in Older People recently updated the definition of sarcopenia, and its diagnosis is confirmed based on the presence of low muscle quantity or quality [5].
The tongue is composed of striated muscles such as those in the limbs; thus, the tongue muscle is also affected by aging [6, 7]. In tongue muscles, unlike other age-related changes in tongue composition, decrease in tongue pressure has received considerable attention [6,7,8,9,10,11,12,13]. Changes in tongue muscle mass may also be associated with aging [14], nutritional status [15], and tongue pressure [6, 15]. Moreover, previous studies have evaluated tongue thickness and volume. However, intramuscular fatty infiltration has remained seldom investigated. To our knowledge, only Butler et al. [16], Ogawa et al. [17], and Humbert et al. [18] have evaluated tongue fat mass. According to them, tongue fat mass is associated with tongue pressure changes [16] and sarcopenic dysphagia [17]. Furthermore, Humbert et al. [18] suggested that future studies should clarify whether significant differences exist between healthy elderly and young adults to answer important clinical questions about tongue sarcopenia. However, no study had compared tongue composition between elderly and young individuals; thus, age-related tongue muscle composition change remains imperfectly understood. We considered that decrease in tongue pressure caused by aging is related to fatty change of tongue musculature.
MRI examination provides a useful approach for the differentiation of normal and abnormal muscle composition. In particular, Dixon MRI is fat-suppression MRI technique that provides intramuscular fat volumetric image. Dixon MRI is highly repeatable [19] and accurate [20], and it has been used for the evaluation of intramuscular fatty infiltration in whole-body sarcopenia [3, 21]. Nevertheless, no study has evaluated age-related tongue muscle abnormality using Dixon MRI.
This study investigated changes in tongue composition using Dixon MRI in young and elderly individuals to detect the effect of tongue fat mass on tongue pressure and swallowing function with respect to aging.
Materials and Methods
Study Design
A prospective cross-sectional study of age-related tongue muscle change using Dixon MRI was performed at Hyogo College of Medicine Hospital, and it was approved by the Ethics Committee of Hyogo College of Medicine. Written informed consent was obtained from all participants.
Participants
A total of 40 individuals participated in this study. Of them, 20 were community-dwelling elderly individuals aged > 65 years. The remaining 20 participants were healthy young participants aged < 40 years. Participants with a history of head and neck cancer, stroke, neuromuscular disease, or aspiration pneumonia were excluded. The occlusal contact of all participants was class A (contact in all four support zones) according to the Eichner Index [22]. The following assessments were performed within a week.
Tongue MRI
MRI was performed on a 3.0 T MRI scanner (Siemens MAGNETOM Skyra, Germany) using 20-ch head–neck coil. The radiologic technologist instructed the following steps to the participants: “Keep the tongue relaxed and position the tip of the tongue to the back of the front teeth. Also, do not swallow and speak during scanning to reduce motion artifacts.” Tongue MRI images were obtained using sagittal T1-weighted 3D MRI (FOV = 200 × 200 mm2, TR = 500 ms, TE = 12 ms, resolution = 192 × 192, voxel size = 1 × 1 × 1 mm3, and TA = 3.38 min) and Dixon MRI (FOV = 220 × 220 mm2, TR = 635 ms, TE = 10 ms, resolution = 320 × 256, voxel size = 0.7 × 0.7 × 4.0 mm3, and TA = 2.55 min). Dixon MRI obtained four images (in-phase, opposite-phase, fat, and water), and the in-phase and fat images were analyzed.
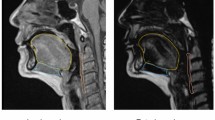
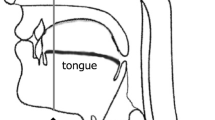
The radiologist manually assessed the tongue contour per image using the Osirix™ image analysis software. Tongue volumes were measured based on sagittal T1-weighted 3D MRI (Fig. 1). To normalize the tongue volume after the 3D reconstruction, we divided such volume by the hard palate length. Tongue fat mass, tongue lean muscle mass, and tongue fat percentage were determined using Dixon MRI and were defined as follows (Fig. 2):
In addition, the anterior and posterior coronal Dixon MRI images were analyzed to assess the distribution of tongue fat percentage. The anterior and posterior images were defined as sites 20.0 mm (4.0 mm × 5 frames) away from the tip and posterior ends of the tongue (Fig. 3).
The analysis of tongue volume, tongue fat mass, tongue lean muscle mass, and tongue fat percentage had good inter-reproducibility; the intraclass correlation coefficient ranged from 0.929 to 0.984, 0.976 to 0.995, 0.947 to 0.988, and 0.981 to 0.996 (p < 0.001), respectively.
Tongue Strength
Using the JMS tongue pressure machine (JMS, Hiroshima, Japan), we measured the maximum anterior tongue pressure (tongue strength). During the measurement, the probe was placed between the front of the palate and the tongue. Then, all participants pushed their tongue up to the hard palate and pressed on the probe with the maximum voluntary effort. Tongue pressure was measured three times to obtain the mean value.
Whole-body Sarcopenia
Whole-body sarcopenia was evaluated using the hand grip strength, gait speed, and skeletal muscle mass according to the Asian Working Group for Sarcopenia criteria [1]. The hand grip strength (T.K.K. 5401, Takei Apparatus Industry, Osaka, Japan) was examined in both hands, and the higher value was defined as the maximum hand grip strength. The gait speed was measured using the 10-m gait speed test. The participants were instructed to walk a 10-m straight course at their usual comfortable gait speed. Meanwhile, the skeletal muscle mass was calculated using bioelectrical impedance analysis (InBody S10, InBody Japan Inc. Tokyo, Japan). Skeletal muscle mass index (SMI) was calculated by dividing skeletal muscle mass by height in meters squared (kg/m2). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Swallowing Function
All participants were evaluated using the water swallowing test (WST), food test (FT), and repeated saliva swallowing test (RSST), which are used in the general clinical evaluation of patients with dysphagia. The RSST is an examination of repeated voluntary swallowing of saliva, and < 3 dry swallows within 30 s is considered abnormal [25]. The WST is a 5-point scale examination that assesses swallowing function by swallowing 30 ml of water [26]. The FT is also a 5-point scale, but it is conducted by eating 4 g (1 teaspoon) of jelly [27].
Thereafter, only elderly participants were examined by videofluorography (VFSS) because this procedure involves radiation exposure. Using a DVD recorder (DIGA DMR-XP200; Panasonic Cor-poration, Osaka, Japan), we recorded the lateral projection of VFSS, which was performed in a sitting position, using 3, 5, and 30 ml of 40% weight/volume barium sulfate. Aspiration was not observed while swallowing 3 and 5 ml of barium liquids; thus, we analyzed the VFSS data by swallowing 30 ml of barium liquids.
Then, the VFSS was examined for the occurrence of aspiration and pharyngeal residue. We evaluated the aspiration status by using the 8-point penetration–aspiration scale (PAS), which assesses the depth to which the material passes in the airway and whether the material entering the airway is expelled during the swallowing (PAS score 1 = no aspiration, 2–5 = penetration, 6–8 = aspiration) [23]. Moreover, using the normalized residue ratio scale (NRRS), we measured the amount of pharyngeal residue. The NRRS is an anatomically referenced scale used for capturing residue severity by pixel-based measurements. It is also used to objectively quantify residue in the valleculae (NRRSv) and pyriform sinuses (NRRSp) [24].
Statistical Analysis
Normal data distribution was examined using the Shapiro–Wilk test. The effects of aging and gender on tongue muscle composition, tongue pressure, gait speed, hand grip, SMI, and BMI between both factors were assessed using the two-way repeated-measures analysis of variance (ANOVA). For hypothesis testing, the aligned rank transform (ART) procedure was employed if the data were not normally distributed. For nonnormally distributed data, the data of adjusted ART ANOVA were more robust and powerful than those of the traditional ANOVA [28]. The distribution of the anterior and posterior tongue fat percentage was compared using the paired t test. Furthermore, the coefficients between tongue pressure and tongue fat mass/fat percentage were calculated by Pearson’s correlation coefficients analysis. Meanwhile, the coefficients between tongue pressure and tongue volume/tongue lean muscle mass and the coefficients between tongue composition and NRRS were calculated using Spearman correlation analysis. Tongue composition and PAS were compared using one-way ANOVA and Tukey’s test. All statistical data were analyzed using SPSS 24.0 J (SPSS Japan, Tokyo, Japan). ART was performed using ARTool 1.6.2 (https://depts.washington.edu/acelab/proj/art/index.html). Moreover, p < 0.05 was considered to be statistically significant.
Results
The mean age of the 10 elderly male participants was 76.9 ± 6.3 years and that of the 10 elderly female participants was 73.7 ± 5.2 years. The mean age of the 10 healthy young males was 29.5 ± 4.1 years and that of the 10 healthy young females was 28.9 ± 4.5 years. None of the participants were diagnosed with whole-body sarcopenia.
Table 1 summarizes the effects of aging and gender on tongue muscle composition, tongue pressure, gait speed, hand grip, SMI, and BMI between both factors. Aging significantly affected the tongue fat mass, tongue fat percentage, and tongue pressure (η2 = 0.66, η2 = 0.62, and η2 = 0.49, respectively) but did not affect the tongue volume and tongue lean muscle mass. Regarding gender, the tongue volume, tongue lean muscle mass, and tongue fat percentage were significantly affected (η2 = 0.15, η2 = 0.19, and η2 = 0.13, respectively). The fat percentage of the posterior tongue was significantly greater than that of the anterior tongue in both elderly and young participants (Table 2, Fig. 4).
Distribution of fat tissue in the tongue muscle. Fat-phase image of a 74-year-old man; fat marbling increased in the posterior tongue. Fat-phase images show fat tissue as high contrast, whereas the boundaries of the soft tissues are not as distinct. Fat tissue was identified with the Osirix image software using a threshold method; the high-contrast region is represented by the green color
Figure 5 shows the bivariate scatterplot for tongue pressure as a function of tongue MRI. A significant negative correlation was observed between tongue fat mass and tongue fat percentage as well as tongue pressure (r = − 0.51, p = 0.001 and r = − 0.60, p < 0.001, respectively). A positive correlation was observed between tongue lean muscle mass and tongue pressure (r = 0.54, p < 0.001). By contrast, tongue volume was not significantly associated with tongue pressure (r = 0.27, p = 0.09).
The results of WST, FT, and RSST were normal in all participants. On VFSS, 11, 6, and 3 elderly participants exhibited no aspiration (PAS score: 1), penetration (PAS score: 2–5), and aspiration (PAS score: 6–8), respectively. However, no significant difference in tongue composition was found between these three groups (Table 3). On NRRS, NRRSv exhibited 0.13 ± 0.12, whereas NRRSp exhibited 0.02 ± 0.04. NRRS had no significant correlation with tongue composition (Table 4).
Discussion
This is the first study to investigate the age-related tongue muscle change using Dixon MRI and the impact of changes in tongue composition on tongue pressure. The main findings of this study associated with aging were as follows: (a) tongue fatty infiltration was higher in the aged group and that lean muscle mass did not differ significantly with age, (b) both of young and elderly participants exhibited fatty infiltration increase in the posterior tongue, (c) fatty infiltration was associated with low tongue pressure.
Age-Related Tongue Atrophy and Tongue Fatty Infiltration
No study compared tongue composition between elderly and young individuals; thus, age-related tongue muscle composition change had been imperfectly understood. In this study, tongue fat percentage of the elderly participants was 20%, which was two times greater than that of young participants. In two-way ANOVA, tongue fat percentage was affected by gender as well as aging, but the effect size of aging was greater than that of gender (η2 = 0.62 and η2 = 0.13, respectively). Therefore, fatty infiltration of the tongue is susceptible to aging rather than gender. In whole-body muscle, aging is one of the risk factor of intramuscular fatty infiltration [29], so that the pathological changes in tongue muscle might be similar to those of whole-body muscle among elderly individuals.
In whole-body muscle, intramuscular fatty infiltration is considerably more rapid than concomitant loss of muscle mass [30]. In this study, the elderly exhibited tongue fat mass was increased despite tongue volume and tongue lean muscle mass maintained compared with the younger; hence, we assumed tongue fatty infiltration is more rapid than muscle atrophy, similar to skeletal muscle. However, the association between tongue muscle atrophy and tongue fatty infiltration remains imperfectly understood because this study was a cross-sectional study, not longitudinal study. Further studies are required in order to confirm these associations.
The present study confirmed that intramuscular fatty infiltration increased in posterior tongue in both young and elderly participants. Several studies have indicated that fatty degeneration was mainly observed in type I muscle fibers [31,32,33], and most of posterior tongue is composed type I fibers [34], which substantiates the view that fatty infiltration is highly observed in posterior tongue.
Interrelationship Between Tongue Fatty Infiltration and Tongue Pressure
A few studies investigated the association between tongue muscle composition and tongue pressure [14,15,16] Of these studies, only Butler et al. [16] focused on tongue fat mass, and they evaluated posterior tongue adiposity with a 1 cm elliptical region of interest using CT. By contrast, the other studies evaluated tongue thickness using ultrasonography [14, 15]. However, the analysis of these studies was not primarily based upon the unremitting imaging data of tongue muscle body. Providing unremitting and dynamic images reflected pathological tongue muscle is suitable to detect the accurately interrelationship between tongue muscle composition and tongue pressure. The present study analyzed 3D MR images, and exhibited that a significant correlation was observed between tongue fat mass, tongue fat percentage, and tongue lean muscle mass as well as tongue pressure (r = − 0.51, − 0.60, and 0.54, respectively). However, tongue volume was not significantly correlated with tongue pressure. Accordingly, tongue fatty infiltration was associated with low tongue pressure, which indicates the functional importance of fat tissue. Intramuscular fatty infiltration is correlated with changes in muscle quality [2,3,4]. Our results suggest that attention must be paid not only to tongue quantity but also to the quality of tongue muscles.
Association Between Tongue Fatty Infiltration and Presbyphagia
We had predicted that tongue fatty infiltration can result in adverse effects on presbyphagia because (1) tongue fatty infiltration induces sarcopenic dysphagia [17] and (2) the increase of intramuscular fatty infiltration is affected by aging and sarcopenia [2,3,4]. However, our study did not clearly demonstrate the interrelationship of tongue fat mass and tongue muscle mass with swallowing function. Therefore, presbyphagia is not as strongly linked to the increase of tongue fatty infiltration as sarcopenic dysphagia.
Limitations
This study has several limitations. First, this study was limited by the small cohort of participants. Moreover, none of the participants were diagnosed with whole-body sarcopenia. Therefore, future studies with a larger cohort diagnosed with whole-body sarcopenia should be conducted. Second, the posterior tongue pressure should have been measured. However, the JMS tongue pressure machine could only measure the anterior tongue force; thus, we only measured and recorded the anterior tongue pressure. Third, the VFSS was examined only in elderly participants because of radiation exposure; thus, the swallowing function between younger and elderly participants could not be compared.
Conclusion
Fatty infiltration of the tongue is a common condition associated with aging and is associated with low tongue pressure. Therefore, we must pay careful attention not only to the quantity but also to the quality evaluation of the tongue muscle.
References
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H, Asian Working Group for Sarcopenia. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.e2. https://doi.org/10.1016/j.jamda.2019.12.012.
Akima H, Yoshiko A, Tomita A, Ando R, Saito A, Ogawa M, Kondo S, Tanaka NI. Relationship between quadriceps echo intensity and functional and morphological characteristics in older men and women. Arch Gerontol Geriatr. 2017;70:105–11. https://doi.org/10.1016/j.archger.2017.01.014.
Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637. https://doi.org/10.1155/2012/629637.
Tuttle LJ, Sinacore DR, Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J Aging Res. 2012;2012:172957. https://doi.org/10.1155/2012/172957.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2, Writing Group for the European. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–311. https://doi.org/10.1093/ageing/afy169.
Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53:1483–9. https://doi.org/10.1111/j.1532-5415.2005.53467.x.
Namasivayam AM, Steele CM, Keller H. The effect of tongue strength on meal consumption in long term care. Clin Nutr. 2016;35:1078–83. https://doi.org/10.1016/j.clnu.2015.08.001.
Buehring B, Hind J, Fidler E, Krueger D, Binkley N, Robbins J. Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J Am Geriatr Soc. 2013;61:418–22. https://doi.org/10.1111/jgs.12124.
Butler SG, Stuart A, Leng X, Wilhelm E, Rees C, Williamson J, Kritchevsky SB. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci. 2011;66:452–8. https://doi.org/10.1093/gerona/glq234.
Maeda K, Akagi J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia. 2015;30:80–7. https://doi.org/10.1007/s00455-014-9577-y.
Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50:M257–M262262. https://doi.org/10.1093/gerona/50a.5.m257.
Ono T, Kumakura I, Arimoto M, Hori K, Dong J, Iwata H, Nokubi T, Tsuga K, Akagawa Y. Influence of bite force and tongue pressure on oro-pharyngeal residue in the elderly. Gerodontology. 2007;24:143–50. https://doi.org/10.1111/j.1741-2358.2007.00172.x.
Todd JT, Lintzenich CR, Butler SG. Isometric and swallowing tongue strength in healthy adults. Laryngoscope. 2013;123:2469–73. https://doi.org/10.1002/lary.23852.
Tamura F, Kikutani T, Tohara T, Yoshida M, Yaegaki K. Tongue thickness relates to nutritional status in the elderly. Dysphagia. 2012;27:556–61. https://doi.org/10.1007/s00455-012-9407-z.
Tamura F, Kikutani T, Furuya H, Okayama H, Takahashi N. A study of tongue thickness in elderly people in Japan. Med Res Arch. 2017;5:1–14.
Butler SG, Lintzenich CR, Leng X, Stuart A, Feng X, Carr JJ, Kritchevsky SB. Tongue adiposity and strength in healthy older adults. Laryngoscope. 2012;122:1600–4. https://doi.org/10.1002/lary.23290.
Ogawa N, Mori T, Fujishima I, Wakabayashi H, Itoda M, Kunieda K, Shigematsu T, Nishioka S, Tohara H, Yamada M, Ogawa S. Ultrasonography to measure swallowing muscle mass and quality in older patients with sarcopenic dysphagia. J Am Med Dir Assoc. 2018;19:516–22. https://doi.org/10.1016/j.jamda.2017.11.007.
Humbert IA, Reeder SB, Porcaro EJ, Kays SA, Brittain JH, Robbins J. Simultaneous estimation of tongue volume and fat fraction using IDEAL-FSE. J Magn Reson Imaging. 2008. https://doi.org/10.1002/jmri.21431.
Grimm A, Meyer H, Nickel MD, Nittka M, Raithel E, Chaudry O, Friedberger A, Uder M, Kemmler W, Engelke K, Quick HH. Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J Cachexia Sarcopenia Muscle. 2018;9:1093–100. https://doi.org/10.1002/jcsm.12343.
Kovanlikaya A, Guclu C, Desai C, Becerra R, Gilsanz V. Fat quantification using three-point Dixon technique: in vitro validation. Acad Radiol. 2005;12:636–9. https://doi.org/10.1016/j.acra.2005.01.019.
Yang YX, Chong MS, Tay L, Yew S, Yeo A, Tan CH. Automated assessment of thigh composition using machine learning for Dixon magnetic resonance images. Magma. 2016;29:723–31. https://doi.org/10.1007/s10334-016-0547-2.
Eichner K. Renewed examination of the group classification of partially edentulous arches by Eichner and application advices for studies on morbidity statistics. Stomatol DDR. 1990;40:321–5.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. https://doi.org/10.1007/BF00417897.
Pearson WG Jr, Molfenter SM, Smith ZM, Steele CM. Image-based measurement of post-swallow residue: the normalized residue ratio scale. Dysphagia. 2013;28:167–77. https://doi.org/10.1007/s00455-012-9426-9.
Sakayori T, Maki Y, Hirata S, Okada M, Ishii T. Evaluation of a Japanese “Prevention of long-term care” project for the improvement in oral function in the high-risk elderly. Geriatr Gerontol Int. 2013;13:451–7. https://doi.org/10.1111/j.1447-0594.2012.00930.x.
Kubota T, Mishima H, Hanada M, Minami I, Kojima Y. Paralytic dysphagia in cerebrovascular disorder—screening tests and their clinical application. Sogo Rehabil. 1982;10:271–6.
Osawa A, Maeshima S, Tanahashi N. Food and liquid swallowing difficulty in stroke patients: a study based on the findings of food tests, a modified water swallowing test and videofluoroscopic examination of swallowing. The Jpn J Rehabil Med. 2012;49:838–45. https://doi.org/10.2490/jjrmc.49.838.
Leys C, Schumann S. A nonparametric method to analyze interactions: the adjusted rank transform test. J Exp Soc ol. 2010;46:684–8. https://doi.org/10.1016/j.jesp.2010.02.007.
Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570. https://doi.org/10.1155/2014/309570.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. https://doi.org/10.1093/gerona/61.10.1059.
St-Jean-Pelletier F, Pion CH, Leduc-Gaudet JP, Sgarioto N, Zovile I, Barbat-Artigas S, Reynaud O, Alkaterji F, Lemieux FC, Grenon A, Gaudreau P, Hepple RT, Chevalier S, Belanger M, Morais JA, Aubertin-Leheudre M, Gouspillou G. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle. 2017;8:213–28. https://doi.org/10.1002/jcsm.12139.
Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol (Lausanne). 2016;7:69. https://doi.org/10.3389/fendo.2016.00069.
Essén-Gustavsson B, Karlsson A, Lundström K, Enfält AC. Intramuscular fat and muscle fibre lipid contents in halothane-gene-free pigs fed high or low protein diets and its relation to meat quality. Meat Sci. 1994;38:269–77. https://doi.org/10.1016/0309-1740(94)90116-3.
Sanders I, Mu L, Amirali A, Su H, Sobotka S. The human tongue slows down to speak: muscle fibers of the human tongue. Anat Rec (Hoboken). 2013;296:1615–27. https://doi.org/10.1002/ar.22755.
Acknowledgements
This study was partly supported by the Japan Society for the Promotion of Science (KAKENHI [17K11927]).
Author information
Authors and Affiliations
Contributions
Y.N, T.Y, K.H, Y.N, and K.D contributed to conceptualization and data curation. Y.N contributed to the evaluation of tongue strength and whole-body sarcopenia. T.K contributed to the analysis of tongue MRI. T.K, Y.H, K.A, and R.I contributed to MRI data collection. K.H and Y.N contributed to the evaluation of occlusal contact. T.Y, N.K, and Y.U contributed to the evaluation of VFSS. All authors contributed to the writing of the original draft, review, and editing. K.D contributed to the final approval of the article.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakao, Y., Yamashita, T., Honda, K. et al. Association Among Age-Related Tongue Muscle Abnormality, Tongue Pressure, and Presbyphagia: A 3D MRI Study. Dysphagia 36, 483–491 (2021). https://doi.org/10.1007/s00455-020-10165-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-020-10165-4