Abstract
Objective
The aim of this study was to investigate the relationship between tongue muscle quality index, which was represented as tongue muscle pressure divided by tongue muscle mass, and swallowing speed in community-dwelling older women.
Methods
The inclusion criteria for this cross-sectional study were that participants be community-dwelling older women aged 65 years and above without dysphagia. The exclusion criteria were stroke and Parkinson’s disease that directly cause dysphagia. We measured tongue muscle thickness and maximum tongue pressure and the tongue muscle quality index, which was defined as the maximum tongue pressure divided by tongue muscle thickness. We investigated swallowing speed via a 100 ml water swallowing test. To assess the relationship between tongue muscle characteristics and swallowing speed, we performed stepwise multiple regression analysis.
Results
Ninety-three participants were enrolled in this study (mean age: 84.2 ± 4.7 years). A stepwise multiple regression analysis showed that age (β = − 0.292, p < 0.01) and tongue muscle quality index (β = 0.267, p < 0.01) were related to swallowing speed.
Conclusion
We found that tongue muscle quality index was related to swallowing speed in community-dwelling older women. According to our findings, it is possible that the tongue muscle quality index is a useful parameter for assessing swallowing speed in older women without dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of swallowing function is a serious problem in older adults. Previous studies have reported that swallowing function declines with age [1,2,3], and that the prevalence of dysphagia among community-dwelling older adults is 15.0% [2]. Older adults’ swallowing function undergoes both physiological and anatomical changes, for example, the declines in muscle mass and strength and oral sensory perception [4]. These age-related changes lead to deteriorating swallowing function, such as slower swallowing [1, 4]; in fact, several studies have reported that swallowing speed assessed by water swallowing test decreases with advancing age [5, 6]. Older adults are vulnerable to swallowing difficulties, such that it is common for acute illness to lead to the development of dysphagia. Dysphagia leads to severe complications, including malnutrition, choking and aspiration pneumonia [3, 7]. Therefore, it is important to clarify the factors associated with loss of swallowing function in older adults.
The tongue is one of the most important muscles for swallowing. It plays an important role in feeding and swallowing processes such as chewing, bolus formation, transfer of the bolus and generating pressure during the swallow. There are several measures to assess tongue muscle characteristics, for example, the evaluation of tongue muscle mass via ultrasound device and tongue pressure via balloon and manometer. It is evident that tongue muscle mass and strength decrease with age [8, 9], and several studies have reported the relationship between tongue muscle characteristics and swallowing function. For example, Butler et al. demonstrated that reduced tongue muscle strength was closely associated with lower swallowing function in healthy older adults [10]. Additionally, tongue muscle mass was decreased among hospitalized older patients with dysphagia [11]. While these studies have provided a powerful basis for the assumption that poor tongue characteristics are associated with low swallowing function, this relationship has not been well established.
Recently, muscle quality which was used to explain micro- and macroscopic changes in muscle architecture and composition has been emphasized in the assessment of skeletal muscle characteristics and the diagnosis of sarcopenia [12]. Although both skeletal muscle mass and strength decline with age, there are differences in aging changes. Some previous findings have indicated that muscle strength decreases more rapidly than muscle mass [13, 14], and that maintaining or gaining muscle mass does not prevent the loss of muscle strength [13]. Thus, muscle quality may explain the discordant relationship between muscle mass and strength [15, 16]. This is a new concept related to physical function. Previous studies have reported that there are several ways to assess muscle quality, such as determining infiltration of fat into muscle by using ultrasound devices and calculating the ratio of muscle strength to muscle mass. Additionally, as the assessment of either muscle mass or strength alone is considered an inadequate indicator of physical performance [17, 18], muscle quality is considered important in the assessment of skeletal muscle [12].
There is a similar age-related change between tongue muscle and skeletal muscle character. Numerous surveys have reported a close relationship between tongue muscle mass and strength, and whole-body muscle mass and strength [3, 5, 9,10,11, 19, 20]. Furthermore, our previous study revealed that older adults with sarcopenia and dynapenia showed both poorer whole-body muscle quality and tongue muscle pressure/motor function than healthy older adults [21]. Accordingly, it is reasonable to assume that assessment of muscle quality, which is considered the important assessment in skeletal muscle, is also necessary for tongue muscle characteristics. In fact, tongue muscle quality has been focused as one of the tongue muscle characteristics and was assessed by various evaluations [11, 22]. However, tongue muscle quality has not been sufficiently verified in studies on tongue muscle function and the relationship between tongue muscle quality and swallowing function in older adults remains unclear.
The aim of this study was to investigate the relationship between tongue muscle quality and swallowing speed, which was one of the swallowing functions in community-dwelling older women. We hypothesized that low tongue muscle quality could be associated with a decline in swallowing speed and that this new parameter of tongue character may be useful for maintaining swallowing function in older adults.
Methods
Study design and participants
This cross-sectional study, conducted in May 2018, involved community-dwelling older women who came to a visiting care facility in Nagano city, Japan. The inclusion criteria were aged 65 years and older and without dysphagia. The exclusion criteria were as follows: stroke, Parkinson’s disease, head and neck cancers, neuromuscular diseases that directly cause dysphagia and a Mini-Mental State Examination (MMSE) score of less than 18 points [23]. This study was conducted in accordance with the guidelines proposed by the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of Tsukuba University Graduate School of Comprehensive Human Sciences.
Assessment of tongue muscle mass, strength and quality
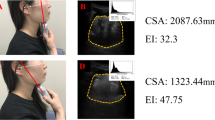
We measured tongue muscle thickness as an indicator of tongue muscle mass via an ultrasound device (ProSound2; Hitachi-Aloka Medical, Tokyo, Japan) with a 3.5 MHz convex array probe. The participant lay on a reclining chair and remained relaxed during the examination. A well-trained examiner placed the probe perpendicular to the Frankfurt plane of the lower chin surface (Fig. 1). We defined tongue muscle thickness as the distance from the lower end of the mylohyoid muscle to the tongue dorsum (cm), in accordance with a previous study [11]. The ultrasound images were recorded twice, with the average value used as the representative for analysis. The reliability of ultrasonographic examinations of tongue muscle thickness has already been verified [9]. The ultrasound images were stored in an ultrasound device as Digital Imaging and Communication in Medicine (DICOM) files. Tongue muscle thickness was calculated using the DICOM file viewer in OsiriX Lite version 9.5 (Pixmeo, Geneva, Switzerland). This analysis was performed by a single investigator who was well trained in the operation technique for ultrasound image analysis.
Maximum tongue pressure, as an indicator of tongue muscle strength, was measured by a handheld balloon probe and manometer (JMS tongue pressure measurement instrument; GC, Tokyo, Japan). Participants were asked to place the balloon on the anterior part of the palate and raise their tongue to compress the balloon on to the palate with maximum voluntary effort [24]. The tongue pressures were recorded three times, and the highest was used as the representative value (kPa).
Tongue muscle quality was assessed by the tongue muscle quality index. In a previous study about skeletal muscle, muscle quality index, which represents the ratio of strength to muscle mass, was one of the parameters for the assessments of muscle quality, and this indicator well correlated with physical performance [16]. For example, the lower extremity muscle quality index was measured by the ratio of knee extension torque to leg muscle mass and the upper extremity was measured by the ratio of grip strength to arm muscle mass [25, 26]. In this study, we operationally defined tongue muscle quality index as the maximum tongue pressure divided by tongue muscle thickness (kPa/cm).
Assessment of swallowing speed
We measured objective swallowing difficulties by swallowing speed. The participant, seated upright, was asked to drink a cup of 100 ml distilled water as quickly as possible. The examiner measured the time from the verbal command to begin the task until completion by stopwatch. We also recorded signs of choking, defined as coughing from the beginning of the task until 1 min after the end of the test, or a wet-hoarse voice after testing [6]. If the participant choked during swallowing, we immediately stopped the test. We measured the amount of water consumed and calculated the swallowing speed, which was defined as the amount of water divided by swallowing time (ml/s).
Assessment of subjective swallowing difficulties
We measured subjective swallowing difficulties by the Eating Assessment Tool (EAT-10), a self-reported questionnaire to screen for dysphagia. It consists of 10 questions about swallowing difficulties. Participants scored their swallowing on a 5-point scale, with a score of 0 indicating no problem and a score of 4 indicating a severe problem. The maximum score is 40 points and a score of ≧ 3 is considered abnormal and suggests the presence of dysphagia [27].
Assessment of oral function
Skills of the tongue tip and tongue dorsum were measured by oral diadochokinesis. Oral diadochokinesis, involving the articulation of the syllables “ta” and “ka” using the anterior and posterior tongue, is a simple measurement of tongue motor function. Several reports have defined tongue motor function in community-dwelling older adults according to oral diadochokinesis [28, 29]. Participants were asked to repeat each syllable (“ta,” “ka”) as quickly as possible for 5 s. The number of articulations was counted using a digital counter (T.K.K. 3351 digital counter, Takei Scientific Instruments Co., Ltd.). Oral diadochokinesis was calculated separately for each syllable as the number of articulations per second [28]. We assessed use of dentures (total dentures, partial dentures, no use) by a self-reported questionnaire. We defined the participant as use of total dentures if a participant used the total dentures on the upper and/or the lower teeth.
Assessment of other parameters
The participants’ age, height, weight, body mass index (BMI) and body fat percentage were assessed. BMI was calculated as weight divided by height squared (kg/m2). Body fat percentage was calculated as total fat mass divided by total body weight via bioelectrical impedance analysis measurements (MC-780A; TANITA Co., Ltd., Tokyo, Japan). Nutritional status was assessed by the Nutrition Screening Initiative (NSI) [30]. The NSI total score is the sum of each item’s score weighted by nutritional risks. We defined scores of 0–2 as “good,” 3–5 as “moderate nutritional risk” and 6 or more as “high nutritional risk” according to the previous studies [30, 31]. Cognitive function was assessed by the MMSE.
Statistical analysis
Variables were assessed for normality by the Shapiro–Wilk test, based on which we used either Pearson’s correlation coefficient or Spearman’s rank correlation coefficient. We assessed the correlations between age, tongue muscle characteristics and oral diadochokinesis and the correlations between age, tongue muscle characteristics and swallowing speed. Then, we performed stepwise multiple regression analysis with swallowing speed as a dependent variable, and age and tongue muscle characteristics, which were significant in the bivariate analysis, as the independent variables. All statistical analyses were performed using IBM SPSS version 25.0 (IBM Japan, Tokyo, Japan). A p value < 0.05 was considered statistically significant.
Results
Of the 118 women who met the inclusion criteria, 25 were excluded because of diseases such as stroke (n = 5), Parkinson’s disease (n = 2) and missing data (n = 18). None of the respondents scored lower than 18 on the MMSE. A total of 93 participants were finally enrolled in this study (Fig. 2). The characteristics of the participants are shown in Table 1. The mean age ± standard deviation (SD) was 84.2 ± 4.7 years. The median NSI score [interquartile range] was 3.0 [1.0–5.0] which indicated moderate nutritional risk. The median EAT-10 score was 0 [0–2.0], which represented the absence of subjective swallowing difficulties. In the bivariate correlation analysis (Table 2), oral diadochokinesis was not significantly correlated with age and any of the tongue muscle characteristics. Swallowing speed was negatively correlated with age (r = − 0.331, p < 0.01) and was significantly correlated with maximum tongue pressure (r = 0.287, p < 0.01), but tongue muscle thickness was not. The tongue muscle quality index was significantly correlated with swallowing speed (r = 0.294, p < 0.01). A stepwise multiple regression analysis was performed with swallowing speed as a dependent variable, and age, maximum tongue pressure and tongue muscle quality index as independent variables (Table 3). Age (β = − 0.292, 95% confidence interval [CI] = − 0.596 to − 0.124, p = 0.003) and tongue muscle quality index (β = 0.267, 95% CI = 0.177–1.077, p = 0.007) were factors related to swallowing speed.
Discussion
In this study, we investigated the relationship between tongue muscle characteristics and swallowing speed in community-dwelling older women. We found that age and tongue muscle strength and quality were significantly correlated with swallowing speed. In addition, stepwise multiple regression analysis demonstrated that tongue muscle quality, not tongue muscle strength, independently affected swallowing speed. Recently, the revised European consensus on sarcopenia proposed that muscle quality is also better indicator for sarcopenia as well as muscle quantity and strength [12]. Therefore, current findings suggested to assess the tongue muscle quality may be important for evaluating on swallowing speed in older women.
We found that maximum tongue pressure and tongue muscle quality index were related to swallowing speed, whereas tongue muscle thickness was not. Regarding skeletal muscle, muscle quality is an important indicator because of the discordant relationship between skeletal muscle mass and strength [12]. Similarly, our results revealed that tongue muscle mass and strength did not always show concordant results. Several studies have also reported that tongue muscle mass is not related to tongue muscle strength [32, 33], and there is a similar pattern with tongue muscle characteristics and skeletal muscle characteristics [21]. Although the tongue differs from skeletal muscle in terms of muscle composition and the similarity between tongue muscle and skeletal muscle has been discussed [34, 35], these results indicated that age-related changes in the tongue muscle are similar to those of the skeletal muscle in clinical findings. Therefore, tongue muscle quality may affect the discordant relationship between tongue muscle mass and strength, and it is important to assess not only tongue muscle quantity and strength but also quality. We measured tongue muscle quality using structural and kinematic assessment of tongue muscle mass and strength, whereas we did not investigate tongue muscle quality in detail. Previous studies have reported that the composition of the tongue muscle changes with age [34], and that the echo intensity of the tongue muscle in sarcopenic dysphagia patients is lower than in non-sarcopenic dysphagia patients [11]. In the future research, it is necessary to verify the relationship between swallowing function and tongue muscle quality by physiological, radiological and histological evaluations.
The results of this study demonstrate the importance of focusing on tongue muscle quality for improving swallowing function. Previous studies have reported that low tongue muscle strength is related to swallowing disorder [10, 19] and hypothesized that improving tongue muscle strength contributes to improvements in swallowing function. Although researchers and clinicians believe that interventions for the tongue lead to improvement in swallowing function, the effects remain unclear [36]. Generally, tongue pressure during swallowing, that is, swallowing pressure, is an important factor in swallowing. While several studies have revealed age-related changes in swallowing pressure, there has been a lack of consensus owing to variations in assessment methods [37,38,39,40]. Robbins et al. have pointed out that the decreases in swallowing pressure and maximum tongue pressure occur in parallel, although the rates varied [40]; therefore, tongue muscle quality index, represented as tongue muscle pressure divided by tongue muscle mass, might be related to the generation of swallowing pressure. Several studies on skeletal muscle have revealed that muscle quality is a more influential indicator of performance than muscle quantity and strength [18, 26]. However, few studies have investigated the effectiveness of tongue muscle quality for tongue muscle strength including swallowing pressure and swallowing function. Our results indicate that tongue muscle quality affects swallowing function; further research is needed to confirm whether tongue muscle training changes tongue muscle quality, and whether this has utility for improving maximum tongue pressure, swallowing pressure and swallowing function.
We used swallowing speed as an indicator of swallowing function, which might be able to reflect the slight swallowing changes in older women without dysphagia. A previous study demonstrated that swallowing speed decreases with advancing age and that slows swallowing speed could be due to a reduction in average bolus volume, or a prolongation of average time per swallow, or a combination of both [41]. Mulheren et al. reported that older adults have decreased swallowing, such as anterior hyoid excursion and pharyngoesophageal segment opening, compared with younger adults [1]. These age-related changes lead to a reduction in average bolus volume and decreased swallowing speed. Swallowing speed was used as a representative indicator of swallowing performance for patients with neurogenic dysphagia [41, 42] and head and neck cancer [43] and revealed that dysphagic patients had slower swallowing speed than healthy older adults. Although a few studies have involved swallowing speed for community-dwelling older adults, this assessment does not need special medical tools such as videofluoroscopic and videoendoscopic examinations, and it might be a simple and sensitive evaluation of swallowing function for community-dwelling older adults without dysphagia. To our knowledge, this is the first study to provide valuable data on swallowing speed in Japanese older women without dysphagia. Swallowing speed is affected by age, sex and several dysphagic diseases such as neurogenic disease and head and neck cancers [41,42,43]. In addition, it is affected by the properties of bolus (i.e., volume, viscosity and temperature) [4]. As we did not assess the number of swallows, we were unable to determine whether the volume per swallow affected the results. Also, as there are few studies investigating swallowing speed, we could not sufficiently discuss the validity of our results. Especially, as the data regarding Japanese older adults were insufficient, there is a need to accumulate the required data.
There were several limitations to this study. First, we investigated only the tongue muscle and did not assess other swallowing-related muscles such as the geniohyoid muscle. As the tongue is just one of the various muscles involved in the complicated process of swallowing, our results are limited to a single aspect of the swallowing speed. Second, we used tongue muscle thickness as an indicator of tongue muscle mass. A previous study measured tongue muscle mass via tongue muscle area [11], and others used tongue muscle thickness [9, 32, 33]. However, previous research is not sufficient to mandate standardized measurements in tongue muscle mass. We should investigate which measurement is most useful for the assessment of tongue muscle mass in older adults. Third, we enrolled only older women without dysphagia. Therefore, our findings are generalizable only to older women without dysphagia, and the relationship between tongue muscle characteristics and swallowing function in older men and adults with dysphagia remains unclear. Finally, we conducted a cross-sectional study. Further research with a longitudinal design is required to clarify whether tongue muscle quality affects tongue muscle strength and swallowing function.
Conclusion
We developed a tongue muscle quality index, which is a new indicator of swallowing speed, and found that it was related to swallowing speed in community-dwelling older women. According to our findings, it is possible that the tongue muscle quality index is a useful parameter for swallowing speed in older women without dysphagia. Further research is required to clarify the relationship between tongue muscle quality and swallowing speed in older men, and dysphagia patients and longitudinal studies are needed to determine whether tongue muscle quality affects swallowing speed and is an intervenable factor to improve swallowing speed.
References
Mulheren RW, Azola AM, Kwiatkowski S et al (2018) Swallowing changes in community-dwelling older adults. Dysphagia 33:848–856
Madhavan A, LaGorio LA, Crary MA et al (2016) Prevalence of and risk factors for dysphagia in the community dwelling elderly: a systematic review. J Nutr Health Aging 20:806–815
Baijens LW, Clavé P, Cras P et al (2016) European society for swallowing disorders—European Union geriatric medicine society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging 11:1403–1428
Humbert I, Robbins A (2008) Dysphagia in the elderly. J Phys Med Rehabil Clin N Am 19:853–866
Mendes AE, Nascimento L, Mansur LL et al (2015) Tongue forces and handgrip strength in normal individuals: association with swallowing. Clinics (Sao Paulo) 70:41–45
Wu MC, Chang YC, Wang TG et al (2004) Evaluating swallowing dysfunction using a 100-ml water swallowing test. Dysphagia 19:43–47
Clavé P, Shaker R (2015) Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol 12:259–270
Robbins J, Humpal NS, Banaszynski K et al (2016) Age-related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia 31:90–96
Tamura F, Kikutani T, Tohara T et al (2012) Tongue thickness relates to nutritional status in the elderly. Dysphagia 27:556–561
Butler SG, Stuart A, Leng X et al (2011) The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci 66:452–458
Ogawa N, Mori T, Fujishima I et al (2018) Ultrasonography to measure swallowing muscle mass and quality in older patients with sarcopenic dysphagia. J Am Med Dir Assoc 19:516–522
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Writing Group for the European working group on Sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Aging 48:16–31
Goodpaster BH, Park SW, Harris TB et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064
Delmonico MJ, Harris TB, Visser M et al (2009) Health, aging, and body. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90:1579–1585
McGregor RA, Cameron-Smith D, Poppitt SD (2014) It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3:9
Barbat-Artigas S, Rolland Y, Zamboni M et al (2012) How to assess functional status: a new muscle quality index. J Nutr Health Aging 16:67–77
Pinto RS, Correa CS, Radaelli R et al (2014) Short-term strength training improves muscle quality and functional capacity of elderly women. Age (Dordr) 36:365–372
Gadelha AB, Neri SGR, Nóbrega OT et al (2018) Muscle quality is associated with dynamic balance, fear of falling, and falls in older women. Exp Gerontol 104:1–6
Sakai K, Nakayama E, Tohara H et al (2017) Tongue strength is associated with grip strength and nutritional status in older adult inpatients of a rehabilitation hospital. Dysphagia 32:241–249
Buehring B, Hind J, Fidler E et al (2013) Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J Am Geriatr Soc 61:418–422
Suzuki M, Koyama S, Kimura Y et al (2018) Relationship between characteristics of skeletal muscle and oral function in community-dwelling older women. Arch Gerontol Geriatr 79:171–175
Chantaramanee A, Tohara H, Nakagawa K et al (2019) Association between echo intensity of the tongue and its thickness and function in elderly subjects. J Oral Rehabil 46:634–639
Tombaugh TN, McIntyre NJ (1992) The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40:922–935
Mori T, Fujishima I, Wakabayashi H et al (2017) Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clin Rep 2:1–10
Newman AB, Haggerty CL, Goodpaster B et al (2003) Health aging and body composition research group. Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc 51:323–330
Yamada M, Kimura Y, Ishiyama D et al (2017) Differential characteristics of skeletal muscle in community-dwelling older adults. J Am Med Dir Assoc 18:807.e9–807.e16
Wakabayashi H, Kayashita J (2014) Translation, reliability, and validity of the Japanese version of the 10-item Eating Assessment Tool (EAT-10) for the screening of dysphagia. JJSPEN 29:871–876 (in Japanese)
Watanabe Y, Hirano H, Arai H et al (2017) Relationship between frailty and oral function in community-dwelling elderly adults. J Am Geriatr Soc 65:66–76
Tanaka T, Takahashi K, Hirano H et al (2018) Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci 73:1661–1667
Posner BM, Jette AM, Smith KW et al (1993) Nutrition and health risks in the elderly: the nutrition screening initiative. Am J Public Health 83:972–978
Sugiura Y, Tanimoto Y, Imbe A et al (2016) Association between functional capacity decline and nutritional status based on the nutrition screening initiative checklist: a 2-year cohort study of Japanese community-dwelling elderly. PLoS ONE 11:e0166037
Fujimoto K, Honda T, Suito H et al (2018) Tongue thickness and its clinical significance. J Oral Health Biosci 31:32–38
Furuya H, Tamura F, Yoshida M et al (2016) Tongue muscle mass and strength relate to whole-body muscle in the community-dwelling elderly. J Jpn Assoc Oral Rehabil 29:1–9 (in Japanese)
Miller JL, Watkin KL, Chen MF (2002) Muscle, adipose, and connective tissue variations in intrinsic musculature of the adult human tongue. J Speech Lang Hear Res 45:51–65
Fujishima I, Fujiu-Kurachi M, Arai H et al (2019) Sarcopenia and dysphagia: position paper by four professional organizations. Geriatr Gerontol Int. https://doi.org/10.1111/ggi.13591
McKenna Victoria S, Zhang Bin, Haines Morgan B et al (2017) A systematic review of isometric lingual strength-training programs in adults with and without dysphagia. Am J Speech Lang Pathol 26:524–539
Nicosia MA, Hind JA, Roecker EB et al (2000) Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci 55:M634–M640
Steele CM (2013) Optimal approaches for measuring tongue-pressure functional reserve. J Aging Res 2013:542909
Todd JT, Lintzenich CR, Butler SG (2013) Isometric and swallowing tongue strength in healthy adults. Laryngoscope 123:2469–2473
Robbins J, Humpal NS, Banaszynski K et al (2016) Age-related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia 31:90–96
Hughes TA, Wiles CM (1996) Clinical measurement of swallowing in health and in neurogenic dysphagia. QJM 89:109–116
Nathadwarawala KM, Nicklin J, Wiles CM (1992) A timed test of swallowing capacity for neurological patients. J Neurol Neurosurg Psychiatry 55:822–825
Patterson JM, McColl E, Carding PN et al (2009) Swallowing performance in patients with head and neck cancer: a simple clinical test. Oral Oncol 45:904–907
Acknowledgements
The authors acknowledge Mr. Buichi Tanaka and Mr. Yasuhito Obuchi for their contributions to the data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors have no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suzuki, M., Koyama, S., Kimura, Y. et al. Relationship between tongue muscle quality and swallowing speed in community-dwelling older women. Aging Clin Exp Res 32, 2073–2079 (2020). https://doi.org/10.1007/s40520-019-01388-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01388-z