Abstract
The Eating Assessment Tool-10 (EAT-10) is a 10-item patient-reported outcome measure (PROM) for dysphagia patients. The objective of this study was to translate and validate the EAT-10Heb and to test for a correlation between its score and residue, penetration and aspiration on Fiberoptic Endoscopic Examination of Swallowing (FEES). 136 patients visiting two specialized dysphagia clinics and undergoing FEES between April 2015 and August 2017, filled the EAT-10Heb. 23 patients refilled the EAT-10Heb during a 2-week period following their first visit. FEES were scored for residue (1 point per consistency, maximum 3 points) and penetration and aspiration (1 point for penetration, 2 points for aspiration per consistency, maximum 6 points). 51 healthy volunteers also filled the EAT-10Heb. Internal consistency and test–retest reproducibility were examined for reliability testing. Validity was established by comparing EAT-10Heb scores of dysphagia patients to healthy controls. The EAT-10Heb score was then correlated with the FEES score. Internal consistency of the EAT-10Heb was high (Cronbach’s alpha = 0.925) as was the test–retest reproducibility (Spearman’s correlation coefficient = 0.82, p < 0.0001). The median EAT-10Heb score was significantly higher in the dysphagia group compared to healthy controls (13, IQR 7–22 points for dysphagia patients compared to 0, IQR 0–0 points for healthy controls, p < 0.0001). A weak correlation was found between the EAT-10Heb scores and the FEES score (Pearson’s correlation coefficient = 0.376, p < 0.0001). While the EAT-10Heb was found to be a reliable and valid PROM, it only weakly correlates with the pathological findings on FEES examination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia affects up to a fifth of the general population [1, 2], and in designated populations, such as stroke patients, its prevalence is much higher [3,4,5]. Despite its prevalence, dysphagia is under-reported by patients and often neglected by clinicians [6]. Dysphagia has many possible etiologies ranging from neurologic diseases to old age and head and neck (H&N) surgery or radiation [7,8,9,10]. Individuals with dysphagia are affected both on a physical level, with development of malnutrition, dehydration, aspiration pneumonia, and asphyxia [11, 12] and on functional and emotional levels, inducing anxiety, depression, and social isolation [6, 10,11,12].
A variety of patient-reported outcome measures (PROM) assessing dysphagia are available [13]. Some PROM are disease-specific, such as the M.D. Andersons Dysphagia Inventory (MD-ADI) which targets H&N cancer survivors [14] and the Dysphagia Goal Handicap (DGH) for patients with esophageal dysphagia [15]. Others are more generic, designed for any-cause dysphagia, such as the Swallowing Quality of Life Questionnaire (SWAL-QOL) [16] or the Dysphagia Handicap Index (DHI) [17].
The Eating Assessment Tool-10 (EAT-10) is a PROM comprised of 10 items addressing the main aspects of dysphagia [18]. Each statement is scored by the patient on a 5-level scale ranging from 0 (no problem) to 4 (severe problem), with a total score range of 0–40. The EAT-10 is designed for any-cause dysphagia and has been applied to different dysphagia populations, including oropharyngeal and esophageal dysphagia [18].
The EAT-10 has been shown to correlate with findings on instrumental swallowing assessments (ISA), such as videofluoroscopy or Fiberoptic endoscopic evaluation of swallowing (FEES). Special emphasis has been put on the ability of the EAT-10 to predict aspiration [19,20,21,22,23,24]. Being a short and easy-to-administer questionnaire, the EAT-10 has often been chosen for dysphagia screening [19, 20, 25] and has been translated to many languages [21, 26,27,28,29,30,31,32,33].
There are several PROM for Hebrew-speaking dysphagia patients. The Swallowing Disturbance Questionnaire (SDQ) was developed in Hebrew as a screening tool and adjunct to bedside evaluation in patients with dysphagia [34]. Recently, the Hebrew-DHI [35] as well as a Hebrew version of the EAT-10 [36] has been validated. However, the recently published Hebrew-EAT10 has several drawbacks. The published version of the Hebrew-EAT10 contains a misspelling in item 10 rendering that item unintelligible in Hebrew. Furthermore, The Hebrew-EAT10 was validated on patients who did not undergo formal dysphagia evaluations, with heterogeneous dysphagia etiologies which were not compared, and was not correlated with objective findings on instrumental swallowing examinations.
The goal of this study was to establish the reliability and validity of another Hebrew version of the EAT-10 (EAT-10Heb), and to examine how the EAT-10Heb correlates with penetration–aspiration and pharyngeal residue on FEES.
Methods
Translation
Translation of the EAT-10 questionnaire into Hebrew was carried out according to the principles of good practice for the translation and cultural adaptation process for patient-reported outcome measures, as defined by the International Society for Pharmacoeconomics and Outcome Research (ISPOR) Task Force for translation and cultural adaptation [37]. The questionnaire was forward translated from English to Hebrew by two independent translators. The translations were reconciled to a single forward translation, which was then back-translated into English by another independent translator. The questionnaire was then reviewed by three subjects who are fluent in both English and Hebrew. The author of the EAT-10 gave his approval to translate the EAT-10.
Population
The study group was recruited from two medical centers. It included patients visiting the dysphagia clinic at the Kaplan Medical Center, Rehovot or the Sheba Medical Center, Tel Hashomer, between the years 2015 and 2017. Exclusion criteria were age under 18, declined cognitive state precluding the patient from filling the questionnaire, non-Hebrew-speaking patients, and patients who were disinclined to participate in the study. Illiterate patients or those who were unable to write their answers due to physical limitations were assisted by a family member, physician, or speech language pathologist (SLP) who read them the EAT-10Heb items. The participant verbally scored the item, which was subsequently filled in their stead. The study was approved by the institutional ethics committees. All participants signed an informed consent form. Data collected included age and sex, as well as Functional Oral Intake Score (FOIS) [38] prior to the clinic visit and the cause of dysphagia, if known.
Eligible patients filled the EAT-10Heb questionnaire during their visit to the dysphagia clinic. A random sample of dysphagia patients was contacted via telephone by a physician or SLP which were blind to the first EAT-10Heb score to fill the questionnaire a second time. Refilling the questionnaire took place during a 2-week period following the date after first filling the questionnaire and without major therapeutic interventions taking place between the two times.
Missing items were given the mean score of the other items in the PROM.
The control group included healthy volunteers recruited from hospital personnel, patients’ companions, and patients admitted to the hospital for ambulatory surgery in areas other than the H&N (e.g., elective orthopedic or urologic surgery). Exclusion criteria for the control group were a personal history of dysphagia, H&N malignancy/radiation therapy or operations (except adenotonsillectomy), history of neurologic disease stroke, inability to fill the questionnaire, and age under 18.
Dysphagia Evaluation
All patients in the study group underwent a full history taking and physical examination followed by an FEES on the same day they filled the EAT-10Heb. The standard FEES protocol was followed with slight modifications as will be described later in detail [39]. A flexible digital video rhinolaryngoscope (ENF-V2, Olympus Medical System Corporation, Tokyo, Japan or Pentax Fiber naso pharyngo laryngoscope FNL 15RP3, Japan, or Storz video rhinolaryngoscope VP 11101, Germany) was passed through the patient’s most patent naris with the administration of a small amount of topical anesthetic (2% Lidocaine hydrochloride gel) which has been shown not to significantly alter the FEES results [40, 41]. Swallowing was evaluated directly with nine bolus challenges, three of each consistency (liquid, purée, and solid) of approximately 5 cc volume each, presented in the following order: Three boluses of purée consistency (green-dyed apple purée) followed by a solid consistency challenge of whole wheat bread (two pieces without crust and one with the crust) and concluded with three thin liquid boluses (green-dyed 3% fat milk). Patients were encouraged to feed themselves, with assistance as needed, i.e., liquid with a straw or cup and purée with a spoon. All patients were allowed to swallow spontaneously, i.e., without a verbal command to swallow.
Each bolus challenge was evaluated for the presence of penetration or aspiration, and was scored using the Penetration–Aspiration Scale (PAS) [42]. Penetration was defined as PAS 2–5 and aspiration was defined as PAS 6–8. The worst PAS out of all bolus challenges in all consistencies was used for analysis. In order to evaluate the overall dysphagia severity, each FEES received a score incorporating both penetration–aspiration and pharyngeal residue. Residue presence was scored using a binary scale. A score of 0 was given if residue was absent in all consistencies tested and a score of 1 was given for each consistency in which any severity residue was observed, with a maximal score of 3 if residue was present in all three consistencies tested. For penetration and aspiration—for every consistency tested, a score of 0 was given if no airway penetration or aspiration was observed, a score of 1 was given if penetration was observed, and 2 if aspiration was observed. The scores for penetration–aspiration and residue were then summated. The possible FEES scores ranged from 0 (no residue, penetration or aspiration in either of the three consistencies) to 9 (maximum 3 points for residue and 6 points for aspiration).
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software version 20. Continuous variables are described as mean with standard deviation. Non-continuous variables were compared using Chi-square test. Continuous variables were compared using Mann–Whitney test if two variables were compared or Kruskal–Wallis test if three or more variables were compared.
The reliability of the questionnaire was tested by two methods: Internal consistency and test–retest reproducibility. Cronbach’s alpha coefficient was used to evaluate internal consistency, with a minimum acceptable value of 0.7. Test–retest reproducibility was analyzed by correlating initial score results and subsequent questionnaire scores using Spearman’s correlation coefficient. A minimum test–retest correlation coefficient of 0.7 was acceptable.
The validity of the questionnaire, comparing dysphagia patient and healthy individuals, was tested using non-paired T test. Concurrent criterion validity was evaluated by comparing the EAT-10Heb score results with the score given to the pathological findings on FEES, as detailed above.
Correlations are presented with either the Spearman’s correlation coefficient (SCC) or the Pearson’s Correlations Coefficient (PCC). To determine the discriminatory capacity of the EAT-10Heb for patients with and without aspirations, the area under the receiver operating characteristics curve (AUC-ROC) was calculated using MedCalc Statistical Software version 14.12.0 software (Ostend, Belgium). p values of < 0.05 were considered statistically significant results.
Results
The Hebrew version of the EAT-10Heb is presented in Fig. 1.
Population
The study group consisted of 136 dysphagia patients: 25 recruited from the Kaplan Medical Center and 111 recruited from the Sheba Medical Center (Table 1). 55 patients (38.4%) were males. Mean age of the cohort was 63.5 ± 14.73 years. Dysphagia etiology was heterogeneous, and consisted of neurogenic etiology, including stroke, head trauma or neuromuscular degenerative diseases (n = 54, 39.7%), H&N surgery related, as well as post-radiation changes (n = 35, 25.7%), esophageal dysphagia, including Zenker’s diverticulum, esophagitis, esophageal surgery, or motility disorders (n = 12, 8.8%), and other/unknown including presbyphagia, globus sensation, chronic cough work-up, and affective disorders (n = 35, 25.7%). No statistically significant differences were observed between populations from both study sites in regards to age, gender, or dysphagia etiology. The control group included 51 healthy volunteers, with a median age of 62.19 ± 9.28, 25 of which (50%) were males.
23 patients refilled the questionnaire, with a mean age of 62.95 ± 13.03, 73.9% males (n = 17), 5 with neurogenic etiology (21.7%), 13 with H&N etiology (56.5%), 1 with esophageal etiology (4.3%), and 4 with unknown/other etiology (17.3%).
There were only 6 missing items from all 1360 items of the questionnaires from the dysphagia group, and none from the control group.
Reliability
The internal consistency (Cronbach’s alpha) for the EAT-10Heb total score was high (0.925). The internal consistency for each of the 10 questions of the EAT-10Heb was also high, ranging from 0.909 to 0.924 (see Table 2). Test–retest reproducibility of the EAT-10Heb was high (SCC = 0.82) based on 23 patients who refilled the questionnaire.
Validation
The EAT-10Heb score was significantly higher for dysphagia patients compared to healthy controls (15 ± 10.17 points compared to 0.27 ± 0.90, p < 0.0001). Moreover, a statistically significant difference was observed between the dysphagia group and healthy controls when comparing the score for each individual question independently (see Table 3).
EAT-10 Scores and Dysphagia Etiologies
The mean EAT-10Heb score was 15.87 ± 8.98 points for the neurogenic dysphagia group, 18.63 ± 11.2 for the H&N group, 12.75 ± 10.58 for the esophageal dysphagia group, and 11.11 ± 9.7 for other/unknown cause dysphagia. Since the unknown/other group included a variety of diagnoses unrelated to one another, we compared results only between the groups of homogenous dysphagia etiologies. There were no statistically significant differences in the EAT-10Heb scores between the esophageal etiology, H&N, and neurogenic etiology groups. The characteristics of each dysphagia etiology group are presented in Table 4. The neurogenic dysphagia group was older (p = 0.006) and showed higher PAS scores compared to the H&N group (p = 0.048). Neurogenic and H&N groups showed worse FEES scores compared to the esophageal dysphagia groups (p = 0.024, p = 0.014 respectively).
Comparison of EAT-10Heb with FEES
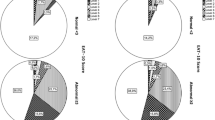
A weak correlation was observed between the overall FEES score and the EAT-10Heb score with a PCC of 0.376 (p < 0.0001). When examining each component of the FEES score independently, both the score for penetration (SCC = 0.394, p < 0.0001), for aspiration (PCC = 0.254, p = 0.003), and for pharyngeal residue (PCC = 0.248, p = 0.004) showed weak statistically significant correlations with the EAT-10Heb score. Figure 2 shows EAT-10Heb score distribution according to presence of pharyngeal residue. There was a statistically significant difference in mean EAT-10Heb scores between patients who received a FEES score of 0–1 (EAT-10Heb = 12.29 ± 9.90, n = 55, 40.4%), a score of 2–3 (EAT-10Heb = 13.95 ± 8.82, n = 42, 30.8%), and a score of 4 or higher (EAT-10Heb = 20.26 ± 9.98, n = 39, 28.6%), p = 0.001. Figure 3 shows EAT-10Heb score distribution according to FEES scores.
Mean EAT-10Heb score distribution according to the presence of pharyngeal residue. RES residue. RES score represents the number of consistencies in which any severity residue was observed. Bolus consistencies were either liquid, semi-solid (purée), or solid. 46 patients (34.5%) received a residue score of 0; 23 patients (16.9%) a residue score of 1; 47 patients (34.5%) a residue score of 2; and 20 patients (14.7%) a score of 3
EAT-10Heb scores distribution according to FEES scores. FEES Fiberoptic Endoscopic Evaluation of Swallowing. For FEES score calculation method, see “Methods”
69 patients (50.7%) received a PAS of 1, 26 patients (19.1%) received 2 ≤ PAS ≤ 5, and 41 patients (30.1%) a PAS of 6 ≥. A weak correlation was observed between the PAS and the EAT-10Heb scores with a PCC = 0.258 (p = 0.003). A statistically significant difference was found in EAT-10Heb scores between patients with PAS = 1, 2 ≤ PAS ≤ 5, PAS of 6 ≥ (p = 0.003). Table 5 presents the EAT-10Heb score distribution according to the FEES scores and PAS.
The ROC for evaluating the discriminatory capacity of the EAT-10Heb for aspiration (based on a group of 41 aspirators and 95 non-aspirators) showed an AUC-ROC of 0.639 (95% CI 0.551–0.72, p = 0.0054) (Fig. 4). The prevalence of aspiration in the studied population was 29.3%. The ROC for pharyngeal residue (based on a group of 46 residue-free patients and 90 patients with pharyngeal residue) was 0.609 (95% CI 0.52–0.639, p = 0.036) (Fig. 5). The prevalence of residue in the studied population was 65.6%. We calculated a cut-off point for the detection of aspiration for the EAT-10Heb, preferring higher sensitivity than specificity considering the EAT-10Heb’s potential use as a screening tool. The optimal cut-off score was 8 <, yielding a sensitivity of 87.18% and a sensitivity of 42.55%.
Discussion
The EAT-10 is a dysphagia PROM which excels in its brevity and ease of use. It has been shown to be able to predict dysphagia severity on objective ISA [19,20,21,22,23,24]. For these reasons, it has gained significant popularity as a screening tool for dysphagia. In this study, we examined the psychometric properties of the EAT-10Heb on a group of 136 patients with dysphagia and 51 healthy controls, and how the EAT-10Heb correlates with objective findings on ISA.
We found the EAT-10Heb has high internal consistency (Cronbach’s alpha of 0.925) test–retest reproducibility, similarly to those reported in other translation studies [21, 26,27,28,29,30,31,32,33]. Refilling the questionnaire over the phone required reading it to the patients instead of allowing them to read and fill it by themselves, which might introduce a bias, though the researcher refilling the questionnaire with the patient was blinded to the first EAT-10Heb score. In addition, the retest sample size was relatively small and had an over-representation of H&N etiology at the expense of the neurogenic etiology. These factors might have initially suggested difficulty to generalize the reproducibility test results to all patients. However, since our results, as well as other reports in the literature [18], showed that no significant difference of EAT-10Heb scores exists between different oropharyngeal and esophageal dysphagia etiologies, we concluded that the dysphagia etiology subgroup representation in the test–retest group does not preclude the generalization of the reproducibility results.
The Hebrew EAT-10 was also found to be a valid tool, able to differentiate between dysphagia patients and healthy controls. Scores for normative population were similar to those reported for the EAT-10 and other translation studies [21, 27, 28, 36].
However, when the EAT-10Heb scores were correlated with objective findings observed in FEES, only a weak correlation with overall FEES score (PCC = 0.376, p < 0.0001) and an even weaker correlation with PAS (SCC = 0.258, p < 0.0001) was observed. Other translation studies also showed a similarly weak correlation between the EAT-10 score and PAS [23, 27, 43]. Only The EAT-10spa showed a moderate correlation with PAS (SCC = 0.54, p < 0.001) [21]. While our results showed that a significant correlation does exist between FEES findings or PAS and the EAT-10Heb, these weak correlations lead to the poor discriminatory capacity of the EAT-10Heb as a screening tool for dysphagia or aspiration. Given these suboptimal discriminatory parameters, we chose the cut-off point for detection of aspiration at 8 <, preferring to opt for higher sensitivity at the expense of lower specificity, in order to minimize the false negatives. The suggested cut-off points in other studies range from 2 to 15 [21, 23]. Possible explanations for the heterogeneity in the cut-off values for aspiration in other studies might be due to differences in studied populations, with a different mix of dysphagia etiologies. For example, esophageal dysphagia patients will have EAT-10 scores similar to those with oropharyngeal dysphagia, but lower PAS scores than oropharyngeal dysphagia patients. Another explanation might be cultural differences. We observed that our dysphagia group’s mean EAT-10Heb was higher than for English speakers, but lower than Italian, Spanish, and Swedish speakers. Japanese speakers especially stood out and had significantly lower EAT-10 scores compared to all other translations (Table 6). Even within the two Hebrew translations of the EAT-10, when supposedly the cultural differences should be minimal, there was a two-point difference between mean questionnaire’s scores in the dysphagia group, despite very similar wording [36]. However, there are other differences between the two Hebrew translations of the EAT-10 which might account for their different scores. Abu-Ghanem’s cohort consisted of more H&N patients (42.7% compared to 25.7%), compared to our cohort which was predominantly comprised of neurogenic dysphagia, and included no esophageal dysphagia compared to 8.8% in our cohort. Concerns about cross-cultural validation of the EAT-10 have been raised by Speyer et al., who evaluated the psychometric properties of the EAT-10 using Item-Response Theory (IRT) and Rasch analysis on data from Spain, Turkey, Sweden, and Italy [44]. Speyer showed significantly different responses in 6 out of the 10 items of the questionnaire on Differential Item Functioning based on language of the questionnaire. They further showed that the EAT-10 has significant weakness in structural validity and internal consistency, and recommended redeveloping the EAT-10 using the IRT. Future studies are required to test the reliability and validity of the EAT-10Heb using IRT in addition to the Classical Test Theory.
Our study has several limitations. Our approach to illiterate patients, who were assisted by another person to read them the questionnaire and help them fill it out might have skewed this subpopulation’s results. In addition, our dysphagia patients were selected from those attending our dysphagia clinics. We did not exclude patients from the dysphagia patient group if they had normal FEES examinations or no diet restrictions (FOIS), meaning some patients with no findings on FEES or normal FOIS might have been mislabeled as dysphagia patients and weakened the resulting correlations. While ensuring that patients did not undergo therapeutic interventions during the 2-week test–retest interval, we could not control for the natural course of the patient’s disease, such as after a stroke, which might have changed their dysphagia symptom severity. We did not test the EAT-10Heb for responsiveness, i.e., the ability of the PROM to reflect change after intervention, though this psychometric property has been demonstrated in other studies [18, 21]. Lastly, physicians performing the FEES were not blinded to EAT-10Heb scores.
Conclusion
The EAT-10Heb is a reliable and valid PROM for Hebrew-speaking dysphagia patients. It has a weak correlation with objective findings on ISA, including penetration, aspiration, and pharyngeal residue. As a potential screening tool for aspiration, its discriminatory capacity is poor. The need for an easy-to-apply and accurate dysphagia-screening tool is significant. Future studies redeveloping the EAT-10 using IRT or offering new alternative screening tools are recommended.
References
Lindgren S, Janzon L. Prevalence of swallowing complaints and clinical findings among 50–79-year-old men and women in an urban population. Dysphagia. 1991;6:187–92.
Wilkins T, Gillies RA, Thomas AM, Wagner PJ. The prevalence of dysphagia in primary care patients: a HamesNet Research Network study. J Am Board Fam Med. 2007;20:144–50.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63.
Serra-Prat M, Hinojosa G, Lopez D, Juan M, Fabré E, Voss DS, Calvo M, Marta V, et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J Am Geriatr Soc. 2011;59:186–7.
Cabré M, Serra-Prat M, Force LL, Almirall J, Palomera E, Clavé P. Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly: observational prospective study. J Gerontol A Biol Sci Med Sci. 2013. https://doi.org/10.1093/gerona/glt099.
Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17:139–46.
Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000;10:380–6.
Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. Eur Neurol. 1997;38:49–52.
Bloem BR, Lagaay AM, vanBeek W, Haan J, Roos RAC, Wintzen AR. Prevalence of subjective dysphagia in community residents aged over 87. BMJ. 1990;300:721–2.
Garcia-Peris P, Paron L, Velasco C, de la Cuerda C, Camblor M, Breton I, Herencia H, Verdaguer J, Navarro C, Clave P. Long- term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr. 2007;26:710–7.
Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:772–8.
Campbell BH, Spinelli K, Marbella AM, et al. Aspiration, weight loss and quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg. 2004;130:1100–3.
Timmerman AA, Speyer R, Heijnen BJ, Klijn-Zwijnenberg IR. Psychometric characteristics of health-related quality-of-life questionnaires in oropharyngeal dysphagia. Dysphagia. 2014;29(2):183–98.
Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127:870–6.
Gustafsson B, Tibbling L. Dysphagia, an unrecognized handicap. Dysphagia. 1991;6:193–9.
McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17(2):97–114.
Silbergleit AK, Schultz L, Jacobson BH, Beardsley T, Johnson AF. The dysphagia handicap index: development and validation. Dysphagia. 2012;27:46–52.
Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24.
Regan J, Lawson S, De Aguiar V. The Eating Assessment Tool-10 predicts aspiration in adults with stable chronic obstructive pulmonary disease. Dysphagia. 2017. https://doi.org/10.1007/s00455-017-9822-2.
Arslan SS, Demir N, Kılınç HE, Karaduman AA. The ability of the Eating Assessment Tool-10 to detect aspiration in patients with neurological disorders. J Neurogastroenterol Motil. 2017;23(4):550–4.
Giraldo-Cadavid LF, Gutiérrez-Achury AM, Ruales-Suárez K, et al. Validation of the Spanish version of the Eating Assessment Tool-10 (EAT-10spa) in Colombia. A blinded prospective cohort study. Dysphagia. 2016;31(3):398–406.
Rofes L, Arreola V, Mukherjee R, Clavé P. Sensitivity and specificity of the Eating Assessment Tool and the Volume-Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–65.
Cheney DM, Siddiqui MT, Litts JK, Kuhn MA, Belafsky PC. The ability of the 10-Item Eating Assessment Tool (EAT-10) to predict aspiration risk in persons with dysphagia. Ann Otol Rhinol Laryngol. 2015;124(5):351–4.
Arrese LC, Carrau R, Plowman EK. Relationship between the Eating Assessment Tool-10 and objective clinical ratings of swallowing function in individuals with head and neck cancer. Dysphagia. 2017;32(1):83–9.
Plowman EK, Tabor LC, Robison R, et al. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil. 2016;28:85–90.
Möller R, Safa S, Östberg P. Validation of the Swedish translation of eating assessment tool (S-EAT-10). Acta Otolaryngol. 2016;136(7):749–53.
Schindler A, Mozzanica F, Monzani A, et al. Reliability and validity of the Italian Eating Assessment Tool. Ann Otol Rhinol Laryngol. 2013;122(11):717–24.
Gonçalves MI, Remaili CB, Behlau M. Cross-cultural adaptation of the Brazilian version of the eating assessment tool EAT-10. Codas. 2013;25(6):601–4.
Demir N, Serel Arslan S, İnal Ö, Karaduman AA. Reliability and validity of the Turkish Eating Assessment Tool (T-EAT-10). Dysphagia. 2016;31(5):644–9.
Wang R, Xiong X, Zhang C, Fan Y. Reliability and validity of the Chinese Eating Assessment Tool (EAT-10) in evaluation of acute stroke patients with dysphagia. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(12):1391–9.
Farahat M, Mesallam TA. Validation and cultural adaptation of the Arabic Version of the Eating Assessment Tool (EAT-10). Folia Phoniatr Logop. 2015;67(5):231–7.
Wakabayashi H, Kayashita J. Translation, reliability and validity of the Japanese version of the 10-item Eating Assessment Tool (EAT-10) for the screening of dysphagia. JJSPEN. 2014;29:871–6.
Burgos R, Sarto B, Segurola H, Romagosa A, Puiggros C, Vaz-quez C, Cardenas G, Barcons N, Araujo K, Perez-Portabella C. Translation and validation of the Spanish version of the EAT-10 (Eating Assessment Tool-10) for the screening of dysphagia. Nutr Hosp. 2012;27(6):2048–54.
Cohen JT, Manor Y. Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011;121:1383–7.
Shapira-Galitz Y, Drendel M, MD, Yousovich R, Shtreiffler-Moskovich L, Wolf M, Lahav Y. Translation and Validation of the Dysphagia Handicap Index in Hebrew Speaking Patients. Abstract presented at the annual meeting of the Israeli Association of Speech and Language Pathologists; 2017.
Abu-Ghanem S, Schechter M, Flesh-Eyni H, Litwin L, Makai E, Oestreicher-Kedem Y, Yehuda M. Validation of the Hebrew Version of the Eating Assessment Tool-10 (H-EAT-10). Folia Phoniatr Logop. 2017;68(6):261–7.
Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for translation and cultural adaptation. Value Health. 2005;8(2):94–104.
Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86(8):1516–20.
Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2:216–9.
Fife TA, Butler SG, Langmore SE, Lester S, Wright SC Jr, Kemp S, Grace-Martin K, Lintzenich CR. Use of topical nasal anesthesia during flexible endoscopic evaluation of swallowing in dysphagic patients. Ann Otol Rhinol Laryngol. 2015;124(3):206–11.
O’Dea MB, Langmore SE, Krisciunas GP, Walsh M, Zanchetti LL, Scheel R, McNally E, Kaneoka AS, Guarino AJ, Butler SG. Effect of lidocaine on swallowing during FEES in patients with dysphagia. Ann Otol Rhinol Laryngol. 2015;124(7):537–44.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JC, Wood JL. A. Penetration–Aspiration Scale. Dysphagia. 1996;11:93–8.
Wilmskoetter J, Bonilha H, Hong I, Hazelwood RJ, Martin-Harris B, Velozo C. Construct validity of the Eating Assessment Tool (EAT-10). Disabil Rehabil. 2017;9:1–11.
Cordier R, Joosten A, Clavé P, Schindler A, Bülow M, Demir N, Arslan SS, Speyer R. Evaluating the psychometric properties of the Eating Assessment Tool (EAT-10) using Rasch analysis. Dysphagia. 2017;32(2):250–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Participants signed an informed consent form.
Rights and permissions
About this article
Cite this article
Shapira-Galitz, Y., Yousovich, R., Halperin, D. et al. Does the Hebrew Eating Assessment Tool-10 Correlate with Pharyngeal Residue, Penetration and Aspiration on Fiberoptic Endoscopic Examination of Swallowing?. Dysphagia 34, 372–381 (2019). https://doi.org/10.1007/s00455-018-9964-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-018-9964-x