Abstract
Adults with COPD frequently present with dysphagia, which often leads to clinical complications and hospital admissions. This study investigates the ability of the Eating Assessment Tool (EAT-10) to predict aspiration during objective dysphagia evaluation in adults with stable COPD. Thirty adults (20 male, 10 female; mean age = 69.07 ± 16.82) with stable COPD attended an outpatient dysphagia clinic for a fiberoptic endoscopic evaluation of swallowing (FEES) in an acute teaching hospital (January 2015–November 2016). During evaluations, individuals completed an EAT-10 rating scale followed immediately by a standardised FEES exam. Aspiration status during FEES was rated using the penetration–aspiration scale by clinicians blinded to EAT-10 scores. Data were retrospectively analysed. Significant differences in mean EAT-10 scores were found between aspirators (16.3; SEM = 2.165) and non-aspirators (7.3; SEM = 1.009) (p = 0.000). The EAT-10 predicted aspiration with a high level of accuracy (AUC = 0.88). An EAT-10 cut-off value of >9 presented a sensitivity of 91.67, specificity of 77.78 with positive and negative likelihood ratios of 4.12 and 0.11, respectively. Positive and negative predictive values were 73.30 and 93.30, respectively. Diagnostic odds ratio was 38.50 (p < 0.01, CI 3.75–395.42). EAT-10 is a quick, easy to administer tool, which can accurately predict the presence of aspiration in adults with COPD. The scale can also very accurately exclude the absence of aspiration, helping clinicians to determine the need for onward referral for a comprehensive dysphagia evaluation. This may ultimately reduce clinical complications and hospital admissions resulting from dysphagia in this clinical population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable lung disease characterised by chronic obstruction of lung airflow that interferes with normal breathing and is not fully reversible [1]. COPD affects over five percent of the population and it is the third leading cause of death worldwide [2]. Cardinal features include chronic cough, dyspnoea and sputum production. COPD is characterised by acute exacerbations and multiple comorbidities both of which often require hospital admissions. In fact, COPD is a leading cause of hospital admissions and it is associated with longer hospital stays, increased intensive care admissions and mortality [3, 4]. Given the high prevalence and the chronic course of COPD, the disease has a significant impact on healthcare systems globally [3]. Effective management of the condition in the community is therefore crucial both from the individual and healthcare system viewpoint.
Limited research to date suggests that dysphagia frequently presents in adults with COPD both in a stable phase and during acute exacerbations [5, 6]. One cause of dysphagia in people with COPD is thought to be the disrupted coordination of the exhale-swallow-exhale respiratory cycle during deglutition [7, 8]. Adults with an exacerbation of COPD have been reported to swallow spontaneously during the inspiratory phase or during the transition between inspiration to expiration [7,8,9]. In terms of swallow safety, one study reported that seventy percent of adults with COPD with suspected swallowing problems aspirated during videofluoroscopy [10]. Silent aspiration has also been reported in this clinical group, which can complicate dysphagia detection and management [11]. Other physiological signs of dysphagia reported in people with COPD include reduced tongue control, a delayed pharyngeal swallow, reduced tongue base retraction, impaired hyolaryngeal excursion, cricopharyngeal dysfunction, impaired laryngopharyngeal sensitivity and slower bolus transit [5,6,7,8,9,10, 12,13,14]. The high prevalence of comorbidities in the COPD population including GORD, cachexia, poor nutritional status, cardiovascular disease and polypharmacy may also contribute to dysphagia in this clinical population [15, 16]. Methodological limitations are evident in research conducted to date in this area. Studies investigating dysphagia in adults with COPD typically have not involved control groups and methods to evaluate dysphagia and respiratory coordination have varied widely across studies with inconsistent use of validated rating scales. Perhaps unsurprisingly, findings with regard to the presence and nature of dysphagia and aspiration in adults with COPD vary across studies. Additionally, the interaction between respiratory coordination and swallowing and the role of dysphagia in acute exacerbations of COPD differs across studies.
Dysphagia has been identified as a risk factor for acute exacerbations of COPD [17]. This has implications not just for the individual, but also for the healthcare system worldwide [18]. Despite this, it is often only when the person with COPD is admitted into hospital with an acute exacerbation that they are referred for an objective dysphagia evaluation. Early and accurate identification of adults with COPD who require more in-depth dysphagia assessment is critical in order to minimise clinical complications and hospital admissions. While videofluoroscopy and fiberoptic endoscopic evaluation of swallowing (FEES) are the reference standard tests used to instrumentally evaluate oropharyngeal dysphagia, objective evaluation of all adults with COPD is not feasible. As dysphagia is so often under-reported in adults with COPD, identification of those who require an in-depth dysphagia evaluation can be problematic.
The Eating Assessment Tool (EAT)-10 is a validated, quick and easy to administer self report scale [19]. In brief, patients are presented with 10 statements regarding their swallowing (e.g. “My swallowing problem has caused me to lose weight”), and are asked to rate themselves on a 5-point scale of severity for each statement (from “no problem” to “severe problem”). A score of 0 indicates no problem where as a score of 4 indicates a severe problem. An overall EAT-10 score of ≥3 is deemed abnormal and suggests the presence of dysphagia. The tool has been reported to have excellent internal consistency, test–retest reproducibility and criterion-based validity [19]. The EAT-10 has been found to be predictive of aspiration in adults with motor neurone disease, head and neck cancer and in heterogeneous clinical populations with dysphagia [20,21,22,23]. EAT-10 scores have also been associated with nutritional status and activities of daily living in elderly in long term care settings [24]. The predictive value of the EAT-10 in identifying adults with COPD who are aspirating has not been investigated to date.
The purpose of this pilot research is to begin an investigation into the ability of the EAT-10 to accurately identify adults with stable COPD who are aspirating during swallowing. Identification of a quick, easy to administer scale which can be administered in outpatient respiratory clinics may help to identify adults with COPD who need to be referred on for more in-depth dysphagia evaluation. Authors hypothesise that the EAT-10 will present with acceptable diagnostic accuracy to predict aspiration in this clinical population.
Methods
Participants
All adults with a diagnosis of COPD made by a respiratory physician who attended a dysphagia clinic within an acute teaching hospital for a FEES evaluation over a twenty-two month period (January 2015–November 2016) were included in this study. COPD severity for each participant was obtained from medical notes. Severity ratings were made by medical physicians based on post-bronchodilator forced expiratory volume (FEV)1/forced volume vital capacity (FVC) spirometry scores.
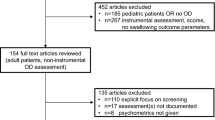
All patients had a clinical swallow evaluation by a speech and language therapist and were attending for a FEES exam. Exclusion criteria included an acute exacerbation of COPD in last six weeks, an inability to complete the EAT-10 rating scale, if a FEES examination was contra-indicated or not tolerated, diagnosis of a respiratory disease other than COPD (e.g. lung cancer, asthma) or history of stroke within the last six months. Data collected were analysed retrospectively.
Protocol
EAT-10 Scale (Index Test)
At the beginning of each procedure, a postgraduate student assisted participants with the completion of the EAT-10 screening tool [19]. The postgraduate student was subsequently not present during the FEES evaluation.
FEES (Reference Standard)
Immediately after the completion of the EAT-10, all patients sat upright in a chair in a clinic room. A standardised FEES examination was completed by one of two experienced clinicians who were blinded to the EAT-10 scores. Patients sat upright in a chair and a Kay Pentax 2.4 mm FEES scope was passed trans-nasally for direct visualisation into the pharynx. Local anaesthetic was not used. As per the department protocol, anatomical structures were initially evaluated as were secretions and airway protection. All participants were subsequently administered fluids from a cup, spoonful of puree and solid consistencies to swallow, unless deemed unsafe by the endoscopist. When the exam was complete, the scope was removed. All exams were recorded and saved on a Kay Pentax Digital Swallow Workstation (Kay Pentax, Lincoln Park, NJ, USA).
Data Analysis
Total EAT-10 scores were calculated by adding scores across the ten questions (maximum 40). After the FEES procedure, two clinicians who were blinded to EAT-10 scores used frame by frame analysis to rate aspiration across consistencies during FEES using the validated Penetration–Aspiration Scale (PAS) [25]. Briefly, the PAS is an eight point ordinal rating scale which captures aspiration status and an individual’s response to aspiration (from 1 = no aspiration up to 8 = silent aspiration). While the PAS was originally designed to measure aspiration during videofluoroscopy [25], it has since been validated for use during FEES [26]. In fact, recent research suggests that PAS ratings have better reliability based on FEES compared to videofluoroscopy [27, 28]. In this study, the worst PAS score across all consistencies and trials was selected for analysis from each examination. As per previous research [20], aspirators were defined as those who received a PAS score of ≥6 on any consistency during the FEES examination, while non-aspirators scored from 1 to 5 on the PAS scale.
Statistical Analysis
EAT-10 scores and worst PAS ratings were entered into an Excel document. Statistical analysis was completed using the statistical processing software MedCalc, Version 16.4.3 (MedCalc Software, Ostend, Belgium) [29]. Mean EAT-10 scores for those who aspirated (EAT-10 score ≥6) were compared to those who did not (EAT-10 score ≤5) using an independent samples t test. A Mann–Whitney U test was used to verify if there were differences in COPD severity between aspirators and non-aspirators. A Kruskal–Wallis test was used to establish if patients with different levels of COPD severity presented differences in their EAT-10 scores. To determine the diagnostic accuracy and optimal cut-off point for the EAT-10, receiver operating characteristic (ROC) curves were created. This allowed the calculation of the area under the curve (AUC), sensitivity, specificity, positive predictive value, negative predictive value and likelihood ratio, as well as 95% confidence intervals (CIs) associated with these values.
Results
Thirty adults with stable COPD met inclusion criteria over the twenty-two month period and were included in this study (20 male, 10 female, mean age = 69.071 ± 16.818). Participants presented with mild (FEV1 ≥ 80% predicted; n = 6), moderate (50% ≤ FEV1 < 80% predicted; n = 14) and severe (30% ≤ FEV1 < 50% predicted; n = 10) COPD.
EAT-10 Scores in Aspirators and Non-aspirators
Using the criterion of PAS score ≥6, it was established that 12 participants aspirated during FEES and 18 did not. Mean EAT-10 scores were significantly lower for safe swallowers (PAS score <6) (7.278, SEM = 1.009) compared to aspirators (mean EAT-10 = 16.333, SEM = 2.165) (t(28) = −4.216, p = 0.000) (see Fig. 1). No significant differences were found in COPD severity between aspirators and non-aspirators (Z = −1.167, p = 0.288). No significant differences in EAT-10 scores were found between the three COPD severity groups (X 2(2) = 1.031, p = 5.97).
Diagnostic Accuracy of EAT-10 in Detecting Aspiration
The EAT-10 predicted aspiration with a very good level of accuracy (AUC = 0.880, p < 0.0001, SE = 0.070, CI 0.709–0.969). Diagnostic accuracy measures for each point in the EAT-10 scale represented in our sample are available in Table 1, and represented in the ROC curve in Fig. 2.
Based on these measures, an optimal cut-off value of a score >9 for prediction of aspiration with the EAT-10 was proposed (Table 1). An EAT-10 cut-off value of >9 presented a sensitivity of 91.67 (CI 61.50–99.80), specificity of 77.78 (CI 52.40–93.60), a positive likelihood ratio of 4.12 (CI 1.70–10.00), a negative likelihood ratio of 0.11 (CI 0.02–0.70), a positive predictive value of 73.30 (CI 44.90–92.20), a negative predictive value of 93.30 (CI 68.10–99.80) and a diagnostic odds ratio of 38.50 (p < 0.01, CI 3.75–395.42).
Discussion
Findings from this initial pilot study suggest that the EAT-10 can accurately predict aspiration in individuals with COPD, as indicated by the diagnostic odds ratio of 38.50, for a cut-off value of 9. In particular, using a cut-off value of 9, aspiration was predicted accurately on 92% of adults with COPD. From the positive predictive value it can be estimated that 77% of individuals with COPD who obtain EAT-10 scores above 9 do, in fact, aspirate. However, this means that it is estimated that the presence of aspiration in this population with this criterion will be somewhat over-estimated, with a false positive rate of about 33%. In fact, the probability of a diagnosis of aspiration is 4.12 times higher in those who aspirate when compared to those who do not. This is a moderately positive likelihood ratio, which indicates that a positive diagnosis of aspiration due to a score above 9 in the EAT-10 is suggestive of true presence of aspiration, but insufficient to establish a definite diagnosis [30].
In addition, with this cut-off value, 78% of those who did not aspirate were correctly classified. This specificity value denotes some of the over-estimation of aspiration discussed above. However, from the negative predictive value of 93%, it is estimated that the test performs exceptionally well in excluding aspiration when it is indeed absent. This translates into a low rate of false negatives expected with this criterion (6.7%). In accordance, based on the negative likelihood ratio of 0.11, a score below the criterion value of 9 can be used to confidently exclude the diagnosis accurately [30]. Altogether, these diagnostic accuracy measures indicate that the EAT-10 has the features of an excellent screening test, as it determines the need for onward referral and additional assessment if scores are above the criterion, and can confidently exclude the diagnosis and determine that there is no need for additional assessment if scores are below the criterion.
Identification of outpatients with COPD who are aspirating, and hence onward referral for appropriate outpatient dysphagia management, may assist in the reduction of aspiration pneumonias and hospital admissions in this clinical group. Aspiration has frequently been reported in adults COPD [5, 10, 11], and it has been associated with acute exacerbations of COPD [17]. Dysphagia has been identified as a risk factor for community-acquired pneumonia in the elderly [31]. Furthermore, COPD is also a leading cause of hospital admissions and has been associated with longer hospital stays [3, 4]. Use of swallowing screening protocols have reduced pneumonia incidence in other clinical populations [32, 33]. As dysphagia is a precipitating factor for malnutrition in COPD, early detection of dysphagia and aspiration may also limit the impact of a swallowing disorder on nutritional status [34]. Despite these facts, awareness of the prevalence of dysphagia in people with COPD remains inconsistent. The inclusion of a short, easy to administer screen in outpatient clinics must surely be a worthwhile step from a respiratory and nutritional viewpoint.
These findings are in keeping with previous research, which investigated the value of the EAT-10 in detecting aspiration in different clinical groups [20, 21, 23]. In one study using a cut-off score of 16, the EAT-10 identified aspiration in a general dysphagia group with 71% sensitivity and 53% specificity, with a positive likelihood ratio of 2.2 in this study [20]. In another study of a general dysphagia population, the EAT-10 predicted aspiration using a cut-off point of 3 with 83% sensitivity and 25% specificity [23]. More recently, in a study of adults with amyotrophic lateral sclerosis, EAT-10 predicted aspiration with 86% accuracy and 72% specificity using a cut-off point of 8. The positive likelihood ratio in this ALS study at 3.1 [21] provided a similar level of confidence to detect the presence of aspiration as the one in our sample (4.12). In this study of adults with COPD, sensitivity and specificity values for aspiration are higher than previous studies (92 and 78% respectively). Furthermore, our very negative predictive value (0.11) indicates that the particular strength of the EAT-10 is in the exclusion of aspiration, a feature that qualifies this test as a tool particularly useful for screening purposes.
Interestingly, all other studies examining the value of the EAT-10 in detecting aspiration or dysphagia have used videofluoroscopy as the reference standard test [20, 21, 23]. However, FEES is considered to be as robust as dysphagia reference standard test. This is the first study to use FEES as the reference standard dysphagia evaluation. Authors purport that FEES has a number of advantages over videofluoroscopy in the COPD population. Unlike videofluoroscopy, FEES does not involve radiation exposure, therefore allowing the effects of endurance or fatigue to be monitored over the course of a mealtime. This may be pertinent for people with COPD who may present with dyspnoea and increased respiratory rate during meals. Use of FEES in the COPD population has the added advantage that the pharyngeal mucosa, laryngopharyngeal sensitivity and vocal cords can be evaluated, which may be of relevance given the increased risk of GORD and lung cancer in this population. FEES has been shown to have better reliability than videofluoroscopy in rating penetration [26,27,28]. Aspiration tends to be scored higher on FEES exams compared to videofluoroscopy, which may be attributed to better visualisation of anatomical structures [28]. However, the potential influence of the nasopharyngeal scope on respiratory rate and coordination in adults with COPD should be a focus of future research in this area.
This research was an initial pilot study conducted to explore the role of the EAT-10 in predicting aspiration in adults with COPD. As such, further research with a larger sample size is required before recommending its integration into clinical practice. The retrospective design of this study was a drawback. However, patients were recruited consecutively and the reference standard test was interpreted without knowledge of EAT-10 scores and vice versa, which addressed the issues of patient selection and bias as per the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [35]. The time interval between the index (EAT-10) and reference (FEES) tests was also minimal which was of value from a flow and timing domain viewpoint [35]. The completion of an item analysis is recommended for future research to determine if specific questions within the EAT-10 differed between aspirators and non-aspirators.
Concerns regarding the structural validity and internal consistency of the EAT-10 have been put forward. The psychometric properties of the EAT-10 scored badly in a recent functional health status questionnaire systematic review, although authors did suggest that the rating system used was quite severe [36]. Additionally, a recent study using Rasch analysis uncovered the limitations of EAT-10 as a stand-alone tool [37]. While these concerns warrant attention in terms of using the EAT-10 as a stand-alone health status scale, they do not affect the use of EAT-10 as a screen to identify those at risk of aspiration who require more in-depth investigation.
In conclusion, EAT-10 scores predicted aspiration in adults with stable COPD during objective dysphagia evaluation in this study. The EAT-10 is a quick and easy to administer tool that it could, with further research, be employed by respiratory physicians in outpatient clinics to identify those who require a more in-depth instrumental dysphagia evaluation. Correct exclusion of aspiration in patients with COPD who are not at risk of aspiration reduces the number of patients attending for unnecessary videofluoroscopy or FEES exams. In addition, a tool that facilitates adequate onward referral in those who are indeed at risk may also assist in the prevention of aspiration pneumonia or an acute exacerbation of COPD, both of which require hospital admission and antibiotic cover.
References
Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35(1):1–6.
Global Strategy for the Diagnosis. Management, and prevention of chronic obstructive pulmonary disease (GOLD). 2008. Accessed 26 Jan 2009.
Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121:121S–6S.
Pifarre R, Falguera M, Vicente-de-Vera C, Nogues C. Characteristics of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. Respir Med. 2007. doi:10.1016/j.rmed.2007.05.011.
Cvejic L, Harding R, Churchward T, Turton A, Finlay P, Massey D, Bardin PG, Guy P. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology. 2011;16:269–75.
Mokhlesi B, Logemann JA, Rademaker AW, Stangl CA, Corbridge TC. Oropharyngeal deglutition in stable COPD. Chest. 2002;121(2):361–9.
Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, Kern MK, Rynders A. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol. 1992;263(5.1):750–5.
Gross DR, Atwood CW, Ross SB, Olszewski JW, Eichhorn KA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:559–65.
Martin-Harris B. Clinical implications of respiratory—swallowing interactions. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):194–9.
Good-Fratturelli MD, Curlee RF, Holle JL. Prevalence and nature of dysphagia in VA patients with COPD referred for videofluoroscopic swallow examination. J Commun Disord. 2000;33(2):93–110.
Zheng Z, Wu Z, Liu N, Chen P, Hou P, Wang X, Fu Y, Liang W, Chen R. Silent aspiration in patients with exacerbation of COPD. Eur Respir J. 2016;48(2):570–3.
Stein M, Williams AJ, Grossman F, Weinberg AS, Zuckerbraun L. Cricopharyngeal dysfunction in chronic obstructive pulmonary disease. Chest. 1990;97(2):347–52.
Clayton NA, Carnaby-Mann GD, Peters MJ, Ing AJ. The effect of chronic obstructive pulmonary disease on laryngopharyngeal sensitivity. Ear Nose Throat J. 2012;91(9):370–82.
Coelho CA. Preliminary findings on the nature of dysphagia in patients with chronic obstructive pulmonary disease. Dysphagia. 1987;2(1):28–31.
Takada K, Matsumoto S, Kojima E, Iwata S, Okachi S, Ninomiya K, Morioka H, Tanaka K, Enomoto Y. Prospective evaluation of the relationship between acute exacerbations of COPD and gastroesophageal reflux disease diagnosed by questionnaire. Respir Med. 2011;105(10):1531–6.
Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549–55.
Kobayashi S, Kubo H, Yanai M. Impairment of the swallowing reflex in exacerbations of COPD. Thorax. 2007;62(11):1017.
Schermer TRJ, Saris CGJ, Van Den Bosch WJHM, Chavannes NH, Van Schayck CP, Dekhuijzen PNR, Van Weel C. Exacerbations and associated healthcare cost in patients with COPD in general practice. Monaldi Arch Chest Dis. 2016. doi:10.4081/monaldi.2006.558.
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24.
Cheney DM, Siddiqui MT, Litts JK, Kuhn MA, Belafsky PC. The ability of the 10-item eating assessment tool (EAT-10) to predict aspiration risk in persons with dysphagia. Ann Otol Rhinol Laryngol. 2015;124(5):351–4.
Plowman EK, Tabor LC, Robison R, Gaziano J, Dion C, Watts SA, Vu T, Gooch C. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil. 2016;28(1):85–90.
Arrese LC, Carrau R, Plowman EK. Relationship between the Eating Assessment Tool-10 and objective clinical ratings of swallowing function in individuals with head and neck cancer. Dysphagia. 2016;32:83–9.
Rofes L, Arreola V, Mukherjee R, Clave P. Sensitivity and specificity of the Eating Assessment Tool and the volume-viscosity swallow test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil. 2014;26(9):1256–65.
Wakabayashi H, Matsushima M. Dysphagia assessed by the 10-item Eating Assessment Tool is associated with nutritional status and activities of daily living in elderly individuals requiring long-term care. J Nutr Health Aging. 2016;20(1):22–7.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration–aspiration scale. Dysphagia. 1996;11(2):93–8.
Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (Fees®) using the penetration–aspiration scale: a replication study. Dysphagia. 2002;17(4):308–15.
Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope. 2007;117(10):1723–7.
Pisegna JM, Langmore SE. Parameters of instrumental swallowing evaluations: describing a diagnostic dilemma. Dysphagia. 2016;31(3):462–72.
MedCalc, Version 16.4.3 (MedCalc Software, Ostend, Belgium). 2016. https://www.medcalc.org.
Dollaghan CA. The handbook for evidence-based practice in communication disorders. Baltimore: Paul H Brookes Publishing Company; 2007.
Almirall J, Rofes L, Serra-Prat M, Icart R, Palomera E, Arreola V, Clave P. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J. 2013;41:923–8.
Ickenstein GW, Riecker A, Höhlig C, Müller R, Becker U, Reichmann H, Prosiegel M. Pneumonia and in-hospital mortality in the context of neurogenic oropharyngeal dysphagia (NOD) in stroke and a new NOD step-wise concept. J Neurol. 2010;257:1492–9.
Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36:1972–6.
Rofes L, Arreola V, Romea M, Palomera E, Almirall J, Cabré M, Serra-Prat M, Clavé P. Pathophysiology of oropharyngeal dysphagia in frail elderly. Neurogastroenterol Motil. 2010;22:851.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Speyer R, Cordier R, Kertscher B, Heijnen BJ. Psychometric properties of questionnaires on functional health status in oropharyngeal dysphagia: a systematic literature review. BioMed Res Int. 2014. doi:10.1155/2014/458678.
Cordier R, Joosten A, Clavé P, Schindler A, Bülow M, Demir N, Serel Arslan S, Speyer R. Evaluating the psychometric properties of the Eating Assessment Tool (EAT-10) using rasch analysis. Dysphagia. 2016;32:250–60.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Regan, J., Lawson, S. & De Aguiar, V. The Eating Assessment Tool-10 Predicts Aspiration in Adults with Stable Chronic Obstructive Pulmonary Disease. Dysphagia 32, 714–720 (2017). https://doi.org/10.1007/s00455-017-9822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-017-9822-2