Abstract
In this study, the submerged culture conditions for exopolysaccharide (EPS) production by Ganoderma cantharelloideum M. H. Liu were screened and optimized, and the physicochemical and biological properties of EPS were investigated. Results showed that the glucose and tryptone were the best C and N sources for the maximum EPS production, respectively. Under the optimal culture conditions, the EPS production achieved 1.60 g/L at day 6 in a 5 L stirred tank reactor. Two purified fractions (i.e., Fr-I and Fr-II) were obtained from the G. cantharelloideum EPS by gel permeation chromatography. Fr-II had a higher yield (87.54%), carbohydrate (95.86%), and protein (3.07%) contents and lower molecular weight (74.56 kDa) than that of Fr-I. Both Fr-I and Fr-II were polysaccharide-protein complexes with different monosaccharide compositions and chemical structures. Fr-II also exhibited stronger radical scavenging abilities, antioxidant capacities, and α-amylase and α-glycosidase inhibitory activities in vitro than that of Fr-I. Therefore, Fr-II obtained from G. cantharelloideum EPS might be explored as potential natural functional components or supplements for applications in food, medicine, and cosmetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungal polysaccharides are isolated from the fruiting body, mycelia, and fermentation broths. A class of active polysaccharides that can control cell division and differentiation and regulate cell growth and senescence. Many fungal polysaccharides play an important pharmacological role in regulating immunity, antitumor, antivirus, hypoglycemic, hypolipidemic, and antioxidation activities (Wasser 2002). Generally, the submerged cultivation and solid culture of fruit body are the main sources in obtaining fungal polysaccharides. However, the formation of fruiting bodies needs considerable time of up to several months. Although similar substrates and ensuring comparable conditions were used, product quality may vary significantly (Fang and Zhong 2002). Increasing studies have demonstrated that the submerged cultivation of the mycelium has been developed as an attractive alternative in producing biomass and obtaining high-value metabolites, such as exopolysaccharides (EPSs), in a compact space and short time without significant contamination risk (Jaros et al. 2018; Kachlishvili et al. 2005). Meanwhile, considerable attention has been paid on bioactive EPS produced using submerged cultures of numerous fungi or mushrooms because they always exhibit similar biological activities to that of the polysaccharides extracted from the fruit body (Kim et al. 2001). For example, Osińska-Jaroszuk et al. (2015) pointed on the bioactive and biological properties of fungal EPS, thereby covering antioxidative properties and immunomodulatory and antitumor activities, as well as potential EPS contributions to biofertilization and bioremediation.
Ganoderma cantharelloideum M. H. Liu is a mushroom-like fungus that is one of the famous traditional Chinese medicinal herbs. G. cantharelloideum that was only found in Guizhou province, China belongs to the phylum Basidiomycota, class Basidiomycetes, order Aphyllophorales, and family Ganoderma. Similar to G. lucidum, G. cantharelloideum has been also customarily used as a health food and medicine in Southwest China for a long time due to its unique pharmacological activities and health benefits, such as activities against hepatitis, hypertension, hypercholesterolemia, and gastric cancer (Tang and Zhong 2001). The pharmaceutical potential of G. cantharelloideum is attributed to its abundant chemical constitutes, especially polysaccharides. By contrast, the study and application of G. cantharelloideum and its polysaccharides were considerably restricted by its lack of natural resources. The submerged fermentation of G. cantharelloideum is a promising alternative for efficient valuable metabolites, that is, EPS. However, to the best of our knowledge, no or only few studies are available on the production, physicochemical, and biological properties of EPS by submerged fermentation of G. cantharelloideum.
Therefore, the objective of this study was to investigate the submerged culture conditions of G. cantharelloideum for EPS production under a shake-flask culture and a 5 L stirred tank reactor. The physicochemical properties and preliminary structural features of the purified EPS fractions were characterized. Meanwhile, the in vitro biological activities of the purified EPS fractions were evaluated.
Materials and methods
Materials and chemicals
Glucuronic acid, trifluoroacetic acid, acarbose, porcine pancreatic α-amylase (≥ 1000 units/mg), α-glucosidase (≥ 10 units/mg), p-nitrophenyl-α-D-glucopyranoside (pNPG), hydrogen peroxide (H2O2), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2΄-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and 2, 4, 6-tris(2-pyridyl)-s-triazine were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals and solvents were of laboratory grade and used without further purification.
Microorganism and growth conditions
G. cantharelloideum obtained from the Henan Province Microbiological Culture Collection Center (HPMCC, No. 251901–2#) and was used throughout this study. The stock culture was maintained on potato dextrose agar (PDA) slants, and then these slants were incubated at 26 °C for 8 days and stored at 4 °C. The seed culture was grown in a 250 mL flask containing 50 mL of basal medium (3% glucose and 0.3% peptone) at 26 °C on a rotary shaker incubator (150 rev min−1) for 4 days. Flask culture experiments were carried out in 250 mL Erlenmeyer flasks containing 50 mL of media after inoculating with 4% (v/v) of the seed culture. G. cantharelloideum was initially grown on PDA medium in a petri dish and transferred into the seed medium by punching out 5 mm of the agar plate culture with a self-designed cutter (Park et al. 2001).
Determination of mycelial dry weight and EPS production
Samples collected regularly from shake flasks, and the mycelia pellets and culture broth were separated through filtration. The mycelial pellets were repeatedly washed with distilled water several times and dried at 70 °C overnight, and then the mycelial dry weight was calculated. The culture broth was directly precipitated by 4 volumes of 95% ethanol, kept overnight at 4 °C, centrifuged at 10,000×g for 15 min, and collected the precipitates to obtain the EPS. EPS was redissolved in distilled water, and the EPS concentration was determined by the phenol–sulfuric acid method (Dubois et al. 1956).
Cultivation in a stirred tank reactor
The batch submerged fermentations were performed in a 5 L stirred tank reactor with a six-blade Rushton turbine impeller. The cultivation medium was inoculated with 4% (v/v) of the seed culture. The culture was aerated at 2 vvm and maintained at 26 °C. During the entire cultivation period, the process parameters, including dissolved O tension, pH, temperature, and air flow, were continuously monitored. The samples were collected at various intervals and subjected to morphology measurements by using an image analyzer (DT2000 System, China) with a software linked to a light microscope (Nikon, Japan) via a CCD camera. Then, the detailed operating conditions and methods were described in our previous study (Cao et al. 2014). Meanwhile, the EPS was harvested and determined as described in Sect. 2.3.
EPS isolation and purification
The crude EPS obtained from the culture broth of G. cantharelloideum by ethanol precipitation was deproteinized using the Sevag reagent, dialyzed (MWCO: 8–14 kDa) against distilled water for 48 h, and freeze dried to yield the pretreated EPS. The resultant EPS was dissolved in 0.2 M NaCl buffer and then centrifuged. The supernatant was applied to a pre-equilibrium Sepharose CL-6B column (2.4 cm × 100 cm, Sigma Chemical Co. St. Louis, MO) and eluted with the same buffer at a flow rate of 0.6 mL/min. Carbohydrates and proteins in each tube (5 mL/tube) were detected by the phenol–sulfuric acid method and the Bradford method at 490 nm and 280 nm, respectively (Bradford 1976; Dubois et al. 1956). The collected fractions were concentrated, dialyzed, and lyophilized to yield the purified EPSs, that is, designated as Fr-I and Fr-II, respectively, and used for further studies.
General chemical properties
The total carbohydrate, uronic acid, and protein contents were determined by the phenol–sulfuric acid method using glucose as a standard (Dubois et al. 1956), via sulfuric acid–carbazole method using glucuronic acid as a standard (Bitter and Muir 1962), and via the Bradford method using bovine serum albumin (BSA) as a standard (Bradford 1976), respectively. Total phenolic content was determined by Folin-Ciocalteu assay reagent using gallic acid as a reference (Siu et al. 2014).
Preliminary structural characterization
The molecular weights (MWs) of Fr-I and Fr-II were determined by Sepharose CL-6B gel-filtration chromatography as described in our previous study (Cao et al. 2014). Dextran MW standards ranging from 10 to 2000 kDa (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were used for calibration and determination of MWs of Fr-I and Fr-II.
The monosaccharide compositions of Fr-I and Fr-II were investigated by a series of acid hydrolysis, derivation, and gas chromatography-mass spectroscopy (GC–MS) on a Varian Star 3600 CX instrument (Varian Co., Lexington, MA, USA) with a fused silica capillary column (Na form, 30 m × 0.25 mm, Supelco Inc., Bellefonte, PA, USA) with the conditions as reported before (Zheng et al. 2014). D-Arabinose (D-Ara), D-glucose (D-Glc), D-galactose (D-Gal), D-mannose (D-Man), L-rhamnose (L-Rha), and D-xylose (D-Xyl) (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were used as monosaccharide standards.
Fourier-transform infrared (FT-IR) spectra of Fr-I and Fr-II were determined using a FT-IR spectrometer (Bruker Tensor 27) in the wavenumber range of 400–4000 cm−1 with KBr pellets and referenced against air.
Antioxidant activity assays
The antioxidant activities in vitro of Fr-I and Fr-II were evaluated by measuring the hydroxyl radical (·OH) scavenging activity, DPPH radical scavenging activity, Trolox equivalent antioxidant capacity (TEAC) assay and ferric reducing ability of plasma (FRAP) assay. For all assays, the samples were predissolved in distilled water and measured at different concentrations (0–3.0 mg/mL) against distilled water as the blank and the vitamin C (Vc) at the same concentration range as a positive antioxidant reference. The details of the operating conditions and methods were described in the previous studies (Wang et al. 2016).
In vitro α-amylase and α-glycosidase inhibitory activity assays
The adapted α-amylase inhibitory activity was performed according to our previous study (Yan et al. 2019). Briefly, 100 μL of samples of various concentrations (0–5.0 mg/mL) and 200 μL of 12.5 U/mL porcine pancreatic α-amylase (prepared by 0.1 mol/L PBS, pH 6.8) were mixed and incubated at 37 °C for 5 min. Then, 500 μL of 1% (w/w) soluble starch was added to initiate the reaction and continued to incubate at 37 °C for 5 min. The reaction was terminated by adding 500 μL of dinitrosalicyclic acid color reagent and incubated at 100 °C for 5 min. After cooling to room temperature, the reaction mixture was diluted to 10 mL with 0.1 mol/L PBS (pH 6.8), and absorbance was measured at 540 nm. The reaction mixture without sample was used as control and the acarbose as a positive reference.
Concerning the α-glycosidase inhibitory activity assay method (Yan et al. 2019), 100 μL samples with different concentrations (0–5.0 mg/mL) were mixed with 3.0 mL 0.1 mol/L phosphate-buffered saline (PBS, pH 6.8) and 20 μL 5 U/mL α-glycosidase (prepared by 0.1 mol/L PBS, pH 6.8) and incubated at 37 °C for 15 min. The reaction was initiated by adding 100 μL 2.5 mmol/L pNPG (prepared by 0.1 mol/L PBS, pH 6.8) and incubated at 37 °C for 15 min, followed by adding 5 mL 0.2 mol/L sodium carbonate to stop the reaction. The mixture absorbance measured at 400 nm against the reaction mixture without sample was used as the control and the acarbose as a positive control.
Statistical analysis
All experiments are conducted in three replicates and the mean ± standard deviation (SD) is used in the analysis. The statistical analysis was performed by Student’s t-test. Data Processing System (DPS Version 3.0) was used for the experimental designs and statistical differences were considered significant at P < 0.05.
Results and discussion
Screening of the optimal C and N sources for EPS production
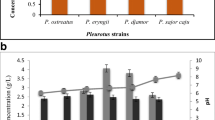
As described in previous studies, the submerged culture conditions for EPS production from Cordyceps militaris and Cordyceps sinensis (Kim and Yun 2005), Morchella esculenta SO-02 (Meng et al. 2010), Funalia trogii (He et al. 2012), and Incutis tamaricis (Zheng et al. 2014) have been screened and optimized. However, the optimal C and N sources in the submerged culture of G. cantharelloideum for mycelial growth and EPS production have not been studied in the present work. Figure 1 shows the effects of various C and N sources on both mycelial growth and EPS production in G. cantharelloideum. Out of all the C sources examined (Fig. 1a), the highest EPS production was 1.90 g/L when glucose was applied as the C source in the culture medium, and the mycelial growth had also the highest level (6.53 g/L) among the tested C sources. Thus, glucose, as the best C source, used for maximum EPS production, which agreed with the results of the previous studies (Chen et al. 2019; Meng et al. 2010), while was the opposite of the results reported by our group in other fungi or mushrooms, such as F. trogii (He et al. 2012) and I. tamaricis (Zheng et al. 2014). As shown in Fig. 1b, different N sources exhibited significant influences on both mycelial growth and EPS production. Among the six tested N sources, tryptone yielded the maximum EPS production (1.87 g/L), whereas the highest mycelial growth (5.93 g/L) was achieved when yeast extract was used. The results suggested that the nutritional requirements for EPS production in G. cantharelloideum were not always consistent with that of mycelial biomass. Similar findings have been also observed in our previous works (He et al. 2012; Zheng et al. 2014). On the basis of the results above, we can conclude that glucose and tryptone were separately regarded as the most favorable C and N sources for the maximum EPS production in the submerged culture of G. cantharelloideum, respectively, and they were selected and applied to EPS production in a 5 L stirred tank reactor.
EPS production in a stirred tank reactor
Figure 2 displays the typical time courses of the EPS production in a 5 L stirred tank reactor under the optimal submerged culture conditions (30 g/L glucose, 3 g/L tryptone, at 26 °C, and pH of 5.0) for EPS production. The EPS production gradually increased and then slowly decreased throughout the submerged fermentation process. The EPS production reached a maximum concentration of 1.60 g/L at day 6. To confirm the EPS production, we observed the typical morphological changes during the entire cultivation period in the stirred tank reactor, and the results are graphically depicted in Fig. 3. The fresh cells mainly formed pellets, and the outer hairy region of the fungal pellets was fluffy on the early stage of the cultivation period (1–4 days). A complex of EPS and mycelium exists in and around the fungal pellet core region. As the submerged culture continues, the fungal pellets became clear, and its hair region thinned out constantly (4–6 days). After 8 days of cultivation, the fungal pellets were compact and had a smooth periphery. The results indicated that the higher shear force in the stirred tank reactor can substantially destroy the hairiness of the fungal pellet and finally resulted in low EPS yield, which agreed with the result shown in Fig. 2. Similar phenomena have been found in other fungi, such as Pycnoporus sanguineus (Cao et al. 2014) and P. tenuipes (Xu et al. 2006).
Purification and physicochemical properties of EPS
The pretreated EPS obtained from the submerged culture of G. cantharelloideum by a series of ethanol precipitation, deproteinization, and dialysis was applied to purify and fractionize by gel permeation chromatography on a Sepharose CL-6B column. As shown in Fig. 4, EPS was fractionized into two purified fractions, namely, Fr-I and Fr-II. The two fractions also showed simultaneous absorbance at 490 and 280 nm, which indicated both Fr-I and Fr-II consisted of polysaccharides and proteins through covalent bonds. The two characteristic peaks at approximately 200 and 280 nm were found in the UV visible spectra of Fr-I and Fr-II (data not shown), respectively, thereby confirming that they were polysaccharide-protein complexes. As shown in Table 1, the Fr-I and Fr-II yields were 6.89% and 87.54%, respectively, and the fraction Fr-II was viewed as the main component. Both Fr-I and Fr-II had high carbohydrate contents (> 95%) and a small amount of proteins, without uronic acids (Table 1). Fr-I and Fr-II also contained small amounts of polyphenols. According to the calibration curve derived by the dextran standards (Fig. 5), the MWs of Fr-I and Fr-II were approximately 627.72 and 74.56 kDa, respectively, which demonstrated that Fr-II had the lower MW than Fr-I. Peng and Zhang (2003) found that the MWs of two fractions of water-soluble polysaccharide-protein complexes from the mycelium of G. tsugae were characterized to be 3550 and 630 kDa, which were larger than those of Fr-I and Fr-II. The monosaccharide compositions of Fr-I and Fr-II were investigated by acid hydrolysis and GC–MS analysis, respectively, and the results are presented in Table 1. Fr-I was mainly composed of D-Xyl, D-Man, D-Glc and D-Gal with the molar ratio of 1.0:49.6:5.8:2.5, and Fr-II was comprised by L-Rha, D-Xyl, D-Man, D-Glc, and D-Gal in a molar ratio of 2.5:1.0:91.6:119.5:15.2. The results revealed that Fr-I and Fr-II were both heteropolysaccharides with different monosaccharide compositions and molar ratios. D-man was detected as the predominant monosaccharide in Fr-I, and L-Rha was absent in Fr-I. In comparison, D-Glc and D-Man were considered as the main monosaccharides in Fr-II, and a small amount of L-Rha was observed in the Fr-II structure. However, Zhen et al. (2018) showed that a homogenous polysaccharide (GAP-3S) purified from G. applanatum consisted of D-Glc, D-Gal, D-Fuc, and D-Xyl in the molar ratio of 7.1: 2.6: 1.1:1. Xu et al. (2019) found that polysaccharides (GLP) and ultrasound-degraded polysaccharides (GLPUD) from G. lucidum were both acidic heteropolysaccharides, comprising by L-Rha, D-Xyl, D-Man, fructose, D-Gal, D-Glc, galacturonic acid and glucuronic acid with various molar ratios. These results were different from our present data, which was due to their different source of materials and separation protocols. Therefore, these results demonstrated that Fr-I and Fr-II purified from the G. cantharelloideum EPS had different MWs and monosaccharide compositions in their molecular structures.
Determination of molecular weight of the EPSs (Fr-I and Fr-II) in Sepharose CL-6B column chromatography. Elutes were analyzed by measuring the absorbance at 480 nm for carbohydrate. Kav = (Ve − Vo)/(Vt − Vo) (Vo; void volume, Vt; total volume, Ve; elution volume). Fr-I and Fr-II were denoted as closed circles
Preliminary structural characterizations of EPS
The preliminary structural features of Fr-I and Fr-II were qualitatively characterized by FT-IR spectroscopy, and the results are displayed in Fig. 6. Fr-I and Fr-II had similar IR spectra. A broad and strong absorption peak at approximately 3277.1 (or 3280.1) cm−1 was assigned to the O–H stretching vibration, and a weak peak at approximately 2927.6 (or 2941.0) cm−1 was attributed to the C–H stretching vibration. No absorption peak was observed at approximately 1730 cm−1. This result indicated the absence of uronic acids in their structure and further confirmed that Fr-I and Fr-II were both neutral heteropolysaccharides. The absorption bands at approximately 1634.4 (or 1632.3) (amide I) and 1548.2 (or 1537.8) cm−1 (amide II) were caused by the presence of proteins (Yan et al. 2017), which agreed with the results presented in Fig. 4 and Table 1. Zhang et al. (2016) demonstrated that two water-soluble polysaccharides (GPS-1 and GPS-2) isolated from the spent mushroom compost of G. lucidum might be amino-polysaccharides due to the -NH-CO- stretching vibration at 1642.63 (or 1623.67) cm−1, which was similar to our present study. The strong absorption bands in the range of 1000–1200 cm−1 were attributed to the C–O–C and C–O–H stretching vibrations. In the FT-IR spectrum of Fr-I, two characteristic peaks were found at approximately 916.0 and 809.2 cm−1 indicate the presence of α- and β-anomeric configurations in Fr-I. By contrast, a characteristic peak at approximately 918.2 cm−1 was observed in Fr-II, thereby suggesting that β-glycosidic bond existed in Fr-II.
In vitro antioxidant activity
The oxidative damage of the kidneys and liver and hemorrhage of the heart and brain are often associated with excess free radicals in the body. Thus, in this experiment, the in vitro antioxidant activities of Fr-I and Fr-II purified from the G. cantharelloideum EPS were investigated and compared with the positive reference Vc by different biochemical methods, such as DPPH and ·OH radical scavenging abilities and TEAC and FRAP assays. As shown in Figs. 7a and b, both Fr-I and Fr-II exhibited DPPH and ·OH radical scavenging abilities in dose-dependent manners. Within the measured concentration range (0.5–3.0 mg/mL), the scavenging activities on DPPH and ·OH radicals of Fr-II were stronger than those of Fr-I but lower than those of Vc. At the concentration of 3.0 mg/mL, the scavenging effects on DPPH and ·OH radicals were 60.25% and 59.87% for Fr-I, and 78.14% and 71.30% for Fr-II, respectively. This finding suggested that the Fr-II showed stronger free radical scavenging ability than Fr-I. Shi et al. (2014) reported that the ·OH radical scavenging abilities of two polysaccharides (GLP-I and GLP-II) obtained from fermented soybean curd residue by G. lucidum were 68.74% and 66.10%, respectively, at the concentration of 10 mg/mL, which was lower than that of Fr-II (71.30%, 3.0 mg/mL). The TEAC and FRAP values for the Fr-II were 233.97 μmol Trolox/g sample and 85.80 μmol Fe2+/g sample, respectively, which were slightly larger than those for Fr-I (157.82 μmol Trolox/g sample and 53.93 μmol Fe2+/g sample, respectively; Fig. 7c). The result indicated that the Fr-II had stronger antioxidant capacities than that of Fr-I, which agreed with the results of DPPH and ·OH radical scavenging abilities possibly due to the fact that Fr-II had the lower MW than Fr-I (Table 1; Fig. 5). Xing et al. (2005) indicated that the antioxidant capacities for low-MW polysaccharides are more pronounced than that of high-MW polysaccharides due to the intramolecular H bond effect. Similarly, Kang et al. (2019) reported that GLPUAE extracted from G. lucidum with ultrasound-assisted extraction with a lower MW (465.55 kDa) exhibited relatively higher levels of radical-scavenging abilities on DPPH and ·OH than that of GLPHWE (703.45 kDa) extracted via hot water extraction. The radical-scavenging ability and antioxidant capacity of Fr-I and Fr-II were also closely related to their carbohydrate and protein contents, monosaccharide compositions, and chemical structures. Therefore, Fr-II with high carbohydrate and protein contents and low MW exhibit evident antioxidant activity.
Inhibitory effects on α-amylase and α-glycosidase
The α-amylase and α-glycosidase inhibitory assays as simple and effective methods have been widely used in the evaluation of hypoglycemic activities of bioactive compounds in vitro (Wu et al. 2016; Yan et al. 2019). Therefore, in the current work, the inhibition effects of Fr-I and Fr-II on the α-amylase and α-glycosidase activities by applying acarbose as the positive control were evaluated, and the results are presented in Fig. 8. Both Fr-I and Fr-II showed concentrate-dependent inhibitory effects on α-amylase and α-glycosidase activities within the determined concentration range (1–5 mg/mL). At 5.0 mg/mL concentration, the inhibitory effects of Fr-II on α-amylase and α-glycosidase activities were 60.70 and 63.06%, respectively, which were stronger than those of Fr-I (40.73 and 50.11%, respectively) but weaker than those of acarbose (78.62 and 90.66%, respectively). The inhibitory effects on α-amylase and α-glycosidase activities were associated with the carbohydrate and protein contents, MWs, monosaccharide compositions, and chemical structures of Fr-I and Fr-II. Fr-I and Fr-II presented strong inhibitory ability against α-glycosidase than that of α-amylase under the same concentrations, which was due to the different mechanisms of action between α-amylase and α-glycosidase, and a moderated inhibition on α-amylase and a strong α-glycosidase inhibition should be preferred. Similar results have been found in our previous study (Yan et al. 2019). Consequently, Fr-II possessed excellent α-amylase and α-glycosidase inhibitory activities in vitro.
Conclusions
This work on the production and characterization of bioactive EPSs from the submerged culture of G. cantharelloideum M. H. Liu has not been conducted elsewhere. Our results demonstrated that glucose and tryptone were the most favorable C and N sources for the maximum EPS production, and the EPS production reached a maximum concentration of 1.60 g/L at day 6 in a 5 L stirred tank reactor under optimal cultivation conditions (30 g/L glucose, 3 g/L tryptone, 26 °C, and pH 5.0). The two fractions (i.e., Fr-I and Fr-II) with yields of 6.89 and 87.54%, respectively, were purified from G. cantharelloideum EPS by gel permeation chromatography on a Sepharose CL-6B column. Fr-II possessed higher carbohydrate (95.86%) and protein (3.07%) contents and lower MW (74.56 kDa) than that of Fr-I (95.35, 2.18%, and 627.27 kDa). They were both neutral heteropolysaccharides and had different monosaccharide compositions and chemical structures. Fr-II exhibited stronger scavenging abilities on DPPH and ·OH radicals, antioxidant capacities, and α-amylase, and α-glycosidase inhibitory activities than that of Fr-I via in vitro assays, which was attributed to its high carbohydrate and protein contents and low MW. Therefore, Fr-II obtained from G. cantharelloideum EPS might be explored as potential natural functional components or supplements for applications in food, medicine, and cosmetics. Further investigations on the structural elucidation, in vivo bioactivity evaluation and molecular mechanism of Fr-II are in progress.
References
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334. https://doi.org/10.1016/0003-2697(62)90095-7
Cao J, Zhang HJ, Xu CP (2014) Culture characterization of exopolysaccharides with antioxidant activity produced by Pycnoporus sanguineus in stirred-tank and airlift reactors. J Taiwan Inst Chem E 45:2075–2080. https://doi.org/10.1016/j.jtice.2014.05.005
Chen L, Wang Z, Zhang B, Ge M, Ng H, Niu Y, Liu L (2019) Production, structure and morphology of exopolysaccharides yielded by submerged fermentation of Antrodia cinnamomea. Carbohyd Polym 205:271–278. https://doi.org/10.1016/j.carbpol.2018.10.070
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric method for determination of sugars and related substances. Anal Chem 28:350–366. https://doi.org/10.1021/ac60111a017
Fang QH, Zhong JJ (2002) Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites-ganoderic acid and polysaccharide. Biochem Eng J 10:61–65. https://doi.org/10.1016/S1369-703X(01)00158-9
He P, Geng L, Wang Z, Mao D, Wang J, Xu C (2012) Fermentation optimization, characterization and bioactivity of exopolysaccharides from Funalia trogii. Carbohyd Polym 89:17–23. https://doi.org/10.1016/j.carbpol.2012.01.093
Jaros D, Köbsch J, Rohm H (2018) Exopolysaccharides from Basidiomycota: Formation, isolation and techno-functional properties. Eng Life Sci 18:743–752. https://doi.org/10.1002/elsc.201800117
Kachlishvili E, Penninckx MJ, Tsiklauri N, Elisashvili V (2005) Effect of nitrogen source on lignocellulolytic enzyme production by white–rot basidiomycetes under solid-state cultivation. World J Microb Biot 22:391–397. https://doi.org/10.1007/s11274-005-9046-8
Kang Q, Chen S, Li S, Wang B, Liu X, Hao L, Lu J (2019) Comparison on characterization and antioxidant activity of polysaccharides from Ganoderma lucidum by ultrasound and conventional extraction. Int J Biol Macromol 124:1137–1144. https://doi.org/10.1016/j.ijbiomac.2018.11.215
Kim DH, Yang BK, Jeong SC, Park JB, Cho SP, Das S, Yun JW, Song CH (2001) Production of a hypoglycemic, extracellular polysaccharide from the submerged culture of the mushroom, Phellinus linteus. Biotechnol Lett 23:513–517. https://doi.org/10.1023/A:1010312513878
Kim HO, Yun JW (2005) A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J Appl Microbiol 99:728–738. https://doi.org/10.1111/j.1365-2672.2005.02682.x
Meng F, Liu X, Jia L, Song Z, Deng P, Fan K (2010) Optimization for the production of exopolysaccharides from Morchella esculenta SO-02 in submerged culture and its antioxidant activities in vitro. Carbohyd Polym 79:700–704. https://doi.org/10.1016/j.carbpol.2009.09.032
Osińska-Jaroszuk M, Jarosz-Wilkołazka A, Jaroszuk-Ściseł J, Szałapata K, Nowak A, Jaszek M, Ozimek E, Majewska M (2015) Extracellular polysaccharides from ascomycota and basidiomycota: production conditions, biochemical characteristics, and biological properties. World J Microb Biot 31:1823–1844. https://doi.org/10.1007/s11274-015-1937-8
Park JP, Kim SW, Hwang HJ, Yun JW (2001) Optimization of submerged culture conditions for the mycelial growth and exo-biopolymer production by Cordyceps militaris. Lett Appl Microbiol 33:76–81. https://doi.org/10.1046/j.1472-765X.2001.00950.x
Peng YF, Zhang LN (2003) Characterization of a polysaccharide-protein complex from Ganoderma tsugae mycelium by size-exclusion chromatography combined with laser light scattering. J Biochem Biophys Methods 56(1–3):243–252. https://doi.org/10.1016/S0165-022X(03)00062-9
Shi M, Yang Y, Hu X, Zhang Z (2014) Effect of ultrasonic extraction conditions on antioxidative and immunomodulatory activities of a Ganoderma lucidum polysaccharide originated from fermented soybean curd residue. Food Chem 155:50–56. https://doi.org/10.1016/j.foodchem.2014.01.037
Siu KC, Chen X, Wu JY (2014) Constituents actually responsible for the antioxidant activities of crude polysaccharides isolated from mushrooms. J Funct Foods 11:548–556. https://doi.org/10.1016/j.jff.2014.08.012
Tang YJ, Zhong JJ (2001) Submerged fermentation of higher fungi for production of valuable bioactive metabolites. J East China Univ SciTechnol 27:704–711
Wang ZB, Chen BB, Luo L, Yan JK (2016) Fractionation, physicochemical characteristics and biological activities of polysaccharides from Pueraria lobata roots. J Taiwan Inst Chem E 67:54–60. https://doi.org/10.1016/j.jtice.2016.07.029
Wasser S (2002) Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol 60:258–274. https://doi.org/10.1007/s00253-002-1076-7
Wu J, Shi S, Wang H, Wang S (2016) Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: a review. Carbohyd Polym 144:474–494. https://doi.org/10.1016/j.carbpol.2016.02.040
Xing RG, Liu S, Guo ZY, Yu HH (2005) Relevance of molecular weight of chitosan and its derivatives and their antioxidant activities in vitro. Bioorgan Med Chem 13:1573–1577. https://doi.org/10.1016/j.bmc.2004.12.022
Xu CP, Kim SW, Hwang HJ, Yun JW (2006) Production of exopolysaccharides by submerged culture of an entomopathogenic fungus, Paecilomyces tenuipes C240 in stirred-tank and airlift reactors. Bioresour Technol 97:770–777. https://doi.org/10.1016/j.biortech.2005.01.042
Xu Y, Zhang X, Yan XH, Zhang JL, Wang LY, Xue H, Jiang GC, Ma XT, Liu XJ (2019) Characterization, hypolipidemic and antioxidant activties of degraded polysaccharides from Ganoderma lucidum. Int J Biol Macromol 135:706–716. https://doi.org/10.1016/j.ijbiomac.2019.05.166
Yan JK, Wang YY, Qiu WY, Shao N (2017) Three-phase partitioning for efficient extraction and separation of polysaccharides from Corbicula fluminea. Carbohyd Polym 163:10–19. https://doi.org/10.1016/j.carbpol.2017.01.021
Yan JK, Wu LX, Qiao ZR, Cai WD, Ma H (2019) Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem 271:588–596. https://doi.org/10.1016/j.foodchem.2018.08.012
Zhang J, Meng G, Zhai G, Yang Y, Zhao H, Jia L (2016) Extraction, characterization and antioxidant activity of polysaccharides of spent mushroom compost of Ganoderma lucidum. Int J Biol Macromol 82:432–439. https://doi.org/10.1016/j.ijbiomac.2015.10.016
Zhen D, Su L, Miao Y, Zhao F, Ren G, Mahfuz S, Song H (2018) Purification, partal characterization and inducing tumor cell apoptosis activity of a polysaccharide from Ganoderma applanatum. Int J Biol Macromol 115:10–17. https://doi.org/10.1016/j.ijbiomac.2018.03.062
Zheng JQ, Mao XJ, Geng LJ, Yang GM, Xu CP (2014) Production optimization, preliminary characterization and bioactivity of exopolysaccharides from Incutis tamaricis (Pat.) Fiasson & Niemela. J Taiwan Inst Chem E 45:725–733. https://doi.org/10.1016/j.jtice.2013.08.006
Acknowledgements
This work was supported by the Major Public Welfare Projects in Henan Province (201300110200), National Science Foundation of China (Grant No U1604176) and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (Grant No 20IRTSTHN022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest relevant to this article.
Rights and permissions
About this article
Cite this article
Long, Z., Xue, Y., Ning, Z. et al. Production, characterization, and bioactivities of exopolysaccharides from the submerged culture of Ganoderma cantharelloideum M. H. Liu. 3 Biotech 11, 145 (2021). https://doi.org/10.1007/s13205-021-02696-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02696-w