Abstract
Biogas, a gaseous effluent from the anaerobic digestion of organic waste, is considered an important source of energy, since it has a composition mainly of methane (CH4; 55–75%) and CO2 (20–60%). Today, CO2 from biogas is an excellent carbon source to induce high microalgal biomass production; however, each microalga strain can have different optimal CO2 concentrations for maximizing their bio-refinery capacity as well as different ability to endure stressful conditions of industrial effluents. This study assessed the bio-refinery capacity of Chlorella sp. and Scenedesmus sp., native of Lago de Chapala, Mexico, from biogas, as well as the effect of high CO2 and methane concentrations on the physiological performance to grow, capture CO2 and biochemical composition of both microalgae cultured under different biogas compositions. The results show that both microalgae have the biotechnological potential to endure biogas compositions of 25% CO2–75% CH4. Under this condition, the biomass production attained by Chlorella sp. and Scenedesmus sp. was 1.77 ± 0.32 and 2.25 ± 0.20 g L−1, respectively, with a biochemical composition mainly of carbohydrates and proteins. Overall, this study demonstrates that both microalgae have the ability to endure the stressful biogas composition without affecting their physiological capacity to capture CO2 and biosynthesize high-value metabolites. Moreover, it is worth highlighting the importance of screening wild-type microalgae from local ecosystems to determine their physiological capacity for each biotechnological application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The supply of CO2 from industrial gases to microalgae culture is a bio-refinery approach used with different purposes, such as increasing biomass production and biosynthesizing high-value metabolites, and reducing production costs and CO2 emissions to the atmosphere [1, 2]. Specifically, biogas, a gaseous effluent from the anaerobic digestion of organic waste, is considered an important source of energy, since it has a composition of methane (CH4; 55–75%), CO2 (20–60%), and sulfidic acid (H2S; 0.005–2%) [3]. To date, several studies have demonstrated that CO2 from biogas is an excellent carbon source to induce high microalgal biomass production and simultaneously perform CH4 upgrading, since CO2 content reduces the calorific value of CH4 preventing meeting the specifications of fuel gas [3,4,5,6,7,8,9]. Nevertheless, this dual purpose varies in each microalga strain, because the potential to endure high CO2 and CH4 concentrations is strain-dependent; besides, the high concentration of both metabolites causes stress on several strains decreasing their physiological activity [3, 10]. Thus, selecting the appropriate strain is the main factor to ensure success of biomass production and cell compound accumulation from CO2 content from biogas [2, 3, 10, 11].
According to Varshney et al. [12], an ideal microalgal strain for CO2 fixation from industrial flue gases must have specific traits, for example, (1) tolerance to high CO2 concentrations and other toxic components that are typically found in flue gases; (2) tolerance to environmental conditions that are present in cultivation systems; and (3) high commercial or calorific value. Although microalgal CO2 fixation can be improved by genetic engineering [4, 13], the use of genetically modified microalgae is limited by safety regulations to prevent environmental contamination and human consumption [2, 11, 14, 15]. In this context, several studies have focused on isolating and identifying wild-type microalgae from local habitats with this novel physiologic and biotechnological performance [11, 12, 14, 15]. Since native microalgae are already adapted to the environmental conditions prevailing in a specific geographical location, they are preferred for bio-refinery and bio-remediation purposes rather than microalgae of collection banks [14] or genetically modified [15]. Specifically, the microalgae of the genus Chlorella and Scenedesmus have been widely used to produce biomass from biogas [3, 5,6,7,8] because of their ability to tolerate high CO2 concentrations [16]. However, microalgae are a very diverse group, and strains from the same genus can have different optimal CO2 concentrations for maximizing their bio-refinery capacity, as well as their ability to tolerate high CO2 and CH4 concentrations from industrial effluents [2, 11, 14]. In this context, Chlorella sp. and Scenedesmus sp. were isolated from Lago de Chapala, Jalisco, the largest lake of Mexico and well known for high phytoplankton diversity [17] to integrate the anaerobic digestion process of agro-industrial waste and microalgal biomass production. To our knowledge, no assessment has been performed on these native microalgal strains for their bio-refinery capacity from CO2 content from biogas and biotechnological potential to endure stressful composition of this effluent.
Considering the above, the aims of this study were to assess both microalgae Chlorella sp. and Scenedesmus sp., on their bio-refinery capacity from biogas, as well as the effect of high CO2 and CH4 concentrations from biogas on their biochemical composition and physiological capacity to grow and capture CO2 cultured under different biogas compositions.
Materials and methods
Microorganisms and culture conditions
Chlorella sp. and Scenedesmus sp. (Fig. 1) were isolated from Lago de Chapala (Jalisco, Mexico; 20°15′27″N, 103°02′33″W) according to the methodology described by Smith et al. [18]. Both microalgae were maintained in C30 + M medium [9] at 27 ± 2 °C, 200 µmol photons m−2 s−1, and stirred at 120 rpm for 14 days.
Experimental growth conditions
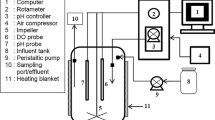
The experiment was set up by adding 50 mL of each microalga previously pre-cultured in 450 mL of C30 + M medium, using a 1-L flask with 500 mL of working volume. Both microalgae were maintained at 27 °C, 200 µmol photon m−2 s−1 and stirred at 120 rpm in an incubator shaker (Innova 43, New Brunswick Scientific, Nürtingen, DE) for 8 days. During all incubation time, a gas mixture was bubbled continuously at the bottom of each flask with a flow rate of 0.006 vvm. Five different gas mixtures were utilized in this study: (1) 75% CH4–25% CO2; (2) 50% CH4–50% CO2 (synthetic biogas; treatments); (3) 75% Argon–25% CO2; (4) 50% Argon–50% CO2; and (5) hydrocarbon-free air (as control). Argon (Ar) was used to avoid the CH4 content from biogas and evaluate the effect of CH4 concentration on each microalga. Each gas mixture was acquired from Praxair, Mexico and sterilized with acrodisc filters of 0.2 µm (Millipore, MA, USA) before feeding each microalgal culture.
Biomass production
Biomass production (g L−1) was quantified by cell dry weight. Briefly, 20 mL of microalgal culture were sampled each 48 h and centrifuged at 10,000 rpm for 10 min; the microalgal pellet was washed twice with distilled water and dried at 80 °C in Thermo Scientific Heratherm™ OGS100 Lab oven (Waltman, MA, USA) for 12 h. Biomass productivity (P; g L−1 day−1) was calculated with Eq. 1 where Xf and Xi corresponded to biomass production (g L−1) at initial (ti) and final time (tf) [9]:
Specific growth rate (µ; day−1) was calculated with Eq. 2 where Xi and Xf were the biomass production (g L−1) at the initial (ti) and final time (tf) of the exponential growth phase:
Determination of CO2 fixation from biogas
Dissolved inorganic carbon (DIC) was quantified at the end of experimental time (8 days) in culture media by a total organic carbon analyzer (Shimadzu-VCSN, Tokyo, JP); the pH in culture medium was determined with a pH meter (Thermo-Orion Model 720A, MA, USA); CO2 fixation rate (\(R_{{{\text{CO}}_{2} }}\); g L−1 day−1) was determined with Eq. 3, according to Tang et al. [19] considering the typical molecular formula of microalgal biomass, CO0.48 H1.83 N0.11 P0.01 [20]:
where P is biomass productivity; Cc is carbon content of the microalgal cell; Mc is carbon molecular weight; and \(M_{{{\text{CO}}_{2} }}\) is the CO2 molecular weight.
Microalgal biomass characterization
Microalgal biomass was measured at the end of each experiment. Total carbohydrates were quantified by the phenol–sulfuric method [21], while protein content was determined by the Lowry method [22]. Total lipids were extracted by the procedure established [23] and quantified in a Rotovapor IKA RV-10 (Staufen, DE).
Experimental designs
To determine biomass production, CO2 fixation rate, biochemical composition, as well as simultaneously evaluate the effect of high CO2 and CH4 concentrations from biogas on both microalgae, each experiment consisted of five gas mixtures: (1) 75% CH4–25% CO2; (2) 50% CH4–50% CO2 (synthetic biogas; treatments); (3) 75% Ar–25% CO2; (4) 50% Ar–50% CO2; and (5) hydrocarbon-free air (as control). Each experiment was performed in triplicate and repeated twice; the results obtained from each experiment were analyzed using ANOVA and then LSD post hoc analysis. Significance was set at P < 0.05, using Statistica 6.0 software (StatSoft, Tulsa, OK).
Results
Carbon dioxide fixation rate by microalgae cultured under different biogas composition
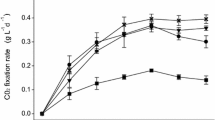
Chlorella sp. recorded the highest CO2 fixation rates at 8 days when it was supplied with 25% CO2–75% CH4 (0.28 ± 0.036 g L−1 day−1) and 25% CO2–75% Ar (0.29 ± 0.018 g L−1 day−1), which was significantly similar when fed with these two gas mixtures (Fig. 2a, lowercase letters). However, when it was provided with 50% CO2, either balanced with 75% CH4 or Ar, the CO2 fixation rate of this microalga significantly decreased, recording 0.18 ± 0.023 and 0.14 ± 0.022 g L−1 day−1, respectively, and it did not show significant differences between both gas mixtures (Fig. 2a, lowercase letters). This same pattern was found in Scenedesmus sp. at eight days, reaching 0.39 ± 0.015 and 0.35 ± 0.037 g L−1 day−1 when it was supplied with 25% CO2–75% CH4 or 25% CO2–75% Ar, respectively, showing to be significantly similar when fed with these gas mixtures (Fig. 2b, lowercase letters). Similarly, at the end of experimental time (8 days), CO2 fixation rate of Scenedesmus sp. also significantly decreased when it was supplied with 50% CO2–50% CH4 (0.28 ± 0.036 g L−1 day−1) and 50% CO2–50% Ar (0.28 ± 0.036 g L−1 day−1), although the results were similar between these two gas mixtures (Fig. 2b, lowercase letters).
CO2 fixation rate by Chlorella sp. (a) and Scenedesmus sp. (b) supplied with different gas mixtures. Points at each time interval denoted by different lowercase letters differ significantly when each microalga grew supplied with different gas mixtures. Statistical analyses were performed using ANOVA and LSD post hoc analysis (P < 0.05). Bars represent standard error
On the contrary, the dissolved inorganic carbon concentration (DIC) in culture media was significantly higher when both microalgae were supplied with 50% CO2 balanced either with CH4 or Ar. In contrast, pH in culture media was significantly higher in the two gas mixtures composed of 25% CO2 balanced either with CH4 or Ar (Table 1).
Biomass production by microalgae cultured under different biogas composition
Similarly, Chlorella sp. recorded the highest biomass production at 8 days, attaining 1.77 ± 0.32 and 1.81 ± 0.17 g L−1 when it was supplied with 25% CO2–75% CH4 and 25% CO2–75% Ar, respectively; it did not show significant differences when supplied with both gas mixtures (Fig. 3a, lowercase letters). Likewise, Scenedesmus sp. also showed the highest biomass production at 8 days, recording 2.25 ± 0.20 and 2.04 ± 0.13 g L−1, respectively, when it was provided with these two gas mixtures; the results were statistically similar (Fig. 3b, lowercase letters). Nonetheless, biomass production in each microalgae significantly decreased when supplied with 50% CO2 either balanced with CH4 or Ar (Fig. 3a, b, lowercase letters), while the two microalgae supplied with air showed the lowest biomass production (Fig. 3a, b, lowercase letters).
Biomass production by Chlorella sp. (a) and Scenedesmus sp. (b) supplied with different gas mixtures. Columns denoted by different lowercase letters differed significantly when each microalga grew supplied with different gas mixtures. Statistical analyses were performed using ANOVA and LSD post hoc analysis (P < 0.05). Bars represent standard error
On the other hand, both microalgae also showed the highest specific growth rates and biomass productivities when they were supplied with 25% CO2 rather than 50% CO2 balanced either with CH4 or Ar (Table 2).
Biochemical composition of microalgae cultured under different biogas composition
Under our experimental conditions, the two microalgae evaluated accumulated mainly carbohydrates and proteins, while lipids were not detected. Both microalgae recorded the highest accumulation of both compounds supplied with 25% CO2 balanced with CH4 or Ar (Fig. 4). In this condition, Chlorella sp. showed a biochemical composition of 24.42 ± 1.10% and 29.10 ± 1.33% of carbohydrates when it was supplied with 25% CO2 balanced with 75% CH4 and Ar, respectively (Fig. 4a), while the protein content was 33.36 ± 1.60% and 27.89 ± 0.74%, respectively (Fig. 4b). Similarly, Scenedesmus sp. recorded 33.41 ± 1.07% and 26.33 ± 1.39% of carbohydrates supplied with 25% CO2–75% CH4 and Ar, respectively (Fig. 4c), and a protein content of 32.96 ± 2.26% and 28.36 ± 1.55%, respectively (Fig. 4d). Likewise, cell composition of both microalgae showed significantly higher differences when supplied with 25% CO2 rather than 50% CO2 balanced either with Ar or CH4 (Fig. 4, lowercase letters).
Biochemical composition by Chlorella sp. (a, b) and Scenedesmus sp. (c, d) supplied with different gas mixtures. Columns denoted by different lowercase letters differed significantly when each microalga grew supplied with different gas mixtures. Statistical analyses were performed using ANOVA and LSD post hoc analysis (P < 0.05). Bars represent standard error
Discussion
Strains from the same group of microalgae can have different ability to tolerate high CO2 and CH4 concentrations, produce biomass, and accumulate cell compounds [2, 11, 14]. Thus, our study hypothesis was that Chlorella sp. and Scenedesmus sp., isolated from and native to Lago de Chapala, might have the biotechnological capacity to endure the stressful biogas composition, capture CO2, produce biomass, and synthesize high-value cell compounds starting from biogas. Therefore, the aims of this study were to assess both Chlorella sp. and Scenedesmus sp., microalgae on their bio-refinery capacity, as well as the effect of high CO2 and CH4 concentrations of this effluent on their physiological capacity and biochemical composition to grow and capture CO2 cultured under different biogas compositions.
Our results demonstrated that Chlorella sp. and Scenedesmus sp. have biotechnological potential to endure high CO2 (25%) and CH4 (75%) concentrations, as well as bio-refinery capacity to produce biomass and high-value metabolites from this effluent. These results might be attributed to the ability of both endemic microalgae to tolerate acid pH induced by CO2 solubility in culture media in the form of dissolved inorganic carbon (DIC: HCO3−, CO −23 , and H2CO3−) [11], since the CH4 concentrations evaluated in this study did not negatively affect their physiological performance. According to Solovchenko and Khozin-Goldberg [16], the mechanisms that allow microalgae to tolerate high CO2 concentrations are (1) ability to prevent acidification of the chloroplast stromal compartment and cytoplasm to maintain ribulose bisphosphate carboxylase–oxygenase activity (Rubisco; EC 4.1.1.39) and (2) ability to rapidly and reversibly shutdown the CO2 concentrating mechanism (CCM), operating under atmospheric CO2 levels but facilitating the drop of pH in cells under elevated CO2 concentrations. Particularly in microalgae, CCM plays a vital role during the carbon fixation process, because it can enhance CO2 level at the Rubisco active site by transporting inorganic carbon into the cell and inducing an increase in photosynthetic rate [10, 11, 16]. The previous information can explain the high CO2 fixation rates obtained by both microalgae evaluated when they were fed with biogas, since microalgae can perform the photosynthetic process under toxic CO2, ammonia, cyanic acid, water vapor, and other toxic gases [24], although their tolerance level to CO2 or CH4 from biogas is dependent on the microalga strain [10, 14]. For instance, Kao et al. [4] claimed that Chlorella sp. MB-9 tolerated high CO2 (20%) and CH4 (80%) concentrations from biogas, while Thiansathit et al. [25] stated that a biogas composition of 40% CO2–60% CH4 was an optimum carbon source for S. obliquus TISTR 8522. Yan et al. [26] reported that the best CH4 concentration during CO2 fixation from biogas by Chlorella sp. was 45–55% (v/v), while Kao et al. [13] stated that growth rate of Chlorella sp. MM-2 decreased proportionally increasing CH4 concentration from 20 up to 80%. In this study, CO2 capture and biomass production were not inhibited when Chlorella sp. and Scenedesmus sp. were fed with biogas composed of 25% CO2 balanced either with 75% CH4 or Ar, indicating that CH4 did not affect them negatively. However, both microalgae decreased their physiologic activity when they were provided with biogas composed of 50% CO2–50% CH4 or Ar, which suggested that this CO2 concentration was adverse for both. Nonetheless, both microalgae assessed can be considered as CO2 tolerant according to the division of CO2-tolerant microalgae as established by Solovchenko and Khozin-Goldberg [16].
The above can be supported by DIC culture media uptake, since its concentration was lower when each microalga was provided with biogas composed of 25% CO2 rather than 50%. It was happened, because the solubility of high CO2 concentration in culture medium decreased pH significantly and inhibited the Rubisco and Carbonic anhydrase (EC 4.2.1.1) activities, vital during CO2 capture by microalgae [19, 27]. For example, Meier et al. [6] demonstrated that the growth of Nannochloropsis gaditana CCMP-527 was completely inhibited with a pH less than 5.00 induced by the high CO2 concentrations supplied. In this study, the pH of culture media decreased to 3.56 and 5.88 when supplied with biogas composed of 50% and 25% CO2, respectively. According to Razzak et al. [27] and Tang et al. [19], a pH of 5–7 is an optimum range for freshwater microalgae and Rubisco and carbonic anhydrase enzymatic activities. This result explains the higher CO2 fixation rates, biomass productivities, and growth rates as recorded by both microalgae provided with 25% CO2, since CO2 capture by microalgae is directly correlated with parameter growth [27, 28], confirming that the 50% CO2 provided in this study was detrimental for the physiological performance of both microalgae. The previous information highlights the importance of assessing and determining the optimal CO2 and CH4 concentrations for each microalga used.
On the other hand, both microalgae assessed in this study also showed the highest metabolite biosynthesis, mainly carbohydrates and proteins, supplied with biogas composed of 25% CO2 balanced either with 75% CH4 or Ar, while lipid accumulation was not detected under the experimental conditions of this study. Supplying the optimal CO2 concentration to the microalga culture increased Rubisco activity and CO2 fixation rate, inducing greater biomass production and high-value compound biosynthesis. However, cell compound biosynthesis is dependent on each microalga strain [29]. Previously, Choix et al. [30] demonstrated that Chlorella sp. and Scenedesmus sp. assessed in this study have the ability to synthesize mainly carbohydrates and proteins but no lipids. Particularly, carbohydrate biosynthesis by microalgae starting from CO2 fixation is more viable energetically than lipid synthesis [31]. Moreover, the 3-phosphoglycerate produced during photosynthesis is a signal for high carbon and energy content within the microalga cell, activating the ADP-glucose pyrophosphorylase enzyme, key in starch biosynthesis by microalgae [32]. In addition, Cheng et al. [33] demonstrated that protein content reflected high metabolic activity, and cells grew constantly; which confirms the bio-refinery capacity of both microalgae assessed in this study starting from CO2 content from biogas and their commercial value.
Conclusions
Overall, our results show that Chlorella sp. and Scenedesmus sp. microalgae, native of Lago de Chapala-Mexico, are CO2-tolerant and both have the biotechnological potential to endure stressful biogas composition (25% CO2 and 75% CH4) without affecting their physiological capacity to capture CO2, grow and biosynthesize high-value metabolites. Besides, these results exhibit the bio-refinery capacity and commercial value of both microalgae accumulating mainly carbohydrates and proteins, useful for obtaining biofuels as ethanol or biogas. Although this study was performed at laboratory scale, it is important to highlight that both wild-type microalgae could be cultured to large scale to produce biomass and biosynthesizing high-value metabolites from secondary effluents generated during anaerobic digestion process of agro-industrial wastes. Finally, this study shows the importance of isolating and identifying microalgae from local ecosystems to determine their physiological capacity for each biotechnological application.
References
Yadav G, Sen R (2017) Microalgal green refinery concept for biosequestration of carbon-dioxide vis-à-vis wastewater remediation and bioenergy production: recent technological advances in climate research. J CO2 Util 17:188–206. https://doi.org/10.1016/j.jcou.2016.12.006
Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sustain Energy Rev 92:394–404. https://doi.org/10.1016/j.rser.2018.04.034
Chen Y-d, Ho S-H, Nagarajan D, Ren N-q, Chang J-S (2018) Waste biorefineries—integrating anaerobic digestion and microalgae cultivation for bioenergy production. Curr Opin Biotechnol 50:101–110. https://doi.org/10.1016/j.copbio.2017.11.017
Kao CY, Chiu SY, Huang TT, Dai L, Hsu L-K, Lin C-S (2012) Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy 93:176–183. https://doi.org/10.1016/j.apenergy.2011.12.082
Van Der Ha D, Nachtergaele L, Kerckhof FM, Rameiyanti D, Bossier P, Verstraete W, Boon N (2012) Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ Sci Technol 46:13425–13431. https://doi.org/10.1021/es303929s
Meier L, Pérez R, Azócar L, Rivas M, Jeison D (2015) Photosynthetic CO2 uptake by microalgae: an attractive tool for biogas upgrading. Biomass Bioenergy 73:102–109. https://doi.org/10.1016/j.biombioe.2014.10.032
Xu J, Zhao Y, Zhao G, Zhang H (2015) Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl Microbiol Biotechnol 99:6493–6501. https://doi.org/10.1007/s00253-015-6537-x
Prandini JM, da Silva MLB, Mezzari MP, Pirolli M, Michelon W, Soares HM (2016) Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour Technol 202:67–75. https://doi.org/10.1016/j.biortech.2015.11.082
Choix FJ, Polster E, Corona-González RI, Snell-Castro R, Méndez-Acosta HO (2017) Nutrient composition of culture media induces different patterns of CO2 fixation from biogas and biomass production by the microalga Scenedesmus obliquus U169. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-017-1828-5
Cheah WY, Show PL, Chang J-S, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol 184:190–201. https://doi.org/10.1016/j.biortech.2014.11.026
Zhou W, Wang J, Chen P, Ji C, Kang Q, Lu B, Li K, Liu J, Ruan R (2017) Bio-mitigation of carbon dioxide using microalgal systems: advances and perspectives. Renew Sustain Energy Rev 76:1163–1175. https://doi.org/10.1016/j.rser.2017.03.065
Varshney P, Beardall J, Bhattacharya S, Wangikar PP (2018) Isolation and biochemical characterisation of two thermophilic green algal species-Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res 30:28–37. https://doi.org/10.1016/j.algal.2017.12.006
Kao CY, Chiu SY, Huang TT, Dai L, Wang G-H, Tseng C-P, Chen C-H, Lin C-S (2012) A mutant strain of microalga Chlorella sp. for the carbon dioxide capture from biogas. Biomass Bioenergy 36:132–140. https://doi.org/10.1016/j.biombioe.2011.10.046
Ghosh A, Khanra S, Mondal M, Halder G, Tiwari ON, Saini S, Bhowmick TK, Gayen K (2016) Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: a review. Energy Convers Manag 113:104–118. https://doi.org/10.1016/j.enconman.2016.01.050
Rocha RP, Machado M, Vaz MGMV, Vinson CC, Leite M, Richard R, Mendes LBB, Araujo WL, Caldana C, Martins MA, Williams TCR, Nunes-nesi A (2017) Exploring the metabolic and physiological diversity of native microalgal strains (Chlorophyta) isolated from tropical freshwater reservoirs. Algal Res 28:139–150. https://doi.org/10.1016/j.algal.2017.10.021
Solovchenko A, Khozin-Goldberg I (2013) High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnol Lett 35:1745–1752. https://doi.org/10.1007/s10529-013-1274-7
Vázquez-García JA, Mora-Navarro MR, Vargas-Rodríguez YL (2004) Ordination of phytoplankton communities in the Chapala Lake, Jalisco-Michoacan, Mexico. Hidrobiológica 14:91–103
Smith DL, Johnson KB, Darmstadt TU (1996) A guide to marine coastal plankton and marine invertebrate larvae, 2nd edn. Kendall/Hunt Publishing Company, Iowa
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. https://doi.org/10.1016/j.biortech.2010.10.047
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Papazi A, Makridis P, Divanach P, Kotzabasis K (2016) Bioenergetic changes in the microalgal photosynthetic apparatus by extremely high CO2 concentrations induce an intense biomass production. Physiol Plant 132:338–349. https://doi.org/10.1111/j.1399-3054.2007.01015.x
Thiansathit W, Keener TC, Khang S (2015) The kinetics of Scenedesmus obliquus microalgae growth utilizing carbon dioxide gas from biogas. Biomass Bioenergy 76:79–85. https://doi.org/10.1016/j.biombioe.2015.03.012
Yan C, Zhang L, Luo X, Zheng Z (2014) Influence of influent methane concentration on biogas upgrading and biogas slurry purification under various LED (light-emitting diode) light wavelengths using Chlorella sp. Energy 69:419–426
Razzak SA, Hossain MM, Lucky RA, Bassi AS, de Lasa H (2013) Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—a review. Renew Sustain Energy Rev 27:622–653. https://doi.org/10.1016/j.rser.2013.05.063
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sustain Energy Rev 31:121–132. https://doi.org/10.1016/j.rser.2013.11.054
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Choix FJ, Ochoa-Becerra MA, Hsieh-Lo M, Mondragón-Cortez P, Méndez-Acosta HO (2018) High biomass production and CO2 fixation from biogas by Chlorella and Scenedesmus microalga using tequila vinasses as culture medium. J Appl Phycol 30:2247–2258. https://doi.org/10.1007/s10811-018-1433-2
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129. https://doi.org/10.1016/j.biortech.2010.06.036
Choix FJ, Bashan Y, Mendoza A, de-Bashan LE (2014) Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J Biotechnol 177:22–34. https://doi.org/10.1016/j.jbiotec.2014.02.014
Cheng P, Wang J, Liu T (2015) Effect of cobalt enrichment on growth and hydrocarbon accumulation of Botryococcus braunii with immobilized biofilm attached cultivation. Bioresour Technol 177:204–208
Acknowledgements
Francisco J. Choix acknowledges CONACYT (National Council for Science and Technology) for the support under the Program-project 2517 Cátedras CONACYT and Diana Fischer for editorial services in English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos-Ibarra, J.R., Snell-Castro, R., Neria-Casillas, J.A. et al. Biotechnological potential of Chlorella sp. and Scenedesmus sp. microalgae to endure high CO2 and methane concentrations from biogas. Bioprocess Biosyst Eng 42, 1603–1610 (2019). https://doi.org/10.1007/s00449-019-02157-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02157-y