Abstract
Purpose
This study aims to examine the biomass production of local microalgae isolates; Chlorella sp., Scenedesmus sp. and Oscillatoria sp. cultivated in high nitrate (NO3 −) concentration of nitrified landfill leachate (NLL).
Methods
NLL concentration of 10–30 % v/v was optimized for maximum microalgae growth, NO3 − removal performance, and biomass productivity. The biomass produced was further characterized for carbohydrate, lipid, and protein composition.
Results
NO3 − was able to be removed by all microalgae isolates with a NO3 − removal rate of 26.5–27.5 mg/L/day and a maximum NO3 − removal percentage of 84 % (20 % NLL) by Oscillatoria sp. Highest biomass productivity (0.11 g/L/day), carbohydrate productivity (2.92 g/L/day), lipid productivity (1.41 g/L/day), and protein productivity (4.87 g/L/day) were observed for Oscillatoria sp. cultured in 10 % NLL. Meanwhile, highest lipid, carbohydrate, and protein content was observed in Chlorella sp. cultured in 10 % NLL (18.23 %), Chlorella sp. cultured in 30 % NLL (37.5 %), and Oscillatoria sp. cultured in 20 % NLL (61.4 %), respectively.
Conclusions
These results suggest that dual application of microalgae for phycoremediation of high NO3 − wastewater and biomass production was feasible particularly for Oscillatoria sp. in term of high nitrate removal capability and biomass productivity. This finding is significant for potential application of microalgae biomass as biofuels feedstock in near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill leachate produced in Malaysia contains high Ammonium (NH4 +) concentration [1]. Under an anaerobic condition, the organic N in the landfill waste, such as protein, is hydrolyzed into amino acids before fermenting further to other compounds including ammonia (NH3). The anaerobic hydrolysis of municipal solid waste (MSW) containing proteins is slower than the one containing carbohydrates, resulting in a slow release of soluble N, and this breeds a high concentration of NH4 + in landfill leachate. The biological leachate treatment method by means of nitrifying activated sludge system (NASS) has been efficient in treating leachate with high NH4 + concentration. So far NASS has successfully treated landfill leachate of 1450 mg/L NH4 + concentration producing NLL with high NO3 − concentration [1]. Hence, there has been growing interests to involve microalgae in the landfill leachate treatment system with the aim to remove nitrate as well as to produce useful biomass.
Microalgae biomass offers a number of advantages over the conventional biomass. It is a photosynthetic microorganism that does not require arable land for cultivation thus it does not compete with other agricultural commodities for growing space. In fact, the facilities for microalgae cultivation can be built on a marginal land that has few other uses [2] and cultivating the species can be performed on any source of water. Some species can thrive in brackish water or seawater, or wastewater from food and the agro-industrial sector [3]. Innovations in microalgae production have allowed the process to become more productive while consuming resources that would otherwise be considered as waste [2]. Hence in some circumstances, the wastewater such as landfill leachate can be a valuable resource for generation of microalgae biomass.
Recently, there is a great interest on utilization of microalgae as a promising feedstock for production of biofuels including bioethanol, biodiesel, methanol and biohydrogen [4]. Several biological and thermochemical conversion technologies such as anaerobic digestion, anaerobic fermentation and transesterification are available in which microalgal biomass can be used as substrate for production of biofuels. However, the use of microalgae biomass in these technologies can be inhibited by their high protein and low carbohydrate content [3]. Yet in most biomass conversion technologies, carbohydrates are the main substrate for the production of biofuels. Nevertheless, the composition of microalgae biomass can be manipulated by several cultivation techniques, such as nutrient depletion and other stressed environmental conditions. Among the environmental stress that received significant attention in influencing the production of biomass content is N-depletion. It has been previously reported that under N depleted conditions, microalgal cells tend to accumulate carbon metabolite as lipids [5]. N limitation also triggers the accumulation of carbohydrate [6]. To date, the capability of microalgae for nutrient removal and biomass production in N rich wastewater particularly using fed-batch cultivation mode has been reported [7, 8].
This paper discusses the capability of local microalgae isolates; Chlorella sp., Scenedesmus sp. and Oscillatoria sp. for high nitrate removal from NLL. The microalgae biomass was also characterized for its biochemical composition and biomass productivity.
Materials and Methods
Microalgae Culture
Three local microalgae isolates; Oscillatoria sp., Chlorella sp., Scenedesmus sp. were used in this study. The microalgae species were isolated from Jeram Sanitary Landfill and identified using 18S rRNA molecular technique [9]. Routine microscopic observation was performed to ensure that the culture was axenic. The microalgae were cultured in an Erlenmeyer flask containing sterile BG-11 plus NO3 − medium. Aeration was provided by a commercial aquarium pump, and light was supplied by a cool, white fluorescent lamp adjusted to 2000–3000 lux intensity and 12:12 light dark cycles. The pH of the cultures was controlled below pH 8 by addition of acetic acid. The inoculum was taken at an exponential growth rate (day 4) with initial cells concentration of 0.01 g/L.
Landfill Leachate
The landfill leachate used in this study was obtained from Equalization Lagoon 1, Jeram Sanitary Landfill Leachate, Selangor, Malaysia. The landfill leachate was collected once in a large volume (50 L) to avoid variation of leachate quality and was kept at 4 °C to avoid biodegradation until further used. The characteristics of landfill leachate are summarized in Table 1.
Chemical Analyses
All parameters were determined according to American Public Health Association (APHA) standard method [10]. BOD5 was measured based on a 5 day period of oxygen consumption at 20 °C. Dissolved oxygen (DO) was measured initially and after an incubation period using Eutech Instruments Cyberscan DO1500 DO meter (USA), and the biochemical oxygen demand (BOD5) was calculated from the difference between initial and final DO. The chemical oxygen demand (COD) was determined by a closed reflux method using HACH COD reactor (USA) and HACH DR 4000 spectrophotometer (USA). Anion (NO3 −, NO2 −, F−, Cl−, PO4 3−, SO4 2−) and cation (NH4 +, Na+, Li+, Mg2+, Ca2+) were analysed using an ion chromatography system (Dionex ICS-1100, USA) and particularly with the ARCS 4-mm anion column and CRCS 4-mm cation column. Specifically, 4.5 mM carbonate and 0.8 mM bicarbonate were used as anion analysis eluent while 20 mM methanesulfonic acid (MSA) was used for cation analysis eluent.
Nitrification of Landfill Leachate
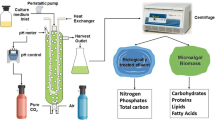
To produce NLL, the landfill leachate was nitrified using activated sludge containing ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) following the process of NASS (Fig. 1) [1]. The system comprises of an aerobic laboratory bioreactor (BIOSTAT A-plus, Germany) with a 2 L working volume culture vessel. The influent (NLL) was fed from the top of the reactor using a peristaltic pump (Masterflex L/S Easy-load II Model No. 77200-60, USA) and the effluent was discharged at the reactor upper port. Oxygen was supplied by an air compressor (Hi-Blow Air Pump, Japan). The DO and pH were controlled at a minimum amount of 2.0 mg/L and 7.5 by adjusting the air flow rate and addition of sodium bicarbonate (NaHCO3), respectively. The characteristics of NLL are summarized in Table 1.
Cultivation of Microalgae Isolates at Different Concentration of NLL
The NLL concentrations were selected based on a concentration range in which the microalgae had survived during the previous screening experiment. The NLL with concentration of 10, 15, 20, 25, and 30 % were prepared by dilution with distilled water. BG-11 medium was used as control as all NLL tested contain NO3 −. All experiments were conducted in a 500 mL Erlenmeyer flask with 350 mL of working volume. The experiments were conducted in triplicate and were repeated twice for all microalgae species (Oscillatoria sp., Scenedesmus sp., and Chlorella sp.). The culture conditions were as previously described. The growth of the cultures was monitored for 14 days, and 3 mL of the samples were taken daily to measure the optical density and NO3 − concentration. After 14 days, the microalgae biomass was harvested and analysed for biochemical analyses.
Microalgae Growth Densities
Cell densities were measured by a spectrophotometric method at an absorbance of 680 nm (OD680) [11]. Each sample was diluted to give an absorbance in the range of 0.1–1.0 if the optical density was greater than 1.0. In all media, the growth curves of the microalgae were developed by measuring the OD680 of the samples every 24 h to determine the growth rate, lag phase, exponential phase, stationary phase, and death phase. The specific growth rate (μ, /day) in exponential period was calculated in the exponential phase of growth using Eq. 1 where N1 and N2 are defined as biomass at time 1 (t1) and time 2 (t2) [12].
To determine the biomass (B, mg/L) of the microalgae, a linear regression relationship between optical density and the dry weight biomass of the microalgae as established earlier was used. The biomass productivity (BP, mg/L/day) was calculated according to Eq. 2 where B1 and B2 represent the biomass density at the time T (day) [13].
Nitrate (NO3 −) Removal
The total NO3 − removal (TNR, mg/L) was calculated using Eq. 3 where NCi is initial NO3 − concentration (day 0) and NCf is final NO3 − concentration (day 14).
NO3 − removal percentage (NRP) was identified from Eq. 4 where TNR is the total NO3 − removal calculated in Eq. 3 and TN is the total available NO3 −.
NO3 − removal rate (NRR, mg/L/day) was calculated using Eq. 5 where TNR is the total NO3 − removal calculated in Eq. 3 and T is the time (day).
Cell Harvesting and Biomass Quantification
Microalgae cells were harvested during the stationary phase (day 14) as cell density was observed highest (OD680). An amount of 20 mL of cultures was harvested by centrifugation at 5000 rpm for 5 min and the pellet was washed at least twice with distilled water [14]. The washed samples were filtered using a pre-weighed filter paper. The dry weight was determined after oven drying at 105 °C until constant weight achieved. The dry weight of the microalgae biomass was expressed as dry weight mg/L. The cell biomass for biochemical composition analysis was freeze-dried using Scanvac Cool Save Freeze Dryer, Denmark. The culture was centrifuged at 5000 rpm for 5 min. The supernatant was removed and the cell was washed twice with deionized water. The microalgae cell was kept overnight in −20 °C freezer before being freeze-dried. The lyophilized microalgae cell was stored at room temperature for further analysis.
Carbohydrate Analyses
Carbohydrate was determined using a phenol–sulphuric acid method [15]. The samples’ absorbance was measured using a spectrophotometer (HACH DR 4000 spectrophotometer, USA) at 490 nm. The total of carbohydrate content (CC, %) was calculated by comparison to a calibration curve using glucose as the standard. Carbohydrate productivity (CP, mg/L/day) was determined according to Eq. 6 where CC denotes the total carbohydrate content (weight/dry weight biomass, %) and BP is biomass productivity calculated in Eq. 2 [16].
Lipid Analyses
A lipid analysis was performed using a modified Bligh & Dyer method [17]. The total lipid extracted was gravimetrically quantified by using an electronic balance. The lipid content (LC, %) was calculated and expressed as % dry cell weight (weight/dry weight biomass). The lipid productivity (LP, mg/L/day) was determined according to Eq. 7 where LC denotes the total lipid content (%) and BP is the biomass productivity calculated in Eq. 2 [16].
Protein Analyses
A protein analysis was performed using Pierce BCA Protein Assay Kit (ThermoScientific, USA), which is based on the modification of original Lowry protein assay procedure. The colour changes of the mixtures were observed at 562 nm absorbance. Total protein content (PC, %) present was determined by comparison to a calibration curve using bovine serum albumin (BSA) as the standard and expressed as % dry cell weight (weight/dry weight biomass). Protein productivity (PP, mg/L/day) was determined according to Eq. 8 where PC denoted the total protein content (weight/dry weight biomass, %) and BP is the biomass productivity calculated in Eq. 2 [16].
Results and Discussion
Characteristics of Landfill Leachate and Nitrified Landfill Leachate (NLL)
The landfill leachate collected was brown in colour, and it had a mean value of pH 8.78, a COD of 2050 mg/L, and a BOD5 of 460 mg/L. The mean available NH4 + was 837.96 mg/L and for nitrite was 1226 mg/L. The concentration for sulphate was 378.32 mg/L and for phosphate was 81.42 mg/L (Table 1). The landfill leachate collected was nitrified using activated sludge containing AOB and NOB following the NASS procedure described in methodology section (Fig. 1). Complete nitrification was achieved with a very high NH4 + removal percentage (~100 %). All NH4 + (837.96 mg/L) and nitrite (1226.00 mg/L) in the landfill leachate were converted to NO3 − (2430 mg/L) by means of nitrification (Table 1). Under aerobic condition, the NH4 + was oxidized by AOB to nitrite, followed by the nitrite oxidization by NOB to produce NO3 −. The NLL produced had a mean value of pH 7.34, a COD of 1624 mg/L, and a BOD5 of 300 mg/L (Table 1). It was expected that the organic carbon in landfill leachate was consumed by denitrifies as BOD5 concentration reduced from 460 to 300 mg/L in NLL. Denitrification requires organic carbon source to reduce nitrate to nitrogen gas and the process is facilitated by facultative anaerobic heterotrophic bacteria.
Cultivation of Microalgae Isolates at Different Concentration of NLL
Cultivation of microalgae isolates at different NLL concentration was carried out to determine the optimum NO3 − concentration for microalgae growth, NO3 − removal performance, and biomass production. Different concentrations of NLL ranging from 10 to 30 % were used in this study.
Microalgae Growth
The growth densities of the microalgae were measured spectrophotometrically at OD680. The growth curve of the microalgae was plotted using ln OD680 over time (Fig. 2). The specific growth rate (µ) of Chlorella sp. cultured in BG-11, 10, 15, 20, 25, and 30 % NLL was 0.80/day, 0.60/day, 0.75/day, 0.70/day, 0.47/day, and 0.62/day, respectively. The highest µ of Chlorella sp. cultured in NLL was observed in 15 % NLL (0.75/day), with maximum biomass production of 344.28 mg/L. The maximum biomass production of Chlorella sp. cultured in 20 % NLL (428.66 mg/L, µ = 0.70/day) was comparable to the maximum biomass production of Chlorella sp. cultured in BG-11 (427.34 mg/L, µ = 0.80/day).
The specific growth rate (µ) of Scenedesmus sp. cultured in BG-11, 10, 15, 20, 25, and 30 % NLL was 0.65/day, 0.65/day, 0.55/day, 0.57/day, 0.61/day, and 0.57/day, respectively. The highest µ of Scenedesmus sp. cultured in NLL was observed in 10 % NLL (0.65/day) was similar to the µ of Scenedesmus sp. cultured in BG-11. However, the maximum biomass production of Scenedesmus sp. cultured in BG-11 (371.52 mg/L) was higher than those recorded in 10 % NLL (160.05 mg/L). Furthermore, Scenedesmus sp. cultured in 25 % NLL showed a comparable increase of growth density to Scenedesmus sp. in BG-11. Biomass productions in all NLL concentrations (<237.04 mg/L) were lower than those achieved in BG-11 culture (371.52 mg/L).
The specific growth rate (µ) of Oscillatoria sp. cultured in BG-11, 10, 15, 20, 25, and 30 % NLL was 0.40/day, 0.43/day, 0.42/day, 0.35/day, 0.71/day, and 0.94/day, respectively. The µ of Oscillatoria sp. cultured in 30 % NLL (0.94/day) and 25 % NLL (0.71/day) was higher than the µ of Oscillatoria sp. cultured in BG-11 (0.40/day). Moreover, the maximum biomass production of 805.96 mg/L was achieved in 20 % NLL culture, which was higher than the one achieved in BG-11 (762.91 mg/L).
Several factors may contribute to the growth of microalgae. Among them is the toxicity of NLL to the microalgae. Our previous study showed that the landfill leachate contained heavy metals which might cause toxicity to microalgae [1]. The presence of dissolved coloured organic compounds in NLL would also probably affect microalgae growth due to the sensitivity of the species to reduced light penetration inside the cultures [18]. Pure NLL (100 % concentration) had an intense brown colour that may limit light penetration, and the same had 68 % of light transmission. In order to reduce the toxicity of NLL to microalgae growth, the NLL was diluted to the appropriate concentration. It was also found that as the microalgae density increased, the light transmission decreased up to 0 %.
Nitrate (NO3 −) Removal Performance
In this study, three strains of microalgae were cultured to NLL with different concentrations, for a culturing period of 14 days. Table 2 summarizes the initial NO3 − concentration of NLL without exposure to microalgae and the final NO3 − concentration after 14 days of culture, TNR, NRP, and NRR. Figure 3 shows the NO3 − removal percentage (%) of Chlorella sp., Scenedesmus sp., and Oscillatoria sp. in different NLL concentrations.
For Chlorella sp., the highest NRP and NRR were observed in 20 % NLL, which were 77.40 % and 26.56 mg/L/day, respectively (Fig. 3 and Table 2). The NRP and the NRR recorded were in the range of 13–77.4 % and 6.69–26.56 mg/L/day, respectively. Meanwhile for Scenedesmus sp., the highest NRP and NRR were observed in 25 % NLL which were 66.34 % and 27.49 mg/L/day, respectively. The NRP and NRR of Chlorella sp. recorded were in the range of 35.24–66.34 % and 5.8–27.49 mg/L/day, respectively. Furthermore for Oscillatoria sp., the highest NRP and NRR were observed in 20 % NLL which were 84 % and 27.39 mg/L/day, respectively. The NRP and NRR recorded were in the range of 43–84 % and 8.78–27.39 mg/L/day, respectively. It was shown that Scenedesmus sp. has the highest NRP and NRR followed by Oscillatoria sp. and Chlorella sp.
A previous study reports that microalgae have the ability to efficiently remove high level of N from wastewater [19]. After 15 days of culture, Kshirsagar [20] observed 78.1 % NO3 − removal by Chlorella vulgaris and 70.3 % by Scenedesmus quadricauda. These findings were slightly higher than those recorded in our study. Hongyang et al. [21] found N removal of 87–89 % with Chlorella pyrenoidosa cultured in industrial wastewater. Meanwhile, Aslan and Kapdan [22] reported that 23–100 % of N could be removed by Chlorella vulgaris with initial N concentration of 13–400 mg/L. A study by Craggs et al. [23] reported that Oscillatoria sp. was tolerant to high NO3 − environment (498 mg/L) and had achieved 100 % NO3 − removal in municipal wastewater. NO3 − uptake from NLL by microalgae is found noteworthy with respect to microalgae growth densities. Since assimilation is the main mechanism of NO3 − removal by microalgae, the microalgae growth rate directly affects the NO3 − removal rate.
Meanwhile, NO3 − can be utilized and removed efficiently only if the available N:P ratio and concentration in the wastewater is in an appropriate range [24]. The optimum N:P ratio differs among microalgae cultures due to strain-varying metabolic pathways [25]. For healthy freshwater environments, the N:P ratio reported is 250:1. However, some of wastewater streams ratios were reported to be as low as 4–5:1 [26]. An optimum N:P ratio for Chlorella vulgaris was reported to be 7:1 [27]. The NLL used in this study has an N:P ratio of 29:1. The elementary composition of microalgae cells can estimate the optimal N:P ratio. According to the Stumm empirical formula for microalgae (C106H263O110N16P), the N:P ratio is 16:1 [28]. This indicates that NLL used in this study has an N:P ratio approximately two times higher than the assumed normal N:P ratio in microalgal cells. However, the average composition of microalgal cells depends on the strain and growth conditions [29]. If the classic Redfield atomic ratio of 16:1 N:P is considered unchangeable, P would remain in the solution when the microalgae are cultured in wastewater with an N:P ratio higher than 16:1 [30].
In this study, at the end of the 14-day culture period, it was found that all phosphate was removed efficiently and there was an over-uptake of N. In Chlorella sp.—20 % NLL, the final nutrient uptake ratio (N:P) was 22.3:1; in Scenedesmus sp.—25 % NLL, 18.4:1; and in Oscillatoria sp.—20 % NLL, 23:1 (Table 3). However, not entire N was removed due to the P limitation. According to Xin et al. [24], the N:P uptake ratio in microalgae cells does not follow the N:P ratio in an empirical elementary composition. They found that Scenedesmus sp. have the ability to over-uptake N or P when the N:P ratio in the growth medium does not match the normal elementary composition.
A study by Zhou et al. [31] reported an over-uptake of N by Chlorella pyrenoidosa grown in landfill leachate. The N:P ratio was 55.4:1, higher than that achieved by Chlorella sp. in this study (22.3:1). Ruiz et al. [32] also reported on over uptake of N up to an N:P ratio of 36:9 by Chlorella vulgaris cultured in municipal wastewater. A study by Kong et al. [33] showed that when the N:P ratio (1.75:1) is lower than that proposed by Stumm (16:1) [28], low phosphate is assimilated by Scenedesmus sp. due to N limitation.
Biomass Production and Biochemical Composition of Microalgae Biomass
Table 4 summarizes the biomass production and biochemical composition of the microalgae biomass produced. In this study, the maximum biomass production achieved for Chlorella sp. was 428.66 mg/L (in 20 % NLL) while the highest biomass productivity was 65.71 mg/L/day (in 15 % NLL). Meanwhile, the maximum biomass production for Scenedesmus sp. was 237.04 mg/L (in 30 % NLL) and the highest biomass productivity for the microalgae was 46.46 mg/L/day (in 25 % NLL). As for Oscillatoria sp., the maximum biomass production was 805.96 mg/L (in 20 % NLL) and the highest biomass productivity was 106.87 mg/L/day (in 10 % NLL). In the aspect of biomass productivity, Oscillatoria sp. in 10 % NLL had the highest productivity. This finding suggests that Oscillatoria sp. might be suitable for a rapid and large-scale microalgae biomass production. Highest carbohydrate, lipid, and protein contents (weight/dry weight biomass, %) were observed in Chlorella sp. cultured in 30 % NLL (37.5 %), in Chlorella sp. cultured in 10 % NLL (18.23 %), and in Oscillatoria sp. cultured in 20 % NLL (61.4 %), respectively.
In addition, the biomass characterization results (Table 4) also showed that Oscillatoria sp. had the highest productivity for carbohydrate, lipid, and protein followed by Chlorella sp. and Scenedesmus sp. The maximum carbohydrate productivity of Oscillatoria sp. was 2921.83 mg/L/day (in 10 % NLL), the maximum lipid productivity was 1414.96 mg/L/day (in 10 % NLL), and the maximum protein productivity was 5498.37 mg/L/day (in 20 % NLL). As for Chlorella sp., maximum carbohydrate productivity was 1511.33 mg/L/day (in 15 % NLL); lipid, 1046.04 mg/L/day (in 10 % NLL); and protein, 3256.22 mg/L/day (in 20 % NLL). For Scenedesmus sp., the maximum carbohydrate productivity was 1212.30 mg/L/day (in 10 % NLL); lipid, 639.77 mg/L/day (in 10 % NLL); and protein, 2476.32 mg/L/day (in 25 % NLL).
Results of this study were in accordance to the percentage of dry biomass characteristic studied by Singh and Olsen [4]. In general, microalgae dry biomass consists of 40–60 % protein, followed by 20–30 % carbohydrate and 10–20 % lipids [4]. Biomass produced in this study had low lipid content (%) but high lipid productivity. From biofuel production point of view, lipids are the most interesting group of biomolecules [34]. More than 20 % of extractable lipid was required for biodiesel generation [35] and the value of lipid percentage obtained from this study species was considered low. Even though the lipid content achieved was low, a large amount of biomass was produced. Thus, the high lipid productivity observed for all species were quite promising for bio-fuel sources. However, a high biomass than lipid ratio would increase the cost required for lipid extraction [36].
The syntheses of lipid and carbohydrate in microalgae cells are highly regulated by the culture conditions [34]. Under unfavourable environmental or stress condition such as N stress and high light intensity, many microalgae species particularly in class Chlorophyceae alter their lipid biosynthesis pathways and tend to accumulate lipid instead of starch [29]. However, in our study high NO3 − wastewater was used, thus producing biomass with low lipid content and significant carbohydrate and protein content. A high protein content of microalgae (>50 %) is suitable for providing animal feed replacement [35]. After the available lipid and carbohydrate content are extracted, the residual biomass can be used as fertilizer, soil amendment, or feed for fish or livestock after ascertaining its toxicological aspects [37].
Conclusions
The selected microalgae isolates have the capability to treat the NLL by reducing the NO3 − concentration for up to 84 %. All the three isolates had almost similar NO3 − removal rate (26.5–27.5 mg/L/day), with Oscillatoria sp. having removed up to 84 % of NO3 − from the NLL. Highest biomass productivity (106.87 mg/L/day), carbohydrate productivity (2921.83 mg/L/day), and lipid productivity (1414.96 mg/L/day) were observed for Oscillatoria sp. cultured in 10 % NLL. The highest protein productivity (5498.37 mg/L/day) was observed for Oscillatoria sp. cultured in 20 % NLL. Meanwhile, highest carbohydrate, lipid, and protein content (weight/biomass dry weight, %) was observed in Chlorella sp. cultured in 30 % NLL (37.5 %), Chlorella sp. cultured in 10 % NLL (18.23 %), and Oscillatoria sp. cultured in 20 % NLL (61.40 %), respectively.
References
Yusof, N., Hassan, M.A., Phang, L.Y., Tabatabaei, M., Othman, M.R., Mori, M., Wakisaka, M., Sakai, K., Shirai, Y.: Nitrification of high-strength ammonium landfill leachate with microbial community analysis using fluorescence in situ hybridization (FISH). Waste Manag. Res. 29(6), 602–611 (2011). doi:10.1177/0734242X10397581
Campbell, M.N.: Biodiesel: algae as a renewable source for liquid fuel. Guelph Eng. J. 1, 2–7 (2008)
Markou, G., Angelidaki, I., Georgakakis, D.: Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 96(3), 631–645 (2012). doi:10.1007/s00253-012-4398-0
Singh, A., Olsen, S.I.: A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energy 88(10), 3548–3555 (2011). doi:10.1016/j.apenergy.2010.12.012
Yeesang, C., Cheirsilp, B.: Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour. Technol. 102(3), 3034–3040 (2011). doi:10.1016/j.biortech.2010.10.013
Dayananda, C., Sarada, R., Shamala, T.R., Ravishankar, G.A.: Influence of nitrogen sources on growth, hydrocarbon and fatty acid production by Botryococcus braunii. Asian J. Plant Sci. 5(5), 799–804 (2006). doi:10.3923/ajps.2006.799.804
Markou, G.: Fed-batch cultivation of Arthrospira and Chlorella in ammonia-rich wastewater: optimization of nutrient removal and biomass production. Bioresour. Technol. 193, 35–41 (2015)
Ji, F., Zhou, Y., Pang, A., Ning, L., Rodgers, K., Liu, Y., Dong, R.: Fed-batch cultivation of Desmodesmus sp. in anaerobic digestion wastewater for improved nutrient removal and biodiesel production. Bioresour. Technol. 184, 116–122 (2015)
Nordin, N., Samsudin, S., Yusof, N.: 18S rRNA molecular characterization of microalgae isolated for high nitrate wastewater treatment and biomass production. In: International Postgraduate Conference on Science and Mathematics 2013 (IPCSM2013), Universiti Pendidikan Sultan Idris, Tanjong Malim, Perak (5th–6th October 2013)
Association, American Public Health: Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association, Washington DC (2005)
Ji, M.K., Kim, H.C., Sapireddy, V.R., Yun, H.S., Abou, S.R.A.I., Choi, J., Lee, W., Timmes, T.C., Inamuddin, R., Jeon, B.H.: Simultaneous nutrient removal and lipid production from pretreated piggery wastewater by Chlorella vulgaris YSW–04. Appl. Microbiol. Biotechnol. 97(6), 2701–2710 (2013). doi:10.1007/s00253-012-4097-x
Guillard, R.R.L., Ryther, J.H.: Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula Confervaceae (cleve). Can. J. Microbiol. 8, 229–239 (1962)
George, B., Pancha, I., Desai, C., Chokshi, K., Paliwal, M., Ghosh, T., Mishra, S.: Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus-a potential strain for biofuel production. Bioresour. Technol. 171, 367–374 (2014). doi:10.1016/j.biortech.2014.08.086
Dayananda, C., Kumudha, A., Sarada, R., Ravishankar, G.A.: Isolation, characterization and outdoor cultivation of green microalgae Botryococcus sp. Sci. Res. Essays 5(17), 2497–2505 (2010)
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28(3), 350–356 (1956). doi:10.1021/ac60111a017
Griffiths, M.J., Harrison, S.T.L.: Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21(5), 493–507 (2009). doi:10.1007/s10811-008-9392-7
Blight, E.J., Dyer, W.J.: A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37(8), 911–9117 (1959). doi:10.1139/o59-099
Depraetere, O., Foubert, I., Muylaert, K.: Decolorisation of piggery wastewater to stimulate the production of Arthrospira platensis. Bioresour. Technol. 148, 366–372 (2013). doi:10.1016/j.biortech.2013.08.165
Tam, N.F.Y., Wong, Y.S.: Effect of immobilized microalgal bead concentration in wastewater nutrient removal. Environ. Pollut. 107(1), 145–151 (2000). doi:10.1016/S0269-7491(99)00118-9
Kshirsagar, A.D.: Bioremediation of wastewater by using microalgae: an experimental study. Int. J. Life Sci. Biotechnol Pharm. Res. 2(3), 339–346 (2013)
Hongyang, S., Yalei, Z., Chunmin, Z., Xuefei, Z., Jinpeng, L.: Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour. Technol. 102(21), 9884–9890 (2011). doi:10.1016/j.biortech.2011.08.016
Aslan, S., Kapdan, I.K.: Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 28(1), 64–70 (2006). doi:10.1016/j.ecoleng.2006.04.003
Craggs, R.J., McAuley, P.J., Smith, V.J.: Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Resour. 31(7), 1701–1707 (1997). doi:10.1016/S0043-1354(96)00093-0
Xin, L., Hu, H.Y., Ke, G., Sun, Y.X.: Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 101(14), 5494–5500 (2010). doi:10.1016/j.biortech.2010.02.016
Cai, T., Park, S.Y., Li, Y.: Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew. Sust. Energy Rev. 19, 360–369 (2013). doi:10.1016/j.rser.2012.11.030
Quiros, R.: The nitrogen to phosphorus ratio for lakes: a cause or a consequence of aquatic biology? In: Cirelli, A.F., Marquisa, G.C. (eds.) El Agua en Iberoamerica: De la Limnologia a la Gestion en Sudamerica, pp. 11–26. CYTED XVII, Centro de Estudios Transdiciplinarios del Agua, Facultad de Veterinaria, Universidad de Buenos Aires, Buenos Aires (2002)
Shi, J., Podola, B., Melkonian, M.: Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J. Appl. Phycol. 19(5), 417–423 (2007). doi:10.1007/s10811-006-9148-1
Stumm, W., Morgan, J.J.: Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters. Wiley, Hoboken (1981)
Li, Y., Han, D., Sommerfeld, M., Hu, Q.: Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour. Technol. 102(1), 123–129 (2011). doi:10.1016/j.biortech.2010.06.036
Samori, G., Samori, C., Guerrini, F., Pistocchi, R.: Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: part I. Water Res. 47(2), 791–801 (2013). doi:10.1016/j.watres.2012.11.006
Zhou, X., Yuan, S., Chen, R., Song, B.: Modelling microalgae growth in nitrogen-limited continuous culture. Energy 73, 575–580 (2014). doi:10.1016/j.energy.2014.06.058
Ruiz-Marin, A., Mendoza-Espinosa, L.G., Stephenson, T.: Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 101(1), 58–64 (2010). doi:10.1016/j.biortech.2009.02.076
Kong, Q.X., Li, L., Martinez, B., Chen, P., Ruan, R.: Culture of microalgae Chlamydomonas reinhardtii in wastewater for biomass feedstock production. Appl. Biochem. Biotechnol. 160(1), 9–18 (2010). doi:10.1007/s12010-009-8670-4
Wen, Z., Liu, J., Chen, F.: Biofuel from microalgae. Compr. Biotechnol. 1, 127–133 (2011)
Brune, D.E., Lundquist, T., Benemann, J.R.: Microalgal biomass for greenhouse gas reductions: potential for replacement of fossil fuels and animal feeds. J. Environ. Eng. 135, 1136–1144 (2009). doi:10.1061/(ASCE)EE.1943-7870.0000100
Brennan, L., Owende, P.: Biofuels from microalgae: a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energy Rev. 14(2), 557–577 (2010). doi:10.1016/j.rser.2009.10.009
Roeselers, G., Loosdrecht, M.C.M., Muyzer, G.: Phototrophic biofilms and their potential applications. J. Appl. Phycol. 20(3), 227–235 (2008). doi:10.1007/s10811-007-9223-2
Acknowledgments
The authors wish to thank Sultan Idris Education University for financing this study through University Research Grant (Code: 2013-0051-102-01). We are also grateful to Worldwide Landfill Sdn. Bhd. for their technical support throughout the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nordin, N., Yusof, N. & Samsudin, S. Biomass Production of Chlorella sp., Scenedesmus sp., and Oscillatoria sp. in Nitrified Landfill Leachate. Waste Biomass Valor 8, 2301–2311 (2017). https://doi.org/10.1007/s12649-016-9709-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9709-8