Abstract
Endoglucanases are an enzyme of cellulases complex that has a great potential for many technological applications. One of the issues of its use concerns the recovery and reuse of this enzyme. Thus, in this study, the use of a surface-modified kaolin was evaluated to immobilize endoglucanase and evaluate the enzyme activity for its reuse. Kaolin was surface modified with 3-aminopropyltriethoxysilane (APTES) and glutaraldehyde (GA). In addition, the properties of the immobilized enzyme were investigated and compared with those of the free enzyme. Results showed that the optimal pH value of endoglucanase was not affected by the immobilization process but showed a broader range of optimal temperature compared to free enzyme. Immobilization on kaolin allowed fast and easy cellulase recovery with a loss of enzyme activity of only 20% after eight cycles of use. These results indicate that kaolin is a promising substitute to the currently synthetic supports studied for cellulases immobilization with the advantage of being abundant in nature, resistant to microbial attack, chemically and mechanically stable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulases are a complex enzymatic capable of hydrolysing cellulose and include at least three types of biocatalysts: endoglucanase, exoglucanase, and β-glucosidases [1, 2]. They are a relevant class of enzymes for the biocatalyst market due to their several applications in important branches of industry, such as pulp and paper, textile, food and beverages, detergent, animal feed, and biofuels industries [3, 4].
Given the high demand for cellulases, different technologies have been studied to make them more attractive and efficient. Between these technologies, immobilization on a solid support has received great attention because it allows easy separation and reuse of the biocatalyst, application of various reactor designs, better control of reactions and easy product recovery with higher purity [5,6,7,8]. Furthermore, immobilization generally stabilizes the structure of the enzyme and may enhance its stability to environmental changes, such as pH or temperature [6, 8].
A wide variety of materials has been considered for cellulase immobilization, e.g., polyacrylamide gel [9], polyaniline microspheres [10], magnetic nanoparticles [11], multiwall carbon nanotubes [12], chitosan-coated magnetic nanoparticles [13, 14], magnetic nanoporous terpolymer [15], magnetic nanoparticles encapsulated in poly(methyl methacrylate) [16], graphene oxide [17], styrene/maleic anhydride copolymer nanoparticles [18]. Although these materials have been described as efficient carriers, many of them are synthetic, expensive or have low mechanical strength, making them not suitable for industrial applications. In this context, kaolin, a clay mineral abundant in nature, inexpensive and non-toxic, seems to be a suitable material for enzymes immobilization. As a result of its inorganic silicate framework, it is chemically and mechanically stable and also resistant to microbial attack [19]. Furthermore, the presence of many hydroxyl groups on the surface of kaolin favors its functionalization with a surface-modifying agent such as 3-aminopropyltriethoxysilane (APTES) for covalent immobilization [20].
For cellulase immobilization, few papers were found using kaolin as support. Sinegani et al. [21] studied the sorption and immobilization of cellulases on different clay minerals (illite, kaolinite, montmorillonite, and palygorskite). The authors also evaluated the influence of coating these clays with hydroxyl aluminum. Results showed that the sorption onto kaolinite surface was the lowest between studied clays. This result can be explained by the lower cation exchange capacity of kaolinite particles. Karagulyan et al. [22] evaluated the adsorption of β-glucosidase on kaolin surface and reported a 95% of enzyme immobilization with a gradual desorption with successive washings. It should be mentioned that β-glucosidases acts on soluble and small substrate, while endo and exoglucanases are responsible for starting the cellulose hydrolysis, and act only on a large and insoluble substrate. Thus, the effects of immobilization on these enzymes can be different. Furthermore, for endo and exoglucanases immobilization, covalent binding is more recommend because these enzymes have a high affinity for the substrate. They tend to leach from the support and adsorb on the substrate surface when weak bonds are used to immobilize them [23, 24]. Consequently, poor reusability results are obtained and several immobilization advantages are lost.
This research aims to investigate the potential of functionalized kaolin as support for endoglucanase immobilization by covalent bonds. Functionalized kaolin particles were characterized, and the effect of immobilization on catalytic properties of the enzyme, such as optimal temperature and pH, thermal and storage stability as well as enzyme derivative were studied. To the best of our knowledge, there have been no reports about immobilization of cellulases on functionalized kaolin.
Materials and methods
Materials
The commercial enzyme preparation (Biokey AKM, rich in endoglucanases) was kindly donated by Akmey Brasil (Indaial, Santa Catarina, Brazil). To remove stabilizers and possible impurities present in the enzyme solution, a dialysis process was performed with phosphate buffer solution, pH 6.0, for 7 days at room temperature. Collagen membranes with 11.625 m2/g of surface area and 21.752 Å of pore diameter (DEVRO) were used in this process. After dialysis, the enzyme solution was lyophilized and stored under refrigeration (4 °C).
Kaolin was obtained from Imerys in (Pará, Brazil). 3-Aminopropyltriethoxysilane (APTES) and sodium carboxymethylcellulose (CMC) were purchased from Sigma-Aldrich. Glutaraldehyde [GA, 25% (w/v) solution in water], acid 3,5-dinitrosalicylic (DNS, P.A), citric acid monohydrate (PA), monobasic anhydrous potassium phosphate (P.A), monobasic anhydrous sodium phosphate (P.A), dibasic sodium dihydrogen phosphate (P.A) and glucose D(+) anhydrous dextrose (P.A) were purchased from Vetec. Sodium hydroxide (NaOH, P.A) was obtained from Lafan Ltda. Potassium and sodium tartrate (P.A) were purchased from Dinâmica.
Methods
Measurement of endoglucanase activity
Enzymatic activity of free and immobilized endoglucanase was determined by a procedure described by Lima et al. (2017) with some modifications. Hydrolysis was carried out with 0.1 mL of enzyme solution and 0.9 mL of 4% CMC solution (dissolved in 0.15 M citrate–phosphate buffer, pH 5.0) at 55 °C for 30 min. The amount of reducing sugars produced during enzymatic hydrolysis was quantified by the DNS method (Miller 1959). Prior to absorbance measurements, the samples were centrifuged at 3130×g for 3 min. All assays were carried in triplicate and mean values are reported.
Immobilization procedure
Surface functionalization of kaolin with APTES
The functionalization of kaolin with APTES followed the methodology described by Batistella et al. (2015) with some modifications. Initially, 50 g of kaolin was dispersed in 500 ml of an ethanol/water mixture (96/4). After that, the APTES was added in a mass concentration of 10% (wt.) related to kaolin mass. The mixture was kept under magnetic stirring for 12 h at 82 °C. At the end, the solvent was evaporated and the sample was macerated with the aid of a pistil, sieved in a 38 µm sieve and stored in an enclosed vial.
Activation of kaolin with GA
For activation with aldehydes groups, 10% (w/v) of functionalized kaolin (KAPTES) was suspended in 2% (v/v) GA solution (prepared in 0.05 M phosphate buffer pH 7.0) and stirred for 1 h, at 30 °C and 150 rpm. After the reaction was finished, solids were washed three times with distilled water to remove the excess of glutaraldehyde.
Endoglucanase immobilization
The immobilization of enzyme was carried out adding (100 mg/mL) endoglucanase solution (prepared in 0.05 M phosphate buffer pH 7.0) to functionalized and activated kaolin (KAPTES-GA). The mixture was stirred for 24 h, at 25 °C and 150 rpm. Finally, the kaolin was recovered by centrifugation (3 min e 3130×g) and exhaustively washed with buffer solution to remove unbounded enzyme. The immobilized enzyme was suspended in buffer solution and stored at 4 °C.
The immobilization efficiency (Y) (Eq. 1) was defined as the percentage ratio of the immobilized endoglucanase activity (AER) to endoglucanase activity before the immobilization process (AE0):
Characterization of kaolin
X-ray diffraction (PXRD) data were collected in an X’PERT-PRO (Panalytical) diffractometer using CuKα radiation (λ = 1.5418 Å). Particle size distribution was measured by the Malvern Mastersizer 2000. Transmission electron microscopy (TEM) analysis was performed on JEM-1011 (100 kV). For TEM analysis, the kaolin samples were suspended in distilled water [1% (w/v)], and the suspension was placed on a carbon-coated copper grid, dried overnight and analyzed. SEM (scanning electron microscope) imaging was performed on a JEOL-JSM-5919LV. Before the SEM analysis, the kaolin sample was coated with gold.
Characterization of free and immobilized endoglucanase
The temperature profile was determined by activity assays at a fixed pH of 5.0 at a temperature ranging from 35 to 75 °C. The pH profile was determined by activity assays at a set temperature of 55 °C at a pH ranging from 4 to 7. Thermal stability was evaluated by measuring the activity of the enzyme incubated at 55 °C in buffer citrate phosphate 0.05 M at pH 5.0 for different intervals of time. Storage stability at 4 °C was monitored by enzymatic activity measurement for 30 days. All activity assays were performed as described above.
All the experiments were carried out in triplicate and the results are expressed by mean. Tukey’s test at a significance level of 5% was used to compare the mean values.
Reusability of immobilized endoglucanase
The reusability of immobilized endoglucanase was evaluated by enzymatic activity assays at the optimal pH and temperature values. After each cycle, the immobilized enzyme was recovered by centrifugation, washed with buffer and dispersed in a fresh CMC solution for the next assay. The enzymatic activity in the first cycle was defined as 100%, and relative activity was calculated for the following cycles.
Results and discussion
Mechanisms of reaction between enzyme and support
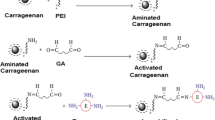
Immobilization by covalent bonds was the method chosen to immobilize the endoglucanase on kaolin due to high affinity of enzyme for the substrate. Three reactions were necessary to achieve the covalent immobilization on kaolin. First, amino groups (functional group) were introduced onto kaolin surface by functionalization using APTES. The functionalization reaction consists in hydrolysis of silane groups of APTES followed by condensation of these groups with hydroxyl surface groups of kaolin [25, 26]. The result is the grafting of the silane onto kaolin surface and the presence of functional amine groups. Second, amino groups were let to react with GA for making them suitable to react with the enzyme. GA is a dialdehyde that reacts rapidly with amine groups at around neutral pH [27]. During the reaction, aldehyde group at one end of GA reacts with the amine group of the silane and forms an imine linkage. Third, the enzyme was let to react with the support. In the reaction, the free aldehyde group of GA reacts with an amine group of the enzyme (N-terminal α-amino group and ε-amino group of lysine), resulting in immobilization of the enzyme [28]. At pH 7, the immobilization tends to proceed through of N-terminal α-amino group (pKa ≈ 7–8) because it is more reactive than lysine ε-NH2 (pKa ≈ 10) [29, 30]. The three reactions involved in covalent immobilization of endoglucanase onto kaolin are schematically shown in Fig. 1.
Activity recovery
The activity recovery was found to be 58 ± 0.8%, that corresponds to 26 U g−1support. This result is similar to those reported for immobilization of cellulase on magnetic nanoparticles using GA for covalent binding [16, 31]. Yu et al. [32] reported a higher immobilization efficiency (75.6 ± 1.2%) for cellulase immobilized on Eudragit S-100 (copolymer based on methacrylic acid and methyl methacrylate) using carbodiimide for the formation of covalent bonding between the amino groups of the enzyme and the carboxylic acid groups of the methacrylic acid. However, as will be discussed later, the reuse results obtained in this study were better than the above study.
Characterization of kaolin
The X-ray diffractograms of K, KAPTES-GA, and KAPETS-GA-E are shown in Fig. 2. The KAPTES-GA and KAPETS-GA-E did not show changes in the positions and intensities of the Bragg reflections arising from the natural kaolin, indicating that the crystallinity of the kaolin was maintained after functionalization and enzyme binding. The peak (001) (related to basal distance) at 12.35° in 2θ do not show a significant shift, suggesting that there was no expansion of the clay after functionalization and enzyme binding in agreement with results found on literature [21]. This is an expected result since kaolin has strong hydrogen bond interactions between platelets which makes difficult to intercalate organic molecules.
Transmission electron microscopy (TEM) images of kaolin before (KAPTES-GA) and after endoglucanase immobilization (KAPETS-GA-E) are shown in Fig. 3. The KAPTES-GA presents well defined and clear shape, while the KAPETS−GA−E showed a thin film around the particles which could be attributed to the enzyme.
Particle size distributions of K, KAPTES-GA, and KAPETS-GA-E are shown in Fig. 4. All samples showed a broad size distribution and the volume mean diameter (d4,3) was 16.7 µm for K and 15.3 µm for KAPTES-GA and KAPETS-GA-E. The broad size distribution of kaolin particles was also observed in the SEM image. The similar results of the particle size distribution suggest that the functionalization and immobilization did not influence the particle size.
Characterization of free and immobilized endoglucanase
The immobilization process usually produces slight distortions in the structure of the enzyme and provides a microenvironment different from the free enzyme [33, 34]. These alterations can affect the stability and catalytic properties of the catalyst, such as optimal conditions. Thus, the effects of temperature and pH on the activity and thermal stability of the free and immobilized endoglucanase were studied.
The effect of pH on the activity of free and immobilized endoglucanase is presented in Fig. 5. As can be observed, the activity of both, free and immobilized enzymes showed the same behavior with pH variation and maximum activity values were observed at pH 4 and 5. According to Tukey’s test, with 95% confidence interval, the activity of both enzymes at pH 4 and 5 were equal. Thus, it can be conclude that optimum pH of free and immobilized cellulase was between pH 4 and 5. This result is consistent with various studies reported in literature [14, 16, 35,36,37,38,39,40] and suggests the immobilization of endoglucanase on kaolin had no significant changes over the optimal pH.
The effect of temperature on hydrolytic activity of free and immobilized endoglucanase is presented in Fig. 6. As shown, the maximal activity of the free enzyme was observed around 45 and 55 °C. Interestingly, according to Tukey’s test, the average activity of the immobilized endoglucanase at 45, 55, 65 and 75 °C was equal. This result suggests that immobilized endoglucanase has a wider range of optimum temperature than the free form. Similar results have also been reported for cellulase immobilized on other supports [15, 16, 35]. The increase in optimum temperature can be explained by the covalent bond of the enzyme which reduces the conformational flexibility and thus stabilizes the enzyme. This property of immobilized endoglucanase opens a broad range of possible industrial applications.
Thermal stability is the ability of the enzyme to maintain its native conformation at relatively high temperatures over time. The results of thermal stability of free and immobilized endoglucanase are presented in Fig. 7. According to Tukey’s test, the average activity of both enzymes over 24 h did not differ statistically. These results suggest the structure of the enzyme was conserved for 24 h at 55 °C. Stability to the storage of an enzyme is an important factor that can limit its application, thus the preservation of its structural stability is a major concern. It refers to the ability of an enzyme to maintain its activity in the period between production and use [41]. The activity of free and immobilized enzyme was monitored for 30 days and there was no observed loss of catalytic activity. A similar result was reported by Zhang et al. [42], where cellulase was immobilized in an APTES-GA modified silica gel, retaining 92.4% of its initial activity after 30 days of storage at 4 °C.
Reusability of the immobilized endoglucanase
Reusability is a mandatory parameter for commercialization of an immobilized enzyme. The number of uses of an immobilized enzyme should be enough to compensate for the additional costs of immobilization. The result of reusability (Fig. 8) shows that the relative activity of the immobilized enzyme gradually decreased with reusing times and retains 80% of its initial activity after eight cycles. The good reusability may be correlated to the covalent bonds between the enzyme and the support, and also to the high thermal stability of the enzyme studied. Thus, the decrease of enzyme activity may be related to the loss of particles in the recovery step.
The result of reuse represented better results when compared to previous works, where cellulase was immobilized in different supports, including magnetic nanoparticles [11, 43,44,45,46]. Yu et al. [32] reported better immobilization efficiency for the immobilization in Eudragit S100, but during the use of the immobilized cellulase in successive cycles of CMC hydrolysis only 37.6% of initial activity was observed after five cycles. Qi et al. [15] reported that cellulase immobilized on magnetic porous terpolymer retained 48.2% of its initial activity after six cycles. Lima et al. [16] studied immobilization of cellulase onto magnetic nanoparticles encapsulated in polymer nanospheres and reported a retention of 69% activity compared to its initial activity after eight cycles of use. In addition to the better reuse results, kaolin also confers easy separation or reusability of the biocatalyst by simple filtration, decantation or centrifugation. Therefore, the use of immobilized cellulase on kaolin would lead to the continuous hydrolysis of CMC without much loss of the activity.
Conclusions
Kaolin functionalized with 3-aminopropyltriethoxysilane and activated with glutaraldehyde was used as solid support for covalent immobilization of cellulase. Immobilization was successfully performed under very mild conditions (25 °C). Immobilized cellulase showed a broader range of optimal temperature compared to its free form, which reflects an important advantage for industrial applications. Reusability results suggest that the immobilized endoglucanase can be efficiently used in several reaction cycles with low loss of catalytic activity. The immobilization of endoglucanase on functionalized and activated kaolin can be considered a potential alternative for covalent immobilization of this class of enzyme. As opposed to the synthetic supports, kaolin is naturally occurring, readily available and also confers easy separation or reusability of the biocatalyst by simple filtration, decantation or centrifugation. To the best of our knowledge, this is the first report presenting the immobilization of cellulase on functionalized kaolin.
References
Henrissat B (1994) Cellulases and their interaction with cellulose. Cellulose 1:169–196
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66:506–577
Zhang XZ, Zhang YHP (2013) Cellulases: characteristics, sources, production, and applications.In: Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers, Wiley, Hoboken, pp 131–146
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial cellulases in industrial applications. Ann Appl Bio-Sci 3:R23–R29
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307
Homaei AA, Sariri R, Vianello F, Stevanato R (2013) Enzyme immobilization: an update. J Chem Biol 6:185–205
Eş I, Vieira JDG, Amaral AC (2015) Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl Microbiol Biotechnol 99:2065–2082
Torres-Salas P, del Monte-Martinez A, Cutiño-Avila B, Rodriguez-Colinas B, Alcalde M, Ballesteros AO, Plou FJ (2011) Immobilized biocatalysts: novel approaches and tools for binding enzymes to supports. Adv Mater 23:5275–5282
Saleem M, Rashid M, Jabbar A, Perveen R, Khalid A, Rajoka M (2005) Kinetic and thermodynamic properties of an immobilized endoglucanase from Arachniotus citrinus. Process Biochem 40:849–855
Ince A, Bayramoglu G, Karagoz B, Altintas B, Bicak N, Arica MY (2012) A method for fabrication of polyaniline coated polymer microspheres and its application for cellulase immobilization. Chem Eng J 189:404–412
Abraham RE, Verma ML, Barrow CJ, Puri M (2014) Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol Biofuels 7:1–12
Mubarak N, Wong J, Tan K, Sahu J, Abdullah E, Jayakumar N, Ganesan P (2014) Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J Mol Catal B Enzym 107:124–131
Sánchez-Ramírez J, Martínez-Hernández JL, Segura-Ceniceros P, López G, Saade H, Medina-Morales MA, Ramos-González R, Aguilar CN, Ilyina A (2017) Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst Eng 40:9–22
Zang L, Qiu J, Wu X, Zhang W, Sakai E, Wei Y (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454
Qi H, Duan H, Wang X, Meng X, Yin X, Ma L (2015) Preparation of magnetic porous terpolymer and its application in cellulase immobilization. Polym Eng Sci 55:1039–1045
Lima JS, Araújo PHH, Sayer C, Souza AAU, Viegas AC, de Oliveira D (2017) Cellulase immobilization on magnetic nanoparticles encapsulated in polymer nanospheres. Bioprocess Biosyst Eng 40:511–518
Gao J, Lu C-L, Wang Y, Wang S-S, Shen J-J, Zhang J-X, Zhang Y-W (2018) Rapid immobilization of cellulase onto graphene oxide with a hydrophobic spacer. Catalysts 8:180 (2073-4344)
Wang Y, Chen D, Wang G, Zhao C, Ma Y, Yang W (2018) Immobilization of cellulase on styrene/maleic anhydride copolymer nanoparticles with improved stability against pH changes. Chem Eng J 336:152–159
Su L, Zeng X, He H, Tao Q, Komarneni S (2017) Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Appl Clay Sci 148:103–108
Zdarta J, Meyer AS, Jesionowski T, Pinelo M (2018) A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts 8:92
Sinegani AAS, Emtiazi G, Shariatmadari H (2005) Sorption and immobilization of cellulase on silicate clay minerals. J Colloid Interface Sci 290:39–44
Karagulyan HK, Gasparyan VK, Decker SR (2008) Immobilization of fungal β-glucosidase on silica gel and kaolin carriers. Appl Biochem Biotechnol 146:39–47
Linder M, Mattinen M-L, Kontteli M, Lindeberg G, Ståhlberg J, Drakenberg T, Reinikainen T, Pettersson G, Annila A (1995) Identification of functionally important amino acids in the cellulose-binding domain of Trichoderma reesei cellobiohydrolase I. Protein Sci 4:1056–1064
Ghose T, Bisaria V (1979) Studies on the mechanism of enzymatic hydrolysis of cellulosic substances. Biotechnol Bioeng 21:131–146
Xu J, Sun J, Wang Y, Sheng J, Wang F, Sun M (2014) Application of iron magnetic nanoparticles in protein immobilization. Molecules 19:11465–11486
Kahraman MV, Bayramoğlu G, Kayaman-Apohan N, Güngör A (2007) α-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chem 104:1385–1392
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–806
Gunda NSK, Singh M, Norman L, Kaur K, Mitra SK (2014) Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl) triethoxysilane (APTES) and glutaraldehyde linker. Appl Surf Sci 305:522–530
Barbosa O, Torres R, Ortiz C, Berenguer-Murcia Á, Rodrigues RC, Fernandez-Lafuente R (2013) Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromolecules 14:2433–2462
Hernandez K, Fernandez-Lafuente R (2011) Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb Technol 48:107–122
Xu J, Huo S, Yuan Z, Zhang Y, Xu H, Guo Y, Liang C, Zhuang X (2011) Characterization of direct cellulase immobilization with superparamagnetic nanoparticles. Biocatal Biotransform 29:71–76
Yu Y, Yuan J, Wang Q, Fan X, Wang P, Cui L (2014) A promising approach for bio-finishing of cotton using immobilized acid-cellulase. Fibers Polym 15:932–937
Rodrigues RC, Ortiz C, Berenguer-Murcia Á, Torres R, Fernández-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307
Secundo F (2013) Conformational changes of enzymes upon immobilisation. Chem Soc Rev 42:6250–6261
Zhang W, Qiu J, Feng H, Zang L, Sakai E (2015) Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J Magn Magn Mater 375:117–123
Zhou J (2010) Immobilization of cellulase on a reversibly soluble—insoluble support: properties and application. J Agric Food Chem 58:6741–6746
Lin Y, Liu X, Xing Z, Geng Y, Wilson J, Wu D, Kong H (2017) Preparation and characterization of magnetic Fe 3 O 4-chitosan nanoparticles for cellulase immobilization. Cellulose 24:5541–5550
Hung T-C, Fu C-C, Su C-H, Chen J-Y, Wu W-T, Lin Y-S (2011) Immobilization of cellulase onto electrospun polyacrylonitrile (PAN) nanofibrous membranes and its application to the reducing sugar production from microalgae. Enzyme Microb Technol 49:30–37
Hartono SB, Qiao SZ, Liu J, Jack K, Ladewig BP, Hao Z, Lu GQM (2010) Functionalized mesoporous silica with very large pores for cellulase immobilization. J Phys Chem C 114:8353–8362
Simon P, Lima JS, Valério A, Oliveira Dd, Araújo PH, Sayer C, Souza AAUd, Souza S (2018) Cellulase immobilization on poly(methyl methacrylate) nanoparticles by miniemulsion polymerization. Braz J Chem Eng 35:649–658
Ó’Fágáin C (2003) Enzyme stabilization—recent experimental progress. Enzyme Microbial Technol 33:137–149
Zhang D, Hegab HE, Lvov Y, Dale Snow L, Palmer J (2016) Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus 5:1–20
Mishra A, Sardar M (2015) Cellulase assisted synthesis of nano-silver and gold: application as immobilization matrix for biocatalysis. Int J Biol Macromol 77:105–113
Wu L, Yuan X, Sheng J (2005) Immobilization of cellulase in nanofibrous PVA membranes by electrospinning. J Membr Sci 250:167–173
Yu Y, Yuan J, Wang Q, Fan X, Ni X, Wang P, Cui L (2013) Cellulase immobilization onto the reversibly soluble methacrylate copolymer for denim washing. Carbohyd Polym 95:675–680
Yang C, Mo H, Zang L, Chen J, Wang Z, Qiu J (2016) Surface functionalized natural inorganic nanorod for highly efficient cellulase immobilization. R Soc Chem Adv 6:76855–76860
Acknowledgements
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support, as well as Laboratório Central de Microscopia Eletrônica at UFSC (LCME-UFSC) for TEM and SEM analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza Lima, J., Costa, F.N., Bastistella, M.A. et al. Functionalized kaolin as support for endoglucanase immobilization. Bioprocess Biosyst Eng 42, 1165–1173 (2019). https://doi.org/10.1007/s00449-019-02113-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02113-w