Abstract
In the current research, endoglucanase, one of the enzymes of the cellulolytic complex, was immobilized on kaolin by two different techniques, adsorption, and covalent bonding. A comparative study was conducted between free, adsorbed, and covalently immobilized endoglucanase. For the covalent bonding, the kaolin particles were functionalized with 3-aminopropyltriethoxysilane (APTES) and activated with glutaraldehyde. Immobilization by adsorption was performed using the kaolin without any treatment. Recovered activities after the endoglucanase immobilization by adsorption and covalent bonding were found to be 60 ± 2.5 and 65 ± 3.5%, respectively. The studies of optima pH and temperature, as well as thermal stability, showed that the catalytic characteristic of the enzyme was maintained after the immobilization by both adsorption and covalent bonding. Even after 8 cycles of use, the endoglucanase immobilized by the two techniques retained about 86% of its initial activity. The results showed that the adsorption was as effective as covalent bonding for the immobilization of endoglucanase on kaolin. However, the adsorption technique seems to have a greater potential for use in future studies, as it is simpler, cheaper, and faster than covalent immobilization. Therefore, in this work it was demonstrated that endoglucanases can be immobilized efficiently on kaolin through a very simple immobilization protocol, offering a promising strategy for performing repeated enzymatic hydrolysis reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulases are an enzymatic complex of three different enzymes (endoglucanases, exoglucanases, and β-glucosidases) capable to cleave the β-1,4-glycosidic bond of the cellulose molecule. They are considered biomolecules with great economic appeal due to various industrial applications, like textiles, pulp and paper, detergent, animal feed, food and beverages, as well as ethanol from biomass [1,2,3]. However, their stability and costs are considered as the barrier to further development of large-scale operations and applications [4,5,6]. Hence, researchers and manufacturers have investigated different approaches to overcome the aforementioned problems and offer an opportunity for making cellulases application more attractive [7]. Among them, enzyme immobilization stands out, as it allows the recovery and reuse of the enzyme and can improve its storage, operational, thermal, and conformational stabilities [8, 9].
The main constituents of an immobilized enzyme are the enzyme, the support, and the immobilization method [8, 10]. Therefore, the selection of the appropriate support and immobilization method is a crucial part of the immobilization protocol and must consider the characteristics of the enzyme and the application [8, 11, 12].
For cellulase immobilization, the characteristics of the substrate is one of the most important factors that must be considered in the immobilization protocol. Due to the insoluble characteristics and the large dimensions of the substrate (cellulose), the support can cause steric restrictions that prevent access to the active site of the enzyme [10, 13]. Therefore, for cellulases, the immobilization protocols that affect the accessibility of the substrate should be avoided. In this context, immobilization on non-porous materials may be preferable, as they cause minimal diffusion limitation [14, 15].
A variety of supports have been used for immobilizing enzymes, like carbon nanotubes [16], synthetic and natural polymers [17,18,19,20,21], magnetic nanoparticles [7, 22,23,24], polymers/clay nanocomposite [25,26,27], electrospun membranes [28, 29], inorganic materials [30,31,32,33,34,35], among others. Kaolin, a natural clay mineral, has been shown to be very attractive since it has many characteristics that are fundamental for a support. Kaolin is an abundant material in nature, cheap, non-toxic, chemically, and mechanically stable, resistant to microbial attacks, and easily recoverable by centrifugation, filtration, or decantation [36]. In addition, excellent results have been reported using kaolin as support for immobilizing enzymes, such as lipases, laccase, inulinase, peroxidase, and cellulases [33, 37,38,39,40,41,42,43,44,45].
The immobilization of enzymes on kaolin can be carried out by adsorption or covalent bonding after its surface functionalization. Both have their advantages and disadvantages. Adsorption has greater commercial potential, as it is usually simple, fast, easy to perform, inexpensive. In addition, the technique causes little or no conformational change and allows the support to be regenerated after the enzyme becomes inactive [8]. However, it has been unrecommended for immobilizing endo and exoglucanases. Due to the weak interaction with the support and the high affinity for the substrate, these enzymes tend to leach from the support and remain adsorbed on the substrate when immobilized by adsorption [46, 47]. Sometimes, the enzymes can be strongly adsorbed on the support or the adsorption can be stable enough to prevent leaching of the enzyme under the application conditions [48]. However, for some applications, such as processes that strictly require the absence of enzyme in the product, covalent bonding may be preferable [10, 35, 49,50,51]. The covalent technique provides a stable bond between the enzymes and the support. Nonetheless, the technique often causes partial enzyme deactivation and, in comparison with adsorption, requires a longer, more complex, and expensive process [52, 53]. In this context, the choice of the attachment method to immobilize an enzyme must be based on experimental analyses and considering all advantages and disadvantages of each technique.
The use of kaolin as support for cellulases immobilization was reported by Karagulyan et al. [54] and Lima et al. [55]. Karagulyan et al. [54] reported the immobilization of β-glucosidase by adsorption. Lima et al. [55] reported the immobilization of endoglucanase on functionalized and activated kaolin by covalent bonding. Both works used the same support, however, it is not possible to compare the effects of the immobilization method because the enzymes studied were different. Endoglucanases initiate the cellulose hydrolysis and act only on a large and insoluble substrate. In contrast, β-glucosidases act on soluble and small molecules of cellulose.
In this context, the main objective of the present study was to compare the suitability of two techniques, adsorption or covalent bonding, for immobilizing endoglucanase on kaolin. The immobilized enzymes were compared in terms of retained activity after immobilization, pH and optimal temperature, thermal stability and reuse using carboxymethylcellulose as substrate. To the best of our knowledge, there are no studies that compare the immobilization of cellulases on kaolin using covalent and adsorption techniques.
Materials and methods
Materials
Kaolin (Saca B; Al2O3 wt.% 40, SiO2 wt.% 46; ρ = 2.58 g/cm3) used as support for endoglucanase immobilization was kindly provided by Imerys (Pará, Brazil). 3-Aminopropyltriethoxysilane (APTES; 99%; ρ = 0.946 g/cm3), and sodium carboxymethylcellulose (CMC; 90,000 Daltons) were acquired commercially from Sigma-Aldrich (São Paulo, Brazil). Citric acid monohydrate (≥ 99%), monobasic anhydrous potassium phosphate (≥ 99%), dibasic sodium dihydrogen phosphate (≥ 98%), monobasic anhydrous sodium phosphate (≥ 98%), acid 3,5-dinitrosalicylic (DNS; ≥ 98%), glutaraldehyde (GA, 25 wt.% solution in water), and glucose were all purchased from Vetec. Potassium and sodium tartrate (99%) were purchased from Dinâmica. All chemicals used in this study were analytical grade.
Enzyme preparation rich in endoglucanases (Biokey AKM) was kindly donated by Akmey Brazil (Indaial, Santa Catarina, Brazil). To remove stabilizers and possible impurities present in the enzyme preparation, a dialysis process with phosphate buffer solution, pH 6.0, was carried out for seven days at room temperature and using a collagen membrane with 11.625 m2/g of surface area and 21.752 Å of pore diameter (DEVRO). At the end of the dialysis, the solution containing the enzyme was frozen at −80 °C (Glacier Ultralow Temperature Freezer, NuAire, Inc.), lyophilized (LIOTOP Lyophilizer, Model L101) for 48 h and then stored under refrigeration (4 ºC).
Determination of endoglucanase activity
The activity of endoglucanase (free and immobilized) was determined by the quantification of reducing sugars released from hydrolysis of sodium carboxymethylcellulose (CMC). The hydrolysis assays were performed as described by Lima, et al. [22]: 100 μL of cellulase solution was incubated with 900 µL of 4% (w/v) CMC solution (0.15 M citrate–phosphate buffer pH 5.0) for 30 min at 55 °C. Reducing sugars were estimated by the dinitrosalicylic acid (DNS) method [56]. For this, 1.5 mL of DNS solution was added to each the hydrolysis reaction. Next, the samples were heated in a boiling water bath for 5 min and cooled in an ice bath. After they were diluted and centrifuged at 3130×g for 3 min. Finally, the absorbance of the samples was measured at 540 nm using a UV–vis spectrophotometer (Cirrus 80, Femto, resolution of 1 nm). The absorbance was converted to reducing sugars concentration through a calibration curve with glucose as standard. Each assay was done in triplicate and the average values were reported.
Enzyme immobilization procedure
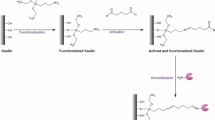
The immobilization by adsorption was performed using the kaolin as received (K). For covalent immobilization, the support was functionalized and activated and named as KFA. The functionalization and activation were carried out according to the methodology previously described by Lima et al. [55]. For support functionalization, 50 g of kaolin was dispersed in 500 mL of ethanol/water mixture (96/4). The coupling agent, APTES, was added with a mass concentration of 10% of the kaolinite mass. The mixture was stirred for 12 h at approximately 80 °C. After the reaction was over, the solvent was evaporated, the particles were washed three times with ethanol to remove excess reagent and then sieved (38 µm). For activation, 10% (w/v) of functionalized kaolin was added to a 2% (v/v) GA solution (prepared in 0.05 M phosphate buffer pH 7.0). The mixture was stirred for 1 h at 30 °C and 150 rpm. At the end of the reaction, the particles were washed three times with distilled water to remove the excess glutaraldehyde.
The immobilization was performed by adding 10% (w/v) of natural kaolin (K) or functionalized and activated kaolin (KFA) to the cellulase solution (100 mg/mL in 0.05 M phosphate buffer pH 7.0). The mixture was continuously mixed at 150 rpm for 24 h, at 25 °C. The immobilized endoglucanase was then separated by centrifugation (3 min and 3130×g) and exhaustively washed with buffer solution to remove unbounded enzyme. Immobilized preparations were suspended in buffer solution and stored at 4 °C until use. The experiments were carried out in triplicate.
The recovered activities were calculated by Eq. 1:
where AER is the activity of endoglucanase immobilized on kaolin, and AE0 is the activity of the supplied enzyme to immobilization.
Characterization of kaolin
X-ray diffraction (XRD) data were obtained by PanAlytical Xpert X-ray diffractometer using a Ni-filtered Cu Kα radiation (λ = 1.5418 Å, generated with 45 kV, 40 mA) with a step size of 0.0334° and speed of 15° min−1, in the angular range 10° ≤ 2θ ≤ 80°. Basal spacing (d-spacing) was calculated using Bragg's Law. The qualitative interpretation of the diffractograms was based on the PDF 01-089-6538 contained in the International Database of Crystalline Structures (ICSD).
The particle size distribution of kaolin particles was determined by the laser diffraction technique on the Mastersizer 3000E (Malvern) equipment. The samples were automatically diluted by the equipment using water and the final values were expressed as mean of three measures. Three diameters were considered for sample analysis: D10, D50 and D90, which are defined as diameter below which 10, 50 and 90%, respectively, of the overall particles are contained [57, 58].
The Zeta potential measurements of the free enzyme and kaolin were made using a Zetasizer Nanosizer instrument (Malvern) using single-point measurements at room temperature. The samples were diluted according to the equipment's turbidity reading limit with 0.05 M phosphate buffer pH 7.0. For each sample, five zeta potential measurements were made and average values are reported.
The morphology analyze of particles and elemental identification were carried out by scanning electron microscopy (SEM) with X-ray energy dispersion spectroscopy (EDX) using the JEOL JSM-6390LV equipment. Before carrying out the analyzes, the samples were coated with gold using a sputter coater.
Catalytic characterization of free and immobilized endoglucanase
The effect of pH on the activity of the free and immobilized endoglucanase was investigated in the range from pH 4 to pH 7 at 55 °C. In the same way, the effect of temperature on the activity of the free and immobilized endoglucanase was evaluated at pH 5 and temperatures between 35 and 75 °C. The thermal stability of the free and immobilized endoglucanases was also investigated by measuring the activity of the enzymes incubated at 55 °C in 0.05 M citrate–phosphate buffer pH 5 for different time intervals. To determine storage stability, the activity of the enzymes stored at 4 °C was monitored for 30 days. All results were converted into relative activities that were calculated using the highest activity recorded for the respective assessed parameter.
Reusability of immobilized endoglucanase
To evaluate the reusability of immobilized endoglucanase, consecutive enzymatic activity assays were conducted at the optimal pH and temperature. At the end of each cycle, the immobilized enzyme was recovered by centrifugation (3130×g for 3 min) and washed with buffer twice to remove any remaining substrate or product. A new cycle was initiated by adding the immobilized enzyme recovered in a fresh CMC solution. The enzymatic activity in the first cycle was defined as 100%, and for the following cycles, it was expressed as a percentage of the initial activity.
Statistical analysis
The analytical experiments were carried out at least in triplicates and data were reported as the means with the associated standard deviation. Statistical analysis of the data was performed using the Tukey test with p ≤ 0.05 as the minimum level of significance.
Results and discussion
Enzyme immobilization
Endoglucanase immobilization on kaolin was carried out by two methods: adsorption and covalent bonding. The immobilization by adsorption was performed using the kaolin as received (K), i. e., kaolin was used without any type of treatment. For pH 7, which was the pH of the buffer solution used during the immobilization, the zeta potential of the kaolin particles was −40 ± 1.1 mV and the endoglucanase was -4.0 ± 0.4 mV. These results indicate the immobilization by adsorption may be not attributed to electrostatic and ionic interactions. These facts imply that the effective immobilization of the biocatalyst occurred mainly through hydrogen bonds and hydrophobic interactions. Hydrogen bonding can be attributed to hydroxyl groups at broken-edges of tetrahedral and octahedral sheets [59]. Hydrophobic interactions can occur with siloxane bonds from the tetrahedral sheet [59].
The immobilization by covalent bonding was performed by the functionalized and activated kaolin (KFA). In this method, the immobilization may be attributed to imine linkage between the N-terminal α-amino group of the enzyme and one end of the glutaraldehyde molecule [55].
The recovered activity of endoglucanase immobilized by adsorption and covalent bonding was 60 ± 2.5 and 65 ± 3.5%, respectively. The recovered activities obtained by covalent bonding and adsorption immobilization can be compared with 58% reported by Lima et al. [55] when endoglucanase was immobilized on kaolin by covalent bonding, and 70.8% reported by Qi et al. [60] for cellulase immobilized on core–shell structured metal–organic framework composites. The results are superior compared to 48% reported for Lima et al. [22] when endoglucanase was immobilized on magnetic nanoparticles encapsulated in poly(methyl methacrylate) nanospheres. These immobilization yields are, however, lower compared to 80% yield reported by Yu et al. [61] and Yu et al. [62] for cellulase immobilized in a commercial copolymer of methyl methacrylate and methacrylic acid (Eudragit) by covalent bonding and adsorption, respectively. It is observed that the results obtained in the present study are in agreement with those found in the literature. However, compared to the supports used in these works (that are synthetic and expensive), kaolin is more attractive and has greater potential to be used on an industrial scale. Kaolin is a naturally occurring material, readily available, inexpensive, mechanical and heat resistant, with reduced susceptibility to microbial attack [35, 41, 64].
The kaolin functionalization and activation steps, necessary to establish the covalent immobilization, make this technique more expensive and time consuming than immobilization by adsorption. However, the immobilization by adsorption can result in leaching of the enzymes from the support, affecting the reusability. Thus, to choose the better method for endoglucanase immobilization on kaolin, other parameters were evaluated.
Characterization of kaolin
XRD patterns of kaolin (K) and the kaolin containing the endoglucanase immobilized by adsorption (KE) and covalent bonding (KFA-E) are shown in Fig. 1. According to ICSD data base file card nº 87771 (PDF-01-089-6538), the highest intensity peaks at 2θ = 12.40 and 24.90° correspond to kaolinite, indicating that it is the main constituent of the kaolin used. Kaolin samples containing enzyme immobilized (KE, and KFA-E) showed d values (or basal spacing) similar to kaolin without enzyme. This suggests that there was no expansion of the kaolin lamellae after immobilization of the enzyme. It means that the immobilization of the enzyme occurred at external surfaces and edges of the kaolin [63]. Such a result was expected since kaolin has a non-expandable structure due to its low ion exchange capacity and the hydrogen bonds between the lamellae [59, 63, 64]. The immobilization of endoglucanases on the surface of kaolin is an important finding and makes kaolin even more attractive for immobilizing these enzymes. Industrially, cellulases are used to hydrolyze macromolecular substrates, so these enzymes must be immobilized on the support surface to ensure enzyme–substrate contact K.
Particle size distributions obtained by laser diffraction of KE, and KFA-E are shown in Fig. 2. All samples had a broad size distribution and similar D50 values, suggesting that neither of the two immobilization methods had influenced in the particle size. The higher D90 values of KE and KFA-E samples may be related to the aggregation of particles caused by the difficulty of redispersion the pellets after the various centrifugations performed to wash the particles after immobilization [65, 66].
SEM micrographs of K, KFA, KE, and KFA-E are shown in Fig. 3. It was observed that the K sample consisted of laminar particles with different sizes, hexagonal shape and well-defined edges. EDX analysis detected characteristic elements that constitute kaolin, silicon, aluminum and oxygen, as well as carbon. The presence of carbon in this sample can be attributed to impurities, such as carbonates. The KFA sample presented laminar particles with rounded edges, which can be attributed to the functionalization of the particles with APTES. The presence of APTES on the surface of the kaolin could be confirmed by EDX analysis, since it was detected the presence of nitrogen.
In the SEM micrograph of both kaolin samples containing endoglucanase, the presence of particles with undefined shapes and edges was observed. Besides the characteristic elements that constitute kaolin (silicon, aluminum, and oxygen), EDX analysis also detected nitrogen and carbon. For KFA-E sample, the presence of carbon can be attributed to functionalization with APTES and enzyme immobilization. For the KE sample, the nitrogen presence can be attributed only to enzyme. The presence of carbon can be attributed to impurities, functionalization of kaolin particles and/or binding of the enzyme to the support. Thus, SEM and EDX results suggest that endoglucanase was successfully immobilized on the kaolin surface.
Catalytic characterization of free and immobilized endoglucanase
After any immobilization procedure, the catalytic properties of the enzyme must be evaluated because the interaction with the support can modify its three-dimensional conformation. In addition, the interaction between the immobilized enzyme and substrate occurs in a microenvironment different from the bulk solution [10]. Thus, the effects of temperature and pH on activity, as well as the thermal stability, of the free and immobilized endoglucanase were studied.
As shown in Fig. 4, the endoglucanase immobilized by covalent bonding and adsorption showed a pH profile similar to that of free endoglucanase, with optimal activity between pH 4 and pH 5. Such similarities suggest that the catalytic activity of endoglucanase, along the pH range studied, was not affected after the immobilization. Similar findings have been reported previously for cellulase immobilized on different supports [18, 22, 47, 55, 67, 68].
The temperature profiles of free and immobilized endoglucanase are shown in Fig. 5. The free and immobilized enzyme by adsorption showed similar temperature profiles with maximum activities observed around 45 and 55 °C. The maintenance of the optimum temperature of enzymes immobilized by adsorption is expected since the three-dimensional structure of the enzyme is almost always preserved because of its weak interaction with the support. Meanwhile, the endoglucanase immobilized by covalent bonding showed higher activities in a wider range of temperatures, which was from 45 to 75 °C. The increase in the optimal temperature range for enzymes immobilized by covalent bonding has also been reported in other papers [22, 55, 69, 70]. This is generally attributed to the reduction of conformational flexibility and thermal vibrations caused by the strong bond between enzyme and support [48, 71]. The increase of optimal temperature range is an important advantage for processes that require temperatures above the optimum temperature of the free enzyme. Otherwise, this is irrelevant because industries always want to operate at the lowest possible temperature to use less energy.
From the application point of view, the thermal stability of immobilized enzymes is more important than the increasing the optimal temperature range. Thermal denaturation is one of the main factors for the loss of catalytic activity during reuse. Therefore, the enzyme must be thermally stable or become highly stabilized during the immobilization process to achieve satisfactory reusability [72]. As presented in Fig. 6, free and immobilized enzymes exhibited a full activity after 24 h at 50 °C. These results showed that the enzyme used in this work is quite stable and the thermal stability was not affected after the immobilization on kaolin by adsorption or covalent bonding. Although covalently immobilized endoglucanase showed a broader optimal temperature range, it was as stable as the enzyme immobilized by adsorption when exposed to temperature for a long time.
The activity of free and immobilized enzymes was monitored for 30 days at 4 °C. All of them exhibited full activity for the conditions studied, indicating that the immobilization techniques used did not affect the storage stability of the enzyme. These results are superior to those reported by Li et al. [73], in which only 71.2% of the activity of cellulase immobilized on carbon nanotubes and sodium alginate was retained The results are also superior to 65% reported for cellulase immobilized on zirconium-based metal–organic frameworks [74].
Reusability of the immobilized endoglucanase
One of the most important advantages of enzyme immobilization is the possibility of recovering or separating the enzymes from the reaction medium and, thus, reusing them [75]. This feature lowers the enzyme's costs and increases its potential use in industrial applications [76, 77]. Endoglucanases immobilized by both covalent bonding and adsorption showed excellent performance in the reuse tests, retaining around 86% of their initial activity after the eighth hydrolysis cycle (Fig. 7). The excellent reuse can be associated with the high thermal stability of the enzyme and the characteristics of the kaolin that allowed the immobilized enzyme to be efficiently recovered [55, 72].
Endoglucanase immobilized by both covalent bonding and adsorption exhibited better reusability (86% of activity retained after eight cycles of use) than previous works [78,79,80,81]. Li et al. [73] reported 71.5% of activity retained after seven cycles for cellulase immobilized on carbon nanotubes and sodium alginate. Lima et al. [22] reported 69% of activity retained after eight cycles for cellulase immobilized on magnetic nanoparticles encapsulated in polymer nanospheres by covalent bonding. Mubarak et al. [82] reported only 26% of activity retained after eight cycles for cellulase immobilized on functionalized multi-walled carbon nanotubes by adsorption. Yu et al. [62] reported less than 40% of activity retained after eight cycles for cellulase immobilized on commercial copolymers of methacrylic acid and methyl methacrylate (Eudragit) by adsorption.
In contrast to the results obtained in the current work, previous studies have reported inferior reusability results for cellulase immobilized by adsorption compared to enzyme immobilized by covalent bonding using a same support [47, 83,84,85]. The immobilization of cellulase on kaolin, studied here, is attributed to hydrogen bonds and hydrophobic interactions, while in the previous works the immobilization of cellulase had been attributed to hydrogen bonds, ionic and/or electrostatic interactions. According to Cao [48], occasionally the interaction forces responsible for the adsorption of the enzyme on the support are stable enough to prevent leaching under application conditions, which may justify the results observed here.
Considering the results of reuse and recovered activity after immobilization, immobilization of kaolin endoglucanases by adsorption has greater potential for use in future studies. The immobilization by adsorption is simpler, cheaper and faster than covalent immobilization. This work showed that adsorption may be adequate to immobilize endoglucanases, but the choice of the immobilization method must be based on experimental data. In addition, the work showed a promising simple protocol for immobilizing endoglucanases using a cheap, natural and inorganic material (kaolin) as a support, along with a simple and inexpensive immobilization method.
Conclusions
Endoglucanase was successfully immobilized on kaolin by adsorption and covalent bonding techniques, with about 60% of the recovered activity. The immobilized endoglucanase exhibited catalytic performance with variations in pH and temperature, as well as thermal stability, similar to the free enzyme. The immobilization of endoglucanase on kaolin can be regarded as a prospective tool for multiple uses of the enzyme since about 86% of initial activity was retained after eight consecutive hydrolysis cycles. In summary, the immobilization of endoglucanase on kaolin by adsorption presented a greater potential for future industrial applications. The adsorption was stable enough to prevent the enzyme leaching from the support during reuse, in addition to being cheaper, simpler and less time-consuming than the covalent method.
References
Sharma A, Tewari R, Rana SS, Soni R, Soni SK (2016) Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol 179:1346–1380
Roth JCG, Hoeltz M, Benitez LB (2020) Current approaches and trends in the production of microbial cellulases using residual lignocellulosic biomass: a bibliometric analysis of the last 10 years. Arch Microbiol 202:935–951
Tushar MSHK, Dutta A (2020) Efficiency analysis of crude versus pure cellulase in industry. In: Srivastava N, Srivastava M, Mishra PK, Gupta VK (eds) Biofuel production technologies: critical analysis for sustainability, 1st edn, pp. 283–298. Springer, Singapore
Jia J, Zhang W, Yang Z, Yang X, Wang N, Yu X (2017) Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules 22:269
Husain Q (2017) Nanomaterials immobilized cellulolytic enzymes and their industrial applications: a literature review. SM Biochemistry 4(3):1029
Califano V, Costantini A (2020) Immobilization of cellulolytic enzymes in mesostructured silica materials. Catalysts 10:706
Khoshnevisan K, Vakhshiteh F, Barkhi M, Baharifar H, Poor-Akbar E, Zari N, Stamatis H, Bordbar A-K (2017) Immobilization of cellulase enzyme onto magnetic nanoparticles: applications and recent advances. Mol Catal 442:66–73
Mohamad NR, Marzuki NHC, Buang NA, Huyop F, Wahab RA (2015) An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip 29:205–220
Jun LY, Yon LS, Mubarak NM, Bing CH, Pan S, Danquah MK, Abdullah EC, Khalid M (2019) An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J Environ Chem Eng 7:102961
Brena B, González-Pombo P, Batista-Viera F (2013) Immobilization of enzymes: a literature survey. In: Methods in molecular biology. Springer, Berlin
Krajewska B (2004) Application of chitin-and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139
Guisan JM (2006) Immobilization of enzymes as the 21st century begins. In: Immobilization of enzymes and cells. Springer, Berlin
Soares JC, Moreira PR, Queiroga AC, Morgado J, Malcata FX, Pintado ME (2011) Application of immobilized enzyme technologies for the textile industry: a review. Biocatal Biotransform 29:223–237
Khoshnevisan K, Bordbar A-K, Zare D, Davoodi D, Noruzi M, Barkhi M, Tabatabaei M (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem Eng J 171:669–673
Cantone S, Ferrario V, Corici L, Ebert C, Fattor D, Spizzo P, Gardossi L (2013) Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem Soc Rev 42:6262–6276
Bilal M, Anh Nguyen T, Iqbal HMN (2020) Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization—a paradigm shift in biocatalyst design. Coord Chem Rev 422:213475
Facin BR, Valério A, Bresolin D, Centenaro G, de Oliveira D, Oliveira JV (2018) Improving reuse cycles of Thermomyces lanuginosus lipase (NS-40116) by immobilization in flexible polyurethane. Biocatal Biotransform 36:372–380
Simon P, Lima JS, Valério A, Oliveira Dd, Araújo PH, Sayer C, Souza AAUd, Souza S (2018) Cellulase immobilization on poly (methyl methacrylate) nanoparticles by miniemulsion polymerization. Braz J Chem Eng 35:649–658
Benucci I, Lombardelli C, Cacciotti I, Liburdi K, Nanni F, Esti M (2016) Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocolloids 61:191–200
Dantas A, Valério A, Ninow JL, de Oliveira JV, de Oliveira D (2019) Potential application of Thermomyces lanuginosus lipase (TLL) immobilized on nonporous polystyrene particles. Environ Prog Sustain Energy 38:608–613
Jampala P, Preethi M, Ramanujam S, Harish B, Uppuluri KB, Anbazhagan V (2017) Immobilization of levan-xylanase nanohybrid on an alginate bead improves xylanase stability at wide pH and temperature. Int J Biol Macromol 95:843–849
Lima JS, Araújo PHH, Sayer C, Souza AAU, Viegas AC, de Oliveira D (2017) Cellulase immobilization on magnetic nanoparticles encapsulated in polymer nanospheres. Bioprocess Biosyst Eng 40:511–518
Zhang Q, Kang J, Yang B, Zhao L, Hou Z, Tang B (2016) Immobilized cellulase on Fe3O4 nanoparticles as a magnetically recoverable biocatalyst for the decomposition of corncob. Chin J Catal 37:389–397
Otari SV, Patel SK, Kim S-Y, Haw JR, Kalia VC, Kim I-W, Lee J-K (2019) Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol 59:105–108
Benucci I, Liburdi K, Cacciotti I, Lombardelli C, Zappino M, Nanni F, Esti M (2018) Chitosan/clay nanocomposite films as supports for enzyme immobilization: an innovative green approach for winemaking applications. Food Hydrocolloids 74:124–131
Duman YA, Tekin N (2020) Kinetic and thermodynamic properties of purified alkaline protease from Bacillus pumilus Y7 and non-covalent immobilization to poly (vinylimidazole)/clay hydrogel. Eng Life Sci 20:36–49
Kumararaja P, Manjaiah K, Datta S, Shabeer TA, Sarkar B (2018) Chitosan-g-poly (acrylic acid)-bentonite composite: a potential immobilizing agent of heavy metals in soil. Cellulose 25:3985–3999
Cacciotti I, Pallotto F, Scognamiglio V, Moscone D, Arduini F (2020) Reusable optical multi-plate sensing system for pesticide detection by using electrospun membranes as smart support for acetylcholinesterase immobilisation. Mater Sci Eng C 111:110744
Li D, Wang Q, Huang F, Wei Q (2019) Electrospun nanofibers for enzyme immobilization. Electrospinning: nanofabrication and applications. Elsevier, Amsterdam
Öztürk H, Pollet E, Phalip V, Güvenilir Y, Avérous L (2016) Nanoclays for lipase immobilization: biocatalyst characterization and activity in polyester synthesis. Polymers (Basel) 8:416
Sanjay G, Sugunan S (2018) Immobilization of α-amylase onto K-10 montmorillonite: characterization and comparison of activity in a batch and a fixed-bed reactor. Clay Miner 40:499–510
Widi RK, Chrisnasari R, Budhyantoro A, Christie SD (2020) Immobilization of glucose oxidase on acid activated-bentonite and its performance examination. UPB Sci Bull Ser B Chem Mater Sci 82:113–124
Garuba EO, Onilude A (2018) Immobilization of thermostable exo-inulinase from mutant thermophilic Aspergillus tamarii-U4 using kaolin clay and its application in inulin hydrolysis. J Genet Eng Biotechnol 16:341–346
Coutinho TC, Tardioli PW, Farinas CS (2020) Hydroxyapatite nanoparticles modified with metal ions for xylanase immobilization. Int J Biol Macromol 150:344–353
Zeuner B, Ovtar S, Persson ÅH, Foghmoes S, Berendt K, Ma N, Kaiser A, Negra MD, Pinelo M (2020) Surface treatments and functionalization of metal-ceramic membranes for improved enzyme immobilization performance. J Chem Technol Biotechnol 95:993–1007
Su L, Zeng X, He H, Tao Q, Komarneni S (2017) Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Appl Clay Sci 148:103–108
Šekuljica NŽ, Prlainović NŽ, Jovanović JR, Stefanović AB, Djokić VR, Mijin DŽ, Knežević-Jugović ZD (2016) Immobilization of horseradish peroxidase onto kaolin. Bioprocess Biosyst Eng 39:461–472
Rahman MBA, Tajudin SM, Hussein MZ, Rahman RNZRA, Salleh AB, Basri M (2005) Application of natural kaolin as support for the immobilization of lipase from Candida rugosa as biocatalsyt for effective esterification. Appl Clay Sci 29:111–116
Tanasković SJ, Jokić B, Grbavčić S, Drvenica I, Prlainović N, Luković N, Knežević-Jugović Z (2017) Immobilization of Candida antarctica lipase B on kaolin and its application in synthesis of lipophilic antioxidants. Appl Clay Sci 135:103–111
Ajayi OA, Nok A, Adefila SS (2012) Immobilization of cassava linamarase on kankara kaolinite clay. J Nat Sci Res 2:55–62
Takahashi J, Kanazawa E, Yamashita Y, Kashiwai T, Takenaka H (1997) Enzyme immobilizing carrier containing kaolin. Google Patents
Iso M, Chen B, Eguchi M, Kudo T, Shrestha S (2001) Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J Mol Catal B Enzym 16:53–58
Dodor DE, Hwang H-M, Ekunwe SI (2004) Oxidation of anthracene and benzo [a] pyrene by immobilized laccase from Trametes versicolor. Enzyme Microb Technol 35:210–217
Hu X, Zhao X, Hwang H-m (2007) Comparative study of immobilized Trametes versicolor laccase on nanoparticles and kaolinite. Chemosphere 66:1618–1626
Wen X, Du C, Wan J, Zeng G, Huang D, Yin L, Deng R, Tan S, Zhang J (2019) Immobilizing laccase on kaolinite and its application in treatment of malachite green effluent with the coexistence of Cd (П). Chemosphere 217:843–850
Linder M, Mattinen ML, Kontteli M, Lindeberg G, Ståhlberg J, Drakenberg T, Reinikainen T, Pettersson G, Annila A (1995) Identification of functionally important amino acids in the cellulose-binding domain of Trichoderma reesei cellobiohydrolase I. Protein Sci 4:1056–1064
Zhang W, Qiu J, Zang L, Sakai E, Feng H (2015) Preparation of functionalized magnetic silica nanospheres for the cellulase immobilization. NANO 10:1550013
Cao L (2006) Carrier-bound immobilized enzymes: principles, application and design. John Wiley & Sons, New York
Benucci I, Lombardelli C, Cacciotti I, Esti M (2020) Papain covalently immobilized on chitosan-clay nanocomposite films: application in synthetic and real white wine. Nanomaterials 10:1622
Cavaco-Paulo A, Gubitz G (2003) Textile processing with enzymes. Elsevier
Sankarraj N, Nallathambi G (2018) Enzymatic biopolishing of cotton fabric with free/immobilized cellulase. Carbohyd Polym 191:95–102
Nguyen HH, Kim M (2017) An overview of techniques in enzyme immobilization. Appl Sci Converg Technol 26:157–163
Vidinha P, Augusto V, Almeida M, Fonseca I, Fidalgo A, Ilharco L, Cabral JM, Barreiros S (2006) Sol–gel encapsulation: an efficient and versatile immobilization technique for cutinase in non-aqueous media. J Biotechnol 121:23–33
Karagulyan HK, Gasparyan VK, Decker SR (2007) Immobilization of fungal β-glucosidase on silica gel and kaolin carriers. In: Biotechnology for fuels and chemicals. Springer, Berlin
Lima JS, Costa FN, Bastistella MA, de Araújo PHH, de Oliveira D (2019) Functionalized kaolin as support for endoglucanase immobilization. Bioprocess Biosyst Eng 42:1165–1173
Miller G (1959) Determination of reducing sugar by DNS method. Anal Chem 31:426–428
Fernandez-Puertas E, Robinson AJ, Robinson H, Sathiyalingam S, Stubbs H, Edwards LJ (2020) Evaluation and screening of spherical Pd/C for use as a catalyst in pharmaceutical-scale continuous hydrogenations. Org Process Res Dev 24:2147–2156
Berton B, Scher J, Villieras F, Hardy J (2002) Measurement of hydration capacity of wheat flour: influence of composition and physical characteristics. Powder Technol 128:326–331
An N, Zhou CH, Zhuang XY, Tong DS, Yu WH (2015) Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl Clay Sci 114:283–296
Qi B, Luo J, Wan Y (2018) Immobilization of cellulase on a core-shell structured metal-organic framework composites: better inhibitors tolerance and easier recycling. Biores Technol 268:577–582
Yu Y, Yuan J, Wang Q, Fan X, Ni X, Wang P, Cui L (2013) Cellulase immobilization onto the reversibly soluble methacrylate copolymer for denim washing. Carbohyd Polym 95:675–680
Yu Y, Yuan J, Wang Q, Fan X, Wang P, Cui L (2015) Noncovalent immobilization of cellulases using the reversibly soluble polymers for biopolishing of cotton fabric. Biotechnol Appl Biochem 62:494–501
Barral S, Villa-Garcia M, Rendueles M, Diaz M (2008) Interactions between whey proteins and kaolinite surfaces. Acta Mater 56:2784–2790
Fiorito TM, Icoz I, Stotzky G (2008) Adsorption and binding of the transgenic plant proteins, human serum albumin, β-glucuronidase, and Cry3Bb1, on montmorillonite and kaolinite: Microbial utilization and enzymatic activity of free and clay-bound proteins. Appl Clay Sci 39:142–150
Meireles M, Bourgeois F, Tourbin M, Guiraud P, Frances C (2010) Review: removal of oversize & recovery of particles from suspensions in the nano size range. Research Report CNRS. hal-01186033
Midelet J, El-Sagheer AH, Brown T, Kanaras AG, Werts MH (2017) The sedimentation of colloidal nanoparticles in solution and its study using quantitative digital photography. Part Part Syst Charact 34:1700095
Rajoka MI, Zia Y (2007) A surface immobilization method of endoglucanase from cellulomonas biazotea mutant improved catalytic properties of biocatalyst during processing. Protein Pept Lett 14:734–741
Zang L, Qiu J, Wu X, Zhang W, Sakai E, Wei Y (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454
Hosseini SH, Hosseini SA, Zohreh N, Yaghoubi M, Pourjavadi A (2018) Covalent immobilization of cellulase using magnetic poly (ionic liquid) support: improvement of the enzyme activity and stability. J Agric Food Chem 66:789–798
Qi H, Duan H, Wang X, Meng X, Yin X, Ma L (2015) Preparation of magnetic porous terpolymer and its application in cellulase immobilization. Polym Eng Sci 55:1039–1045
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38:453–468
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463
Li L-J, Xia W-J, Ma G-P, Chen Y-L, Ma Y-Y (2019) A study on the enzymatic properties and reuse of cellulase immobilized with carbon nanotubes and sodium alginate. AMB Express 9:112
Ahmed IN, Yang X-L, Dubale AA, Li R-F, Ma Y-M, Wang L-M, Hou G-H, Guan R-F, Xie M-H (2018) Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks. Biores Technol 270:377–382
Elnashar M (2011) The art of immobilization using biopolymers, biomaterials, and nanobiotechnology. In: Elnashar MMM (ed) Biotechnology of biopolymers InTech Europe, United Kingdom, Germany
Tischer W, Kasche V (1999) Immobilized enzymes: crystals or carriers? Trends Biotechnol 17:326–335
Asmat S, Husain Q, Azam A (2017) Lipase immobilization on facile synthesized polyaniline-coated silver-functionalized graphene oxide nanocomposites as novel biocatalysts: stability and activity insights. Rsc Adv 7:5019–5029
Bohara RA, Thorat ND, Pawar SH (2016) Immobilization of cellulase on functionalized cobalt ferrite nanoparticles. Korean J Chem Eng 33:216–222
Mishra A, Sardar M (2015) Cellulase assisted synthesis of nano-silver and gold: application as immobilization matrix for biocatalysis. Int J Biol Macromol 77:105–113
Yang C, Mo H, Zang L, Chen J, Wang Z, Qiu J (2016) Surface functionalized natural inorganic nanorod for highly efficient cellulase immobilization. RSC Adv 6:76855–76860
Abraham RE, Verma ML, Barrow CJ, Puri M (2014) Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol Biofuels 7:90
Mubarak N, Wong J, Tan K, Sahu J, Abdullah E, Jayakumar N, Ganesan P (2014) Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J Mol Catal B Enzym 107:124–131
Bayramoglu G, Senkal BF, Arica MY (2013) Preparation of clay–poly (glycidyl methacrylate) composite support for immobilization of cellulase. Appl Clay Sci 85:88–95
Ince A, Bayramoglu G, Karagoz B, Altintas B, Bicak N, Arica MY (2012) A method for fabrication of polyaniline coated polymer microspheres and its application for cellulase immobilization. Chem Eng J 189:404–412
Wang S, Su P, Ding F, Yang Y (2013) Immobilization of cellulase on polyamidoamine dendrimer-grafted silica. J Mol Catal B Enzym 89:35–40
Acknowledgements
The authors are grateful to Coordination for the Improvement of Higher Level Personnel (CAPES PRINT Project number 88887.310560/2018-00 and CAPES PRINT Project number 88887.310727/2018-00) and National Council for Scientific and Technological Development (CNPq) for providing financial support, Central Laboratory of Electron Microscopy (LCME–UFSC) for SEM and EDX analyses, Interdisciplinary Laboratory for the Development of Nanostructures (LINDEN/UFSC) for Zeta potential analysis, and Akmey and Imerys companies that kindly supplied the enzyme and kaolin, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza Lima, J., Boemo, A.P.S.I., de Araújo, P.H.H. et al. Immobilization of endoglucanase on kaolin by adsorption and covalent bonding. Bioprocess Biosyst Eng 44, 1627–1637 (2021). https://doi.org/10.1007/s00449-021-02545-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02545-3