Abstract
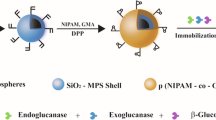
Immobilization of cellulases on magnetic nanoparticles, especially magnetite nanoparticles, has been the main approach studied to make this enzyme, economically and industrially, more attractive. However, magnetite nanoparticles tend to agglomerate, are very reactive and easily oxidized in air, which has strong impact on their useful life. Thus, it is very important to provide proper surface coating to avoid the mentioned problems. This study aimed to investigate the immobilization of cellulase on magnetic nanoparticles encapsulated in polymeric nanospheres. The support was characterized in terms of morphology, average diameter, magnetic behavior and thermal decomposition analyses. The polymer nanospheres containing encapsulated magnetic nanoparticles showed superparamagnetic behavior and intensity average diameter about 150 nm. Immobilized cellulase exhibited broader temperature stability than in the free form and great reusability capacity, 69% of the initial enzyme activity was maintained after eight cycles of use. The magnetic support showed potential for cellulase immobilization and allowed fast and easy biocatalyst recovery through a single magnet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulases, enzymes that convert cellulose into glucose, are essential for several industries, such as food and beverage, textile, animal feed, pulp and paper, as well as in hydrolysis of lignocellulosic raw materials [1]. In the textile industry, the hydrolysis action of the cellulase weakens the fibril removing small fiber ends protruding from the cotton surface and reduces the hairiness or fuzz of the fabrics. This process is known as biopolishing and can be accomplished at any time during textile wet processing but is most convenient performed after bleaching [2]. Although the enzymatic hydrolysis offers numerous advantages, high costs, inhibition by end products and especially the difficulty of recovering from the reaction medium for subsequent reuse have hindered the application of free cellulases on industrial scale. Immobilization on magnetic nanoparticles has been one of the approaches proposed to solve the problems described above and make the cellulases more attractive.
Magnetite (Fe3O4) is one of the magnetic materials most used in enzyme immobilization [3–5]. These materials show excellent magnetic properties, biocompatibility and are easily synthesized [6]. Magnetite nanoparticles with different characteristics have been applied in the immobilization of cellulases [7–11]. Although advances have been made using magnetic nanoparticles for cellulase immobilization, this approach shows some important limitations. Magnetite nanoparticles can oxidize in contact with air and are easily leached by acidic solutions [12]. As a result, the magnetic particles can lose dispersibility and magnetic response, decreasing significantly its useful life. In addition, in some industries, the magnetite may react with other substances and cause significant problems to the product [12, 13]. In addition, in some industries, the magnetite may react with other substances and cause significant problems to the product. One example is in the textile industry, where iron cations from magnetite can react with residual hydrogen peroxide from bleaching process and cause oxidation of dyes present in the fabrics due Fenton reactions [14, 15]. Therefore, providing a suitable surface coating is very important to maintain the stability of magnetite nanoparticles and avoid aforementioned problems. In such context, encapsulation in polymeric colloidal particles is an excellent alternative, not only to stabilize the magnetite nanoparticles providing a protective coating, but also to provide functional groups for further functionalization and linking of other molecules such as enzymes [12, 16]. Chiaradia et al. [14] immobilized lipase B from Candida antarctica (CALB) on poly(urea-urethane) (PUU) nanoparticles with encapsulated superparamagnetic magnetite (Fe3O4). Immobilized enzyme led to 95% of substrate conversion after 8 cycles of application demonstrating high operational stability. Poly(methyl methacrylate), PMMA, is a biocompatible polymer that has been used for enzyme immobilization [15–17]. At the same time, magnetite nanoparticles were successfully encapsulated in PMMA nanoparticles in one single step by miniemulsion polymerization [18]. The aim of this study was to propose the development of a support that retains its magnetic properties during the hydrolysis process. Magnetic nanoparticles encapsulated in polymer particles provide an opportunity of significantly improving the reusability of cellulase, reducing its cost. Magnetic nanoparticles were synthesized, encapsulated in poly(methyl methacrylate) nanoparticles and characterized. Cellulase immobilization was evaluated and the optimum operating conditions were determined.
Materials and methods
Materials
Cellusoft CR (Novozymes, Brazil) was dialyzed and lyophilized to concentrate the cellulase. For the synthesis of magnetic nanoparticles (MNPs) distilled water (H2O), ferric chloride (FeCl3·6H2O), ferrous sulfate (FeSO4·7H2O), ammonia hydroxide (NH4OH), and oleic acid (OA) (C18H34O2), all purchased from Vetec, were used. For the encapsulation of MNPs the following reactants were employed: distilled water, Crodamol (as costabilizer), provided by Alfa Aesar; polyvinyl alcohol 80% hydrolyzed [as stabilizer and to provide functional groups (hydroxyl)], purchased from Poly-sciences, Inc; methyl methacrylate (MMA, 99.5%) (as monomer) and potassium persulfate (KPS, P.A) (as initiator) were provided by Sigma-Aldrich. Glutaraldehyde (GA, 25% w/v solution in water), provided by Vetec, was used for the activation of the nanoparticles. For activity measurement was used sodium hydroxide (NaOH, P.A), acquired from Lafan Ltda, potassium and sodium tartrate (P.A) provided by Synth, and glucose D(+) anhydrous dextrose (P.A), acid 3,5-dinitrosalicylic (DNS, P.A), monobasic anhydrous potassium phosphate (P.A), dibasic sodium dihydrogen phosphate (P.A), and sodium carboxymethyl cellulose (CMC), all provided by Sigma-Aldrich. Cellulose acetate membranes (Unifil, 47 mm, 0.45 µm) were used to determine enzymatic activity. All these reagents were used as received without previous purification.
Methods
Synthesis of magnetic nanoparticles
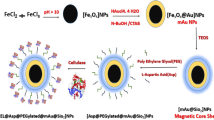
The synthesis of magnetic nanoparticles (MNP) was based on the co-precipitation method described by Feuser et al. [18]. The MNPs coated with oleic acid (OA) were separated by applying an external magnetic field and washed three times with ethanol to remove excess of OA.
Encapsulation of magnetic nanoparticles in poly(methyl methacrylate) by miniemulsion polymerization
The encapsulation of magnetic nanoparticles followed the methodology described by Feuser et al. [18], with some modifications. Organic phase was composed by MNPs (0.6 g), MMA (2.0 g) and Crodamol (0.1 g) and was subjected to ultrasonic bath for 5 min. Aqueous phase composed of water (20 mL), KPS (0.02) and PVA (0.22 g). PVA was used to stabilize the monomer droplets/polymer nanospheres and to introduce functional groups on their surface. After mixing both phases, the dispersion formed was sonifiedusing an ultrasonic probe (Fisher Scientific, Sonic Dismembrator Model 500) for 3 min (10 s on and 1 off) at 70% power intensity to prepare the miniemulsion. During the sonication, the miniemulsion was cooled in an ice bath to avoid the early onset of polymerization. The miniemulsion product was transferred to 20 mL ampoules that were immersed in a thermostatic bath at constant temperature (70 °C) for 3 h. Afterwards, encapsulated magnetic nanoparticles (MNPs-PMMA) were separated using a magnetic field, frozen and lyophilized. The lyophilized powder was stored at room temperature (25 °C).
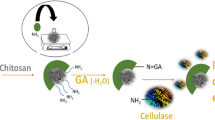
Activation of MNPs-PMMA
For activation, the MNPs-PMMA were added [5% (w/v)] to a glutaraldehyde solution (5% v/v) and maintained under mild agitation at 25 °C. After 12 h, activated nanoparticles were separated using a magnet and washed three times with distilled water to remove excess of glutaraldehyde.
Immobilization of cellulase
Cellulase immobilization was carried out by adding the activated nanoparticles [5% (w/v)] to a suspension of cellulase (100 mg mL−1) in phosphate buffer (50 mM, pH 6.0) at 25 °C. After 24 h, the nanoparticles were separated by applying of an external magnetic field and exhaustively washed with buffer solution to remove unreacted enzyme. The immobilized cellulase was suspended in buffer solution and stored at 4 °C. The binding of the enzyme was confirmed using attenuated total reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR-Bruker TENSOR 27) at range from 600 to 4000 cm−1, with a resolution of 4 cm−1 and 32 scans were recorded per measurement. The analyses were performed with freeze–dried samples.
The immobilization efficiency was determined by Eq. (1), as follows:
where Y is the immobilization efficiency (%), AER is the enzyme activity retained on the support (U mL−1) and AE0 is the enzyme activity before the immobilization process (U mL−1).
Enzyme activity assays
The activities of free and immobilized cellulase were determined by measuring the amount of reducing sugars produced during enzymatic hydrolysis of sodium carboxymethyl cellulose (CMC). The concentration of reducing sugars was quantified by DNS method [19]. The activity assays were carried out according to the following procedure: 900 µL of CMC solution (4% w/v in phosphate buffer 50 mM, pH 6.0) and 100 µL of free or immobilized cellulase were incubated for 30 min at 55 °C. The reaction was stopped by addition of 1.5 mL of DNS solution. The resulting solution was heated in a boiling water bath for 5 min and then cooled in an ice bath. The samples were diluted and filtered in cellulose acetate membranes for particulates removal. Glucose concentration was measured using UV spectrophotometer at 540 nm. The relation between absorbance and reducing sugars concentration was determined through a calibration curve with glucose as standard. Control experiment was performed by adding cellulase and DNS at the same time in CMC solution under the same conditions of hydrolysis assays.
One enzyme activity unit (U) was defined as glucose amount produced per minute (µmol min−1). All measurements were performed in duplicate. The relative activity (AR) (Eq. 2), where noted, was expressed as percentage of enzyme activity at a specific value (AEi) relative to the maximum activity (AEmax). Maximum activity is the highest activity among all activities values obtained for the variable under study
The amount of total protein in free cellulase solutions were determined by Bradford method [20].
Characterization of encapsulated magnetic nanoparticles
The intensity average diameters of particles were measured by dynamic light scattering (DLS; Zetasizer Nano S, from Malvern). Nanoparticles morphology was observed using Transmission Electron Microscopy (TEM, JEOL, JEM 2100F) at 100 kV. For TEM analysis, the nanoparticle suspension [1% (w/v)] was placed on a carbon‐coated copper grid, dried overnight and analyzed. Thermal decomposition was studied by thermogravimetric analysis (TGA, STA 449 F3 Jupiter, NETZSCH). Approximately 11 mg of each dried sample was weighed in a platinum pan and heated from 25 to 800 °C under a nitrogen atmosphere at a heating rate of 10 °C min−1. Magnetization measurements were performed with dried samples and using an electromagnet vibrating sample magnetometer (3473-70, VSM) at 300 K.
Determination of optimal pH and temperature
To determine the optimal pH, the free and immobilized cellulase activity was investigated at different pH values (from 4.0 to 8.0). Optimal temperature was determined by activity assays at different temperatures (from 35 to 75 °C). All assays were conducted with 4% (w/v) substrate solution (CMC).
Determination of thermal and storage stability
Thermal stability was determined by measuring enzymatic activity in different incubation times (30–300 min) at 55 °C. The storage stability of enzymes at 4 °C was monitored by enzymatic activity for 75 days. All assays were conducted with 4% (w/v) substrate solution (CMC) (pH 6.0).
Reusability assay
The reusability of immobilized cellulase was evaluated by measuring the enzymatic activity (see “Enzyme activity assays” section) in eight successive hydrolysis cycles. Immobilized enzymes were magnetically separated at the end of each cycle and washed before a new cycle. The scheme of reusability assay is shown in Fig. 1. The enzymatic activity in the first cycle was defined as 100% and relative activity was calculated for the following cycles.
Results and discussion
Characterization of encapsulated magnetic nanoparticles
The magnetic nanoparticles synthesized were encapsulated in polymeric matrix of poly(methyl methacrylate) (PMMA) to prevent their agglomeration and oxidation. Furthermore, due to the use of PVA as stabilizer, the polymer coating of the magnetite nanoparticles provided hydroxyl groups for the functionalization of the nanoparticles with glutaraldehyde, responsible for binding the enzyme to the support.
The MNPs-PMMA were examined by TEM to verify their shape and MNPs encapsulation. Figure 2 shows that MNPs-PMMA had spherical morphology with PMMA matrix containing dark spots that are attributed to magnetic nanoparticles. TEM images confirm the encapsulation of MNPs in poly(methyl methacrylate) spheres. Therefore, during enzymatic reactions there will be no contact between the magnetic core and the reaction medium. Consequently, it is possible to prevent contamination of the product by iron cations and the magnetic properties of the nanoparticles are expected to remain preserved. According to DLS analysis, the MNPs-PMMA have an intensity average diameter of about 150 nm, which is smaller than that of PVA/Fe2O3 nanoparticles (270 nm) synthesized by microemulsion system for cellulase immobilization [7].
The amount of MNPs that was effectively encapsulated in PMMA was determined by TGA analysis. Figure 3 shows TGA analysis of MNPs and MNPs-PMMA. The amount of magnetite in MNPs sample was 77 wt%. The other 23 wt% corresponds to water (2%) and OA (21%). MNPs-PMMA sample showed the gradual mass loss and it can be clearly seen that the PMMA nanoparticles are completely degraded when reaching about 400 °C. The amount of magnetite in MNPs-PMMA sample was 17 wt%. The other 83 wt% corresponds to the PMMA and OA present in MNPs-PMMA. The results were similar to those obtained by Feuser et al. [18].
The magnetic properties of MNPs and MNPs-PMMA were measured by Vibrating Sample Magnetometer (VSM) at room temperature. The field-dependent magnetization curves of MNPs and MNPs-PMMA are shown in Fig. 4. The main magnetic parameters, such as coercive force, Hc, remanent magnetization, Mr, and saturation magnetization, MS, are shown in Table 1. The remanence magnetization is the residual magnetism that a magnetic material retains after removing the external field, in other words, at zero driving field. The magnetization is drive to zero by applying an opposite direction field. Thus, the amount of inverse conduction field necessary to demagnetize a material is called to coercive force. Saturation magnetization is the maximum of magnetization of a material and an increase in applied external magnetic field cannot increase the magnetization of the material [21]. Saturation magnetization values in emu g−1 were obtained considering the magnetite mass obtained from TGA analysis. The saturation magnetization of the MNPs and MNPs-PMMA was about 71.7 and 44.6 emu g−1 of the MNPs, respectively. Similar results were obtained for nanosized magnetic polystyrene spheres [22]. The lower saturation magnetization value of MNPs-PMMA can be attributed to oxidation of magnetic nanoparticles during the sonication and polymerization steps [23]. When alternating the magnetic field applied to a magnetic material the magnetization curve is obtained in both directions. The lack of retraceability of the magnetization curve is the property called hysteresis and it is related to the existence of magnetic domains in the material [24]. However, the absence of hysteresis loops (Fig. 4) associated with low (almost zero) coercive field and remanent magnetization values indicate the superparamagnetic behavior of both samples (MNPs and MNPs-PMMA). The superparamagnetic behavior ensured a fast response to the external magnetic field and a stable dispersion of nanoparticles in the absence of magnetic field, that are excellent advantages for enzyme immobilization. Figure 5 shows the easy separation of the MNs-PMMA of the aqueous phase when a magnetic field was applied.
Characterization of immobilized cellulase
Cellulase was immobilized on magnetic nanoparticles encapsulated by chemical binding via GA. Covalent attachment was chosen as cellulase can be easily lixiviated from support if the enzyme is weakly bound. This is a consequence of high affinity of cellulase by the substrate [25, 26]. The immobilization efficiency was found to be 49% that corresponds to 0.18 U mL−1 or 3.6 U g−1 support. Xu et al. [27] reported a similar result of activity retention after the immobilization of cellulase in magnetite. To verify cellulase immobilization, the FTIR spectrum of immobilized cellulase was compared to the FTIR spectra of free enzyme and of activated nanoparticles (Fig. 6). The spectrum of the immobilized cellulase showed two absorption regions that were not observed in the spectrum of activated nanoparticles. The slight decline on the band at 1640 cm−1in the spectrum of immobilized cellulase is assigned to the stretching vibration mode of C = O in the amide group [28]. A peak around 1500 cm−1 was expected in immobilized cellulase spectrum that is a characteristic band of proteins and corresponds to amide bonds [8, 9]. The absence of this peak and weak peaks at 1640 cm−1 on immobilized cellulase spectrum may be associated with the limited amount of bound cellulase. According to the Bradford assays, less than 10% of the concentration of enzyme used in immobilization process corresponds to protein. However, the broad absorption band in the range of 3700–3100 cm−1, which is attributed to stretching of O–H and N–H in the structure of cellulase, proves the binding of the enzyme on support [29].
Effect of pH and temperature on cellulase activity
During the immobilization process, the enzyme structure may be altered so as to change its stability, specificity and/or accessibility to the active site. Therefore, as well as the free enzyme, the immobilized enzyme must be carefully characterized to determine its optimal conditions. Figure 7 shows that free and immobilized cellulase exhibit excellent stability in the studied pH range. According to Tukey’s test, with 95% confidence interval, the activity of both enzymes at pH 5.0 and 6.0 were equal. Maximum activities of both cellulases were obtained at these pH values (5.0 and 6.0), suggesting that there is no conformational alteration on cellulase structure by the immobilization process.
The effect of immobilization on the thermal characteristics of the free and immobilized enzyme was evaluated by means of activity assays at different temperatures and the results are shown in Fig. 8 Free cellulase showed the optimal activity at 55 °C. According to Tukey’s test at the 95% confidence interval, the average activity of the immobilized cellulase at 55 and 65 °C was equal. As at these temperatures the highest activity values were obtained, it can be affirmed that the immobilized enzyme has an optimum temperature range between 55 and 65 °C. The present study demonstrated that the stability of the enzyme increased by 10 °C with immobilization. Covalent bond between the enzyme and support restricts the conformational changes during heating. Consequently, higher activation energy is required for disorganizing the structure of the enzyme (denature), thus its stability is improved. In other immobilization studies with magnetic nanoparticles, the immobilized cellulase also showed higher thermal stability at higher temperatures [4, 11]. Furthermore, at 45 °C the relative activity of immobilized cellulase was approximately 90% while the relative activity of free cellulase was lower than 85%. The results suggest that the immobilized cellulase showed better stability in relation to temperature variations, which implies in operational flexibility. This makes immobilized cellulase even more attractive from an application point of view.
Thermal and storage stability
The thermal stabilities of free and immobilized cellulase were compared by measuring their activities over the time while temperature (55 °C), pH (6.0) and concentration of substrate (4% w/v) were kept constant. Figure 9 shows that free and immobilized cellulase exhibited similar profile. The activity of free and immobilized enzyme was monitored for 75 days and there was no loss of catalytic activity of both enzymes. In another study, cellulase immobilized on silica gel surface retained only 48% of its activity after four days [30]. Therefore, the results suggest that the immobilization process did not influence on stability (thermal and storage) of cellulase and conditions used for storage, phosphate buffer pH 6.0 at 4 °C, were suitable for enzyme conservation.
Reusability of the immobilized cellulase
The operational stability or reusability is one of the most important factors for successful application of an immobilized enzyme, and may even determine its economic viability. The reusability of immobilized cellulase was evaluated by measuring enzymatic activity in successive cycles of hydrolysis. After every reaction cycle, the immobilized cellulase was separated by a magnet, washed and reused with fresh substrate solution. Figure 10 shows that the catalytic efficiency of immobilized cellulase was reduced to approximately 31% of the initial value after eight reaction cycles. Earlier studies of cellulase immobilization on magnetic nanoparticles reported that about 40% activity was retained after eight cycles under similar conditions to those used in this study [4]. As noted in other studies of cellulase immobilization on magnetic nanoparticles [4, 9, 31], the catalytic activity of the enzyme decreased gradually in each cycle and the highest decrease (about 10%) was observed after the first reuse. The gradual loss of enzyme activity may be associated with protein denaturation, product inhibition and/or structural modification of the enzyme [9]. The results obtained here suggest that the immobilized cellulase on encapsulated magnetic nanoparticle can be used in several reaction cycles with low loss of catalytic activity. The main advantage of this approach is the easy and fast recovery of cellulase after each hydrolysis cycle by the application of an external magnetic field, without the support losing its magnetic properties.
Conclusions
The results from the present work showed that the cellulase was successfully immobilized on magnetic nanoparticles encapsulated in polymeric nanospheres via glutaraldehyde activation. Immobilized cellulase exhibited higher stability in relation to temperature variations than its free form, which reflects in operational flexibility, an important advantage for industrial hydrolysis. The magnetic support allowed fast and simple recovery of immobilized enzyme using magnets, resulting in promising results of reusability. The protocol used provides an efficient and facile approach for the synthesis of magnetic support with potential to be applied in several industrial processes without loss of its properties. Furthermore, the protocol can be easily adapted for the immobilization of other enzymes, contributing to studies about immobilization of enzymes and enzymatic hydrolysis.
References
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Cavaco-Paulo A (1998) Mechanism of cellulase action in textile processes. Carbohydr Polym 37:273–277
Chiaradia V, Valério A, de Oliveira D, Araújo PHH, Sayer C (2016) Simultaneous single-step immobilization of Candida antarctica lipase B and incorporation of magnetic nanoparticles on poly(urea-urethane) nanoparticles by interfacial miniemulsion polymerization. J Mol Catal B Enzym 131:31–35
Abraham RE, Verma ML, Barrow CJ, Puri M (2014) Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol Biofuels 7:1–12
Sahoo B, Sahu SK, Pramanik P (2011) A novel method for the immobilization of urease on phosphonate grafted iron oxide nanoparticle. J Mol Catal B Enzym 69:95–102
Wei W, Zhaohui W, Taekyung Y, Changzhong J, Woo-Sik K (2015) Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater 16:023501
Liao H, Chen D, Yuan L, Zheng M, Zhu Y, Liu X (2010) Immobilized cellulase by polyvinyl alcohol/Fe2O3 magnetic nanoparticle to degrade microcrystalline cellulose. Carbohydr Polym 82:600–604
Khoshnevisan K, Bordbar A-K, Zare D, Davoodi D, Noruzi M, Barkhi M, Tabatabaei M (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem Eng J 171(2):669–673
Jordan J, Kumar CSSR, Theegala C (2011) Preparation and characterization of cellulase-bound magnetite nanoparticles. J Mol Catal B Enzym 68:139–146
Zang L, Qiu J, Wu X, Zhang W, Sakai E, Wei Y (2014) Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind Eng Chem Res 53:3448–3454
Zhang W, Qiu J, Feng H, Zang L, Sakai E (2015) Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J Magn Magn Mater 375:117–123
Lu A-H, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Mahdavi M, Ahmad M, Haron M, Namvar F, Nadi B, Rahman M, Amin J (2013) Synthesis, surface modification and characterisation of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules 18:7533
Chiaradia V, Soares NS, Valério A, de Oliveira D, Araújo PHH, Sayer C (2016) Immobilization of Candida antarctica lipase B on magnetic poly(urea-urethane) nanoparticles. Appl Biochem Biotechnol 1:1–18
Cunha AG, Besteti MD, Manoel EA, da Silva AAT, Almeida RV, Simas ABC, Fernandez-Lafuente R, Pinto JC, Freire DMG (2014) Preparation of core–shell polymer supports to immobilize lipase B from Candida antarctica: effect of the support nature on catalytic properties. J Mol Catal B Enzym 100:59–67
Li S, Hu J, Liu B (2004) Use of chemically modified PMMA microspheres for enzyme immobilization. Biosystems 77:25–32
Valério A, Nicoletti G, Cipolatti EP, Ninow JL, Araújo PHH, Sayer C, de Oliveira D (2015) Kinetic study of Candida antarctica lipase B immobilization using poly(methyl methacrylate) nanoparticles obtained by miniemulsion polymerization as support. Appl Biochem Biotechnol 175:2961–2971
Feuser PE, Bubniak LdS, Silva MCdS, Viegas AdC, Castilho Fernandes A, Ricci-Junior E, Nele M, Tedesco AC, Sayer C, de Araújo PHH (2015) Encapsulation of magnetic nanoparticles in poly(methyl methacrylate) by miniemulsion and evaluation of hyperthermia in U87MG cells. Eur Polymer J 68:355–365
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cullity BD, Graham CD (2011) Introduction to magnetic materials. Wiley, Oxford
Zheng W, Gao F, Gu H (2005) Magnetic polymer nanospheres with high and uniform magnetite content. J Magn Magn Mater 288:403–410
Landfester K, Ramírez LP (2003) Encapsulated magnetite particles for biomedical application. J Phys Condens Matter 15:S1345
Neunzert H, Siddiqi AH (2013) Topics in industrial mathematics: case studies and related mathematical methods. Springer, US
Hirsh SL, Bilek MMM, Nosworthy NJ, Kondyurin A, dos Remedios CG, McKenzie DR (2010) A comparison of covalent immobilization and physical adsorption of a cellulase enzyme mixture. Langmuir 26:14380–14388
Linder M, Mattinen ML, Kontteli M, Lindeberg G, Ståhlberg J, Drakenberg T, Reinikainen T, Pettersson G, Annila A (1995) Identification of functionally important amino acids in the cellulose-binding domain of Trichoderma reesei cellobiohydrolase I. Protein Sci Publ Protein Soc 4:1056–1064
Xu J, Huo S, Yuan Z, Zhang Y, Xu H, Guo Y, Liang C, Zhuang X (2011) Characterization of direct cellulase immobilization with superparamagnetic nanoparticles. Biocatal Biotransform 1:29–35
Bandekar J (1992) Amide modes and protein conformation. Biochim Biophys Acta (BBA) Protein Struct Mol Enzymol 1120:123–143
Mishra A, Sardar M (2015) Cellulase assisted synthesis of nano-silver and gold: application as immobilization matrix for biocatalysis. Int J Biol Macromol 77:105–113
Zhang D, Hegab HE, Lvov Y, Dale Snow L, Palmer J (2016) Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus 5:1–20
Yu Y, Yuan J, Wang Q, Fan X, Wang P, Cui L (2015) Noncovalent immobilization of cellulases using the reversibly soluble polymers for biopolishing of cotton fabric. Biotechnol Appl Biochem 62:494–501
Acknowledgements
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support as well as Laboratório Central de Microscopia Eletrônica da UFSC (LCME-UFSC) for TEM images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lima, J.S., Araújo, P.H.H., Sayer, C. et al. Cellulase immobilization on magnetic nanoparticles encapsulated in polymer nanospheres. Bioprocess Biosyst Eng 40, 511–518 (2017). https://doi.org/10.1007/s00449-016-1716-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1716-4