Abstract
Polycyclic aromatics hydrocarbons (PAHs) are ubiquitous and toxic pollutants that are dangerous to humans and living organism in aquatic environment. Normally, PAHs has lower molecular weight such as phenanthrene and naphthalene that are easy and efficient to degrade, but high-molecular-weight PAHs such as chrysene and pyrene are difficult to be biodegraded by common microorganism. This study investigated the isolation and characterization of a potential halophilic bacterium capable of utilizing two high-molecular-weight PAHs. At the end of the experiment (25–30 days of incubation), bacterial counts have reached a maximum level (over 40 × 1016 CFU/mL). The highest biodegradation rate of 77% of chrysene in 20 days and 92% of pyrene in 25 days was obtained at pH 7, temperature 25 °C, agitation of 150 rpm and Tween 80 surfactant showing to be the most impressive parameters for HMWPAHs biodegradation in this research. The metabolism of initial compounds revealed that Hortaea sp. B15 utilized pyrene to form phthalic acid while chrysene was metabolized to form 1-hydroxy-2-naphthoic acid. The result showed that Hortaea sp. B15 can be promoted for the study of in situ biodegradation of high molecular weight PAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatics hydrocarbons (PAHs) are environmentally recalcitrant, toxic and persistent organic contaminants produced from petrogenic sources and organic materials of incomplete combustion. PAHs consist of two or more fused benzene rings in a lineal, angular or cluster arrangement. The larger is the number of benzene rings, the more difficult is their biodegradation [1]. Many approaches and techniques were evaluated to degrade or reduce PAHs pollution in the environment, such as advanced oxidation, incineration, ozonation, coagulation, adsorption, precipitation, solvent extraction, and ion. However, these treatments are limited on the metabolism of high molecular weight-PAHs (HMWPAHs) and less information for the generation of secondary toxic compounds and operational costs [2,3,4]. Among HMWPAHs, chrysene and pyrene are typical four fused benzene ring of PAH, has a toxic and carcinogenic properties and very poor solubility in water, which results lower in bioavailability, and inhibit microbial utilization. Bacteria belonging to the genera Stenotrophomonas, Hortaea, Bacillus, Mycobacterium, Achromobacter, Gardona, Rhodococcus, Pseudomonas, Burkholderia, Flavobacterium, Cycloclasticus, Ralstonia and Sphingomonas have been found to utilize some PAHs as a food and energy source [5,6,7].
Recently, biodegradation has received great attention because efficient treatment, less investment cost and energy input to the process, considered environmentally friendly. Biodegradation exploits the ability of microorganisms to metabolize and detoxify various unconventional carbon sources, including PAHs into innocuous compounds such as H2O, CO2, and methane. Biodegradation can be enhanced by bioaugmentation, an addition of specific and efficient pollutant-biodegrading microorganisms. Some parameter applied for the biodegradation experiment was varied to improve the degradation rate of PAHs [8,9,10].
In this present study, effect of environmental parameters such as temperature, pH, agitation, and surfactant was conducted to enhance degradation rate of chrysene and pyrene. Moreover, because there is no existence of detailed published report on identification of metabolites, as well as transformation pathway of pyrene and chrysene by a halophilic bacteria Hortaea sp. B15, the present study was undertaken to explore the pathway of pyrene and chrysene degradation by UV–Vis spectrophotometer and gas chromatography mass spectrometer.
Materials and methods
Reagents, materials and culture media
Chrysene, pyrene, phthalic acid, catechol, 1-hydroxy-2-naphthoic acid and Bushnell–Haas (BH) medium for bacterial culture were procured from Sigma-Aldrich. BH Medium was used due to proving the good result for the examination of fuels for microbial contamination and for studying microbial hydrocarbon deterioration. The solvents, reagents and yeast extract used for the microorganism culture and degradation study were obtained from TCI (Malaysia).
Bacterial culture and degradation study
The utilization of HMW PAHs (100 mg/L pyrene and 100 mg/L chrysene) by Hortaea sp. B15 was acquired by growing the inoculum in a separate 200-mL Erlenmeyer flask comprising 40 mL of Bushnell–Haas (BH) liquid media. After incubation for several time, the supernatant was filtrated from centrifugation (3600g at 4 °C). Resuspension of the bacteria were conducted using new medium with saline solution (1% NaCl, pH 7) prior inoculated to liquid media. Colony forming technique (CFU) was measured to monitor the progress of Hortaea sp. B15 growth on pyrene and chrysene and the unit stated as log cfu/mL. The residual pyrene and chrysene in the liquid media was calculated at every 5 hours’ time intervals. Centrifugation (8000g for 12 min at at 5 °C) was conducted to separate the cells and obtain neutral extract using three equal volumes of ethyl acetate. The ethyl acetate fractions were dried over Na2SO4 (anhydrous) and divided into two parts after dissolved with chloroform. One part was analyzed quantitatively for degradation rate and the other part was derivatized with silylation procedure [2]. Effects of temperature (20–40 °C), pH level (5–9), stationary and agitated condition (50–200 rpm), and surfactant type (Triton X100, Tween 80, Tween 20, sodium dodecylsulphate and tetradecyltrimethylammonium bromide) were examined to define the best parameter for biodegradation of pyrene and chrysene by Hortaea sp. B15 (Table 1). Autoclaved bacterial consortium was performed to prepare the control for representing abiotic losses. All analyses were conducted in triplicate at each time point.
Degradation rate analysis was performed on an Agilent 5975E GCMS fitted with flame ionization detector (FID) and a non-polar and low-bleed DB-1 column (length 30 m, inner diameter 0.25 mm, and 0.25 µm film thickness) using helium as the carrier gas and set at a constant 1 mL/min flow. The temperature conditions of the analysis were 70 °C for initial temperature, and held for 2 min followed by an increase to 150 °C at a rate of 18 °C/min, then gradually increased to 330 °C at a rate of 28 °C/min and holding time at 330 °C for 15 min. Both detector and injector temperatures were maintained at 260 °C. The injection volume was 1 µl. Mass spectrum ranged to 50–500 amu and scanned every second with electron energy of 1.3 eV. Comparison of mass spectra between sample, authentic compounds and Wiley 275L database was the final procedure for metabolite identification [7].
Results and discussion
Growth of Hortaea sp. B15 and batch study
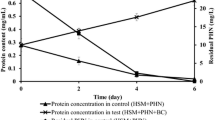
Concentrations of 5, 10, 50 and 100 mg/L were performed to know the effect of pyrene and chrysene concentration on Hortaea sp. B15 accretion. The maximum bacterial growth (over 40 × 1016 CFU/mL) was attained at 100 mg/L of PAH culture in 25–30 days of incubation (Fig. 1). Other lower concentration of pyrene and chrysed showed that the highest total bacteria was nearly 10 × 1016 CFU/mL at 20 and 25 days. Hortaea sp. B15 degraded 77% of chrysene (100 mg/L) in 20 days at a 3.85 mg/d rate while 88% of pyrene (100 mg/L) was degraded in 20 days at 4.4 mg/L rate (Fig. 2). The strain revealed a significant stage at initial 25 days followed by stable growth in cell density with connected reduction in pyrene and chrysene amount. All compounds were not completely degraded by the strain even after the end of experiment at 30 days. The level of pyrene and chrysene degradation by halophilic strain differs upon the bacteria and the growing environments such as pH, initial concentration and temperature. Hortaea sp. B15 is a fast bacterium in degrading HMW PAHs compared with other bacteria informed previously. Table 2 shows the ability of previous pyrene-degrading bacteria such as Mycobacterium sp. utilized 80% pyrene from initial concentration of 25 mg/L in 30 days while Achromobacter xylosoxidans and fused strain (Sphingomonas sp. and Pseudomonas sp.) utilized 80% of 100 mg/L pyrene within 25 days and 10 days. Hortaea sp. B15 was also compared with previous chrysene-degrading bacteria such as A. xylosoxidans utilized 56% chrysene from initial concentration of 50 mg/L in 15 days while consortium of Rhodococcus, Bacillus and Burkholderia utilized 96% of 10 mg/L chrysene in 8 days and two combination of bacteria (Bacillus and Pseudomonas) utilized only 17% of 50 mg/L chrysene in 7 days [11,12,13,14,15,16].
Biodegradation of PAHs varies with culture and environment condition such as pH, temperature, salt, oxygen content, C/N ratio, nutrient, ability of microorganism to metabolize toxic compound and capacity of microorganism to produce surfactant [6, 17]. This study revealed that the ability of Hortaea sp. B15 in degrading HMWPAHs was effected by initial pH value (5–9), incubation temperature (20–40 °C) static and rotatory incubation (50–200 rpm), and type of surfactant (ionic, anionic and cationic). The most effective pH for biodegradation pyrene and chrysene was pH 7 because it shows high removal of pyrene (92%) and chrysene (88%) and the maximum total count at 48 × 106 CFU/mL and 38 × 106 CFU/mL (Fig. 3A). Previous study showed that biodegradation of PAHs at neutral pH was preferred by most of chemoheterotrophic microorganism such as fungi and bacteria. Other study showed that acidic or base condition at extreme level gave a damaging impact on utilization capacity of the microorganism [17]. The optimal pH range for biodegradation of PAHs is 6.5–8.0 while pH 7.5 was the optimum pH level for particular strain and mixture bacteria [7, 18]. The results of the present study showed similar pattern with previous report, that the optimal value of pH was 7.
Effect of stationary and agitated culture on pyrene and chrysene utilization was also investigated. In this manner, the maximum level of biodegradation and bacterial growth was detected in the culture agitated at 150 rpm condition (Fig. 3b). Bacterial growth and degradation rate was higher in agitated culture compared to stationary culture because shaking increased solubility and oxygen concentration for microorganism utilization. Our research was similar with the previous study that the degradation efficiency improved when physiological state of strain and mass transfer between the medium and the cells runs smoothly. The bioavailability of oxygen and contact between the substrate, oxygen, and bacteria cells in the culture was influenced by air flow rate and agitation [19, 20].
The highest degradation rate of pyrene and chrysene and bacterial growth was resulted by the culture incubated at 25 °C. The degradation rate of pyrene and chrysene reduced to minimum level when the temperature increased to 40 °C (61% and 38%) (Fig. 3c). The result revealed that increasing the temperature above 35 °C might decrease the microbial growth and biodegradation rate. Previous study showed that temperature affects the biodegradation process in terms of transformation of physical and chemical structure of contaminant, ability of microorganisms in metabolizing pollutants and conversion of colony composition [8]. Even though utilization of hydrocarbon process is conducted in varying temperature, reduction in the rate of biodegradation is frequent in lower temperature. Previous study claimed that the room temperature is the ideal condition for microbial activity and biodegradation process. On the contrary, it was observed that microbial growth and biodegradation suppressed with the increasing of temperature [21, 22].
Various surfactant type (SDS, Tween 20, Triton X100, Tween 80, TDTMA,) were examined to improve the utilization of pyrene and chrysene by Hortaea sp. B15. The highest utilization of pyrene and chrysene and bacterial growth by Hortaea sp. B15 was observed in the culture supplemented with Tween 80, while the lowest was showed by TDTMA (pyrene) and Tween 20 (Chrysene) (Fig. 3d). Previous study showed that the addition of surfactants in cultures increased the pollutant solubility in liquid media and contribute to higher mixing level and physiology of cell surface [23]. It was determined that microbial growth was improved by reducing the pollutant aggregation during the addition of Tween 80 into the culture. The previous study showed that non-ionic surfactants were destructed into small moieties and increased the bioavailability of PAHs for bacteria, resulting in higher level of degradation during the transformation process. A significant increase of biodegradation of PAHs by Polyporus sp. S133 and Armillaria sp F22 was observed with the addition of Tween 80 in the culture. As distinct from Tween 80, the addition of Tween 20 and TDTMA into the culture results in a major drop of PAHs utilization even though the high microbial growth intensifies [22]. Consequently, Tween 20, SDS and TDTMA are not suggested as additional surfactant for increasing the utilization of pyrene and chrysene in this present study.
Identification of metabolites
The identification of metabolic products produced during the degradation of pyrene and chrysene by Hortaea sp. B15 is shown in Table 3. Mass spectral analysis of TMS-derivatized metabolites extract showed the occurrence of two peaks. Those peaks showed identical retention time as those of standards such as phthalic acid (13.9 min) and 1-hydroxy-2-naphthoic acid (1H2NA) (8.6 min). The metabolites of pyrene and chrysene were further characterized by MS. The peak at 13.9 min has a molecular ion with m/z at m/z 310 and fragmentation ions at m/z 295 (M+–15), 221 (M+–89), sequential loss of –OSi(CH3)3, sequential loss of –COOSi(CH3)3, sequential loss of methyl (–CH3), and 193 (M+−117), as well as the expected fragment ions at 140, 147, 148, 119, 75, and 73 [(CH3)3Si]. This fragmentation configuration matches very well with the structure of phthalic acid. The identification of phthalic acid as metabolic product of pyrene proposes that dihydroxyphenanthrene and 2,2′-diphenic acid was formed as continuation degradation of pyrene via the phthalic acid route. Unfortunately, dihydroxyphenanthrene and 2,2′-diphenic acid was not detected in the Hortaea sp. B15 culture. Further degradation was performed to form pyruvate or succinate through meta and ortho-cleavage of protocatechuic acid [24].
A GC peak at 8.6 min had an m/z 332 (M+) for silylation derivatives and fragment ions of m/z 317 [M+–15] corresponding to the respective sequential losses of methyl (–CH3), as well as m/z 114, 147 as the expected fragment ions, and 73 [(CH3)3Si], which are in accordance with that of the silylation derivative of 1H2NA. Dioxygenase system was assumed as primary enzyme of bacteria for transformation of chrysene to cis- or trans-dihydrodiol, finally form chrysenequinone. Unfortunately, only 1H2NA was identified in this research, showing that the Hortaea sp. B15 produce dioxygenase that plays an important role in chrysene transformation. The degradation pathways of pyrene and chrysene by Hortaea sp. B15 in this research is shown in Fig. 4. The effective degradation of pyrene and chrysene, and its metabolites, by strain Hortaea sp. B15 suggests, it is a potential bacterium for the bioremediation of PAHs and other hydrocarbon contaminated sites.
Conclusions
Hortaea sp. B15 is a halophilic bacterial that has the ability to degrade HMWPAHs pyrene and chrysene at optimum culture conditions of neutral pH (7), room temperature (25 °C), shaking culture at 150 rpm and Tween 80 surfactant. Hortaea sp. B15 degraded 77% of chrysene (100 mg/L) in 20 days at a 3.85 mg/d while 88% of pyrene (100 mg/L) degraded in 20 days at 4.4 mg/L rate. The maximum bacterial growth (over 40 × 1016 CFU/mL) was attained at 100 mg/L of PAH culture in 25–30 days of incubation. The strain could effectively utilize different type of four rings PAHs of as source of carbon and energy. The investigations of metabolites indicated that Hortaea sp. B15 is capable to transform pyrene and chrysene into phthalic acid and 1H2NA. It can be recommended that the application of Hortaea sp. B15 for remediation of petroleum hydrocarbons in the environment will have quite a few advantages.
References
Abdelhay A, Magnin JP, Gondrexon N, Baup S, Willison J (2008) Optimization and modeling of phenanthrene degradation by Mycobacterium sp. 6PY1 in a biphasic medium using response-surface methodology. Appl Microbiol Biotechnol 78:881–888
Lazim ZM, Hadibarata T, Puteh MH, Yusop Z (2015) Adsorption characteristics of Bisphenol A onto low cost modified phyto-waste material in aqueous solution. Water Air Soil Pollut 226:34
Harmsen J, Rietra RPJJ (2018) 25 years monitoring of PAHs and petroleum hydrocarbons biodegradation in soil. Chemosphere 207:229–238
Jiang Y, Zhang Z, Zhang X (2018) Co-biodegradation and pyrene and other PAHs by the bacterium Acinetobacter johnsonii. Ecotoxicol Environ Saf 163:465–470
Hadibarata T, Chuang TZ, Rubiyatno A, Zubir MMFA, Khudhair AB, Yusoff ARM, Salim MR, Hidayat T (2013) Identification of naphthalene metabolism by white rot fungus Pleurotus eryngii. Bioprocrocess Biosyst Eng 36:1455–1461
Chauhan A, Fazlurrahman C, Oakeshott JG, Jain RK (2008) Bacterial metabolism of polycyclic aromatic hydrocarbons: strategies for bioremediation. Ind J Microbiol 48:95–113
Kristanti RA, Hadibarata T, Al Farraj DA, Elshikh MS, Alkufeidy RM (2018) Biodegradation mechanism of phenanthrene by halophilic Hortaea sp. B15. Water Air Soil Pollut 229:324
Khudhair AB, Hadibarata T, Kamyab H, Kristanti RA (2017) Biotransformation of pyrene by Candida sp. S1 under high salinity conditions. Bioprocess Biosyst Eng 40:1411–1418
Singh K, Chandra S (2014) Treatment of petroleum hydrocarbon polluted environment through bioremediation: a review. Pak J Biol Sci 17:1–8
Zhou H, Wang H, Huang Y, Fang T (2016) Characterization of pyrene degradation by halophilic Thalassospira sp. strain TSL5-1 isolated from the coastal soil of Yellow Sea, China. Int Biodeterior Biodegrad 107:62–69
Nzila A, Ramirez CA, Musa MM, Sankara S, Basheer C, Li QX (2018) Pyrene biodegradation and proteomic analysis in Achromobacter xylosoxidans, PY4 strain. Int Biodeterior Biodegrad 130:40–47
Wang S, Li X, Liu W, Li P, Kong L, Ren W, Wu H, Tu Y (2012) Degradation of pyrene by immobilized microorganisms in saline-alkaline soil. J Environ Sci 24:1662–1669
Lu J, Guo C, Zhang M, Lu G, Dang Z (2014) Biodegradation of single pyrene and mixtures of pyrene by a fusant bacterial strain F14. Int Biodeterior Biodegrad 87:75–80
Ghevariya CM, Bhatt JK, Bharti P (2011) Enhanced chrysene degradation by halotolerant Achromobacter xylosoxidans using Response Surface Methodology. Bioresour Technol 102:9668–9674
Vaidya S, Devpura N, Jain K, Madamwar D (2018) Degradation of chrysene by enriched bacterial consortium. Front Microbiol 9:1333
Dhote M, Juwarkar A, Kumar A, Kanade GS, Chakrabarti T (2010) Biodegradation of chrysene by the bacterial strains isolated from oily sludge Monika. World J Microbiol Biotechnol 26:329–335
Sawadogo A, Harmonie OC, Sawadogo JB, Kabore A (2014) Isolation and characterization of hydrocarbon-degrading bacteria from wastewaters in Ouagadougou, Burkina Faso. J Environ Prot 5:1183–1196
Thapa B, Kumar A, Ghimire A (2012) A review on bioremediation of petroleum hydrocarbon contaminants on soil. Kathmandu Univ J Sci Eng Technol 8:167–170
Collina E, Bestetti G, Di Gennaro P, Franzetti A, Gugliersi F, Lasagni M, Pitea D (2005) Naphthalene biodegradation kinetics in an aerobic slurry-phase bioreactor. Environ Int 31:167–171
Hassan SED, Desouky SE, Fouda A, El-Gamal MS, Alemam A (2015) Biodegradation of phenanthrene by Klebsiella sp. Isolated from organic contaminated sediment. J Adv Biol Biotechnol 4:1–12
Adnan LA, Sathishkumar P, Yusoff ARM, Hadibarata T, Ameen F (2017) Rapid bioremediation of Alizarin Red S and Quinizarine Green SS dyes using Trichoderma lixii F21 mediated by biosorption and enzymatic processes. Bioprocess Biosyst Eng 40:85–97
Hadibarata T, Kristanti RA (2014) Effect of surfactants and identification of metabolites on the biodegradation of fluoranthene by basidiomycetes fungal isolate Armillaria sp. F022. Bioprocess Biosyst Eng 37:593–600
Kaczorek E, Olszanowski A (2011) Uptake of hydrocarbon by Pseudomonas fluorescens (P1) and Pseudomonas putida (K1) strains in the presence of surfactants: a cell surface modification. Water Air Soil Pollut 214:451–459
Hadibarata T, Tachibana S, Askari M (2011) Identification of metabolites from phenanthrene oxidation by phenoloxidases and dioxygenases of Polyporus sp. S133. J Microbiol Biotechnol 21:299–304
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG1439-044, Malaysian Ministry of Higher Education through Fundamental Research Grant Scheme no. 5F244.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Farraj, D.A., Hadibarata, T., Yuniarto, A. et al. Characterization of pyrene and chrysene degradation by halophilic Hortaea sp. B15. Bioprocess Biosyst Eng 42, 963–969 (2019). https://doi.org/10.1007/s00449-019-02096-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02096-8