Abstract
Polycyclic aromatic hydrocarbon is a toxic recalcitrant environmental pollutant and its removal from the environment is very essential. In this study, a novel S1 strain isolated from the tropical rain forest was identified as Candida species based on 18S rRNA. The pyrene biodegradation was performed by Candida sp. S1. Pyrene was 35% degraded in 15 days. The percentage of pyrene biodegradation increased up to 75% with 24 g L−1 of sodium chloride and decreased along with increasing salinity. Under the acidic condition, the biodegradation was increased up to 60% at pH 5. It was also found that the increasing glucose concentration of more than 10 g L−1 had no significant effect on pyrene biodegradation, while agitation proved to have greater influence. There was a positive relationship between biomass growth and biodegradation rate of pyrene. One pyrene metabolite was identified from the extract solution and analyzed by a thin-layer chromatography, UV–visible absorption and gas chromatography–mass spectrometry. The metabolite found in the pyrene degradation was benzoic acid. Suitable conditions must be found to promote a successful microbial augmentation in liquid culture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds composed of two or more benzene rings fused together. The arrangement of the rings could be straight linear or linear with angular or cluster. Their existence in environment could be natural from volcanic eruptions and forest fire or anthropogenically, i.e., traffic fumes, incomplete combustion of fossil fuel, industries, organic waste, petroleum seepage and cigarette smoke [1]. Due to PAHs’ carcinogenic and mutagenic effects [2], the Environmental Protection Agencies identified 16 compounds of PAHs on the priority contaminant list [3, 4]. The removal of organic pollutants can be achieved by adsorption, photo-degradation, phytoremediation and biodegradation [5,6,7]. Among these, biodegradation is favorable considering the ecological and economical aspects. In the biodegradation process, the chemical structure of molecules that are introduced to the environment can be changed by microorganisms’ enzymes [8]. Higher molecular weight (HMW) PAHs show more resistance to degradation by microorganisms, which indicates their potential as environmental bio-remediators. Pyrene, a four-ring PAH that has low biodegradability and high persistence in the environment, had been listed as a priority pollutant by the United States Environmental Protection Agency (US EPA). Pyrene can easily enter the human body through skin contact and inhalation. It is toxic toward our organs, including kidney and liver. Due to its toxic characteristic and xenobiotic property, its very important to remove PAHs from environment [9].
Various filamentous fungi had been recognized for their ability to eliminate a wide range of PAHs. Filamentous fungi was chosen for as degrader in this research because of fast growing and resistance to various conditions in the environment. The capability of these fungi in the degradation of persistent pollutants mainly depends on its capacity to secrete the specific enzymes [10, 11]. Many factors such as temperature, pH, agitation, initial concentration of PAH, salinity concentration and carbon source concentration can influence the biodegradation of PAHs by microorganisms. Increasing the temperature would increase the solubility and bioavailability of pollutants to microorganisms [3, 12]. Salinity is one of the important factors considering saline soil and sea water salinity. At high salinity concentration, biological treatment performance is very low due to the loss of cell activity and plasmolysis of microorganisms, which cause most microorganisms to die or live at an attenuated level. On the other hand, some microorganisms are tolerant and resistant to a high salinity and these microogranims are potential for removing the pollutants [13]. In this study, Candida sp. S1, isolated fungus from the tropical rain forest of Malaysia, is used to transfer pyrene, a four-ring PAH. The most important parameters that influence the biodegradation process, such as temperature, pH, agitation, salinity, carbon source, and the initial concentration of PAH, were investigated to accelerate pyrene degradation.
Materials and methods
Chemicals
Pyrene was procured from TCI chemical industry, Japan (Table 1). Ethyl acetate, dichloromethane, N,N-dimethylmethanamide, and glucose were purchased from QREC (Asia). Reactive Black 5 and benzoic acid (purity 98%) were purchased from Sigma-Aldrich. Chloramphenicol was obtained from ACROS Organic.
Isolation of fungi
Reactive Black 5 (RB5) dye has a chemical structure similar to PAHs. It has been used as an indicator to screen dye-decolorizing fungi. In preliminary screening, the fungus was inoculated into MEA amended with 200 mg L−1 of RB5 to measure the decolorization ability of isolated fungal strains. The growth and decolorization rate were monitored daily and the efficient decolorizing fungal strain was selected and stored in a refrigerator prior to further use.
Identification of the fungus by 18S rRNA analysis
Genomic DNA of the selected fungal strain was extracted according to the previous method [18]. The 18S rRNA gene fragment was amplified by polymerase chain reaction (PCR) using a set of universal primers: ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC). The PCR conditions (initial denaturation at 95 °C for 5 min; 25 cycles of 30 s at 94 °C, 30 s at 50 °C and 2 min at 72 °C; and final extension at 72 °C for 10 min) were performed in a PCR thermal cycler (Mastercycler Gradient, Eppendorf). The PCR product of 18S rRNA gene was sent to the First BASE Laboratories Sdn. Bhd., Malaysia, for DNA sequencing. The full length sequence of 18S rRNA genes was submitted to BLAST from NCBI to compare with the nucleotide sequences from Genbank database, and phylogenetic trees were constructed using MEGA4 software.
Microorganisms and culture medium
Our preliminary experiment revealed that Candida sp. S1 was not able to consume pyrene as the sole carbon source or nutrient. Additional nutrient was needed to support the initial degradation of pyrene. Candida sp. S1 was cultured into 20 mL liquid culture and incubated at room temperature. Various liquid media were tested to check the degradation performance as shown in Table 2. Pyrene was completely degraded within 25 days in mineral extract broth (MEB) medium. The composition of growth medium MEB is shown in Table 3. The ability of this isolate to utilize various sugars as co-substrate in pyrene degradation was examined (Table 4). The stock solution of pyrene with a concentration of 20 mg L−1 was prepared by dissolving pyrene in 10 mL N,N-dimethylmethanamide. To prevent bacterial growth, 200 mg L−1 of chloramphenicol was added to the medium. A small piece (~5 mm diameter) of fungus mycelia taken from the solid cultured media was subsequently inoculated into 100 mL flasks containing pyrene solution. All experiments were performed in duplicate and evaluations for each parameter were summarized as shown in Table 5.
Sample extraction
20 mL of ethyl acetate was added to each sample and shaken at 100 rpm for 24 h to extract pyrene from the liquid medium. The liquid portion was separated from the fungal biomass using a filter paper (4A, 7 cm diameter) and the biomass was rinsed with 20 mL of ethyl acetate. The fungal biomass were dried in oven (80 °C) for 24 h, then extracted with 20 mL of ethyl acetate. The extracted pyrene in ethyl acetate was purified by 100 mL of dichloromethane in silica gel and the volume was reduced to 10 mL.
Instrumental analysis
The samples were injected into the Agilent Technology 7820A GC system with the following conditions: the injection volume, 1 µL; the oven temperature heated to 100 °C and held for 2 min, increasing to 260 °C in 30 °C min−1; the column type, HP5; length, 25 m; the inside diameter, 0.1 mm. Derivatization of carboxylic acids for gas chromatography–mass spectrometry (GC–MS) analysis was performed with a trimethylsilyl method [14, 15]. The suspected compound was compared with pyrene in terms of retention time. The retention time for pyrene is 6.625 ± 0.006 min. The MS of the sample was compared with the corresponding authentic standards and the mass spectra library of Wiley 275L.
Results and discussion
Screening of fungi strains
The collected fungal strains (AH3 and S1) showed different ability to decolorize RB5, respectively (data not shown). This result shows that tropical fungi species are different in terms of their ability to decolorize dyes. A similar result was noticed by Nor et al. [16].
Microscopic features of isolate fungal strain S1
The macroscopic features of fungal isolate S1 are smooth and pasty appearance, glistening or dry, wrinkled and dull, and creamish to yellowish color. The microscopic appearance of fungal isolates is shown in Fig. 1. The blastoconidia appeared as small clusters with elongation and the pseudohyphae are long and branched.
Phylogenetic tree analysis
The phylogenetic tree was established using BLAST-Webpage and MEGA4 Software to determine the phylogenetic relatedness of operational taxonomic units (OTUs) [17]. The identifiable homologous characters (nucleic acid bases) of the S1 18s rRNA gene sequence were aligned and only 11 homologous sequences (include S1 sequence) were used for the phylogenetic tree construction in MEGA4. The phylogenetic tree that has been depicted using BLAST-Webpage using neighbor-joining method showed that S1 was closely related to the Candida sp. This is a confirmation that the S1 belonged to Ascomycota yeast, class Saccharomycete, genus Candida as shown in Fig. 2.
Influence of culture conditions on pyrene biodegradation
Effect of temperature and pH on pyrene degradation by Candida sp. S1
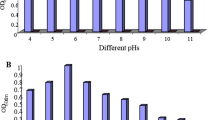
The biodegradation of pyrene and fungal biomass growth at 20, 28 and 37 °C in 15 days were 19, 33 and 10% and 2.4, 3.5 and 1.9 g L−1, respectively, as shown in Fig. 3a. This result clearly shows that the optimum temperature for biodegradation and growth was 28 °C. The biodegradation of RB5 and growth of fungi was decreased in temperature higher and lower than 28 °C. This may be because Candida sp. S1 is considered to be a mesophilic yeast, which prefers 28 °C for best growth and biodegradation. This result is consistent with biodegradation of organic pollutants such as dye, phenol and pyrene by other species of Candida [11, 18,19,20]. The optimum temperature was 30 °C for the growth and biodegradation of many PAHs including four rings by Phanerochaete chrysosporium [3]. The temperature has great effect on the biodegradation of compounds in the aqueous phase. Increasing the temperature would increase the solubility and bioavailability of pollutants to microorganisms. On the other hand, increase of temperature to some level would reduce the solubility of oxygen, which affects the microorganisms’ growth and PAH biodegradation rate. From Fig. 3, it can be concluded that there is a positive relationship between Candida sp. S1 biomass growth and biodegradation rate of pyrene.
The influence of pH on the biodegradation of pyrene and biomass growth was investigated at a range of pH from 3 to 6. The maximum rate of biodegradation (46%) was achieved at pH 5 with 8 g L−1 biomass growth as shown in Fig. 3b, whereas it was decreased at pH values 6 and 3. This result matches with the previous researches [20, 21]. Sood et al. reported that Candida digboiensis can transfer pyrene at pH 3, which may be due to the development of the mechanism of adaptation of this fungus [12]. In general, fungi grow faster under acidic condition than neutral condition, while in alkaline condition the growth was slow due to the inhabitation of fungal growth [22]. Many members of the Candida genus could produce organic acids and decrease the pH of the medium. Therefore, the pH of the medium was checked during the growth of the organism in liquid medium. The result showed that the pH of the medium was slightly decreased; however, it will not affect the biodegradation process.
Effect of agitation and glucose concentration on pyrene degradation by Candida sp. S1
The biodegradation rate of pyrene was 31 and 46% in 15 days in the stationary and agitation phase as shown in Fig. 4a. In the agitation phase, the growth increased from 3.5 to 6.5 g L−1. Agitation has two main roles in microorganisms’ growth and biodegradation rate. Firstly, it increases the contact between the reagents (substrate, oxygen, and biomass) which enhances the mass transfer between microorganisms’ cells and the medium [14]. Secondly, it increases the dissolved oxygen in the liquid medium which is responsible for pyrene oxidation. This result is similar to the biodegradation of pyrene by Armillaria [15].
The biodegradation rate reached 25, 30, 32, 34 and 35% at 2.5, 5, 10, 20 and 30 g L−1 glucose, respectively, while the biomass growth reached 2.5, 3.7, 4.1 and 4.3 g L−1, respectively, as shown in Fig. 4b. In non-glucose addition, there was no biomass growth, and biodegradation of pyrene was observed. This may be because Candida sp. S1 could not utilize pyrene as a sole source of carbon and energy at the beginning of the culture. The microorganism needs glucose for the initial growth; then they consume pyrene as a source of carbon or energy. According to Peng et al., there is no absolute evidence that fungi can utilize PAH as growth substrate [23]. On the other hand, the addition of carbon sources (such as glucose, cellulose and sawdust) can induce a rapid increase of fungal biomass. The addition of glucose enhanced the biodegradation of pyrene up to 42% comparative to 28% without glucose [24]. The relatively fixed biodegradation rate and biomass growth on increasing the amount of glucose additive may be because the glucose amount was enough for fungi growth of Candida sp. S1.
Effect of salinity and pyrene concentration on pyrene degradation by Candida sp. S1
Salinity is one of the important factors that affect the biodegradation of pollutants in industrial saline wastewater, saline soil and sea water. The performance of biological treatment in saline condition is usually low due to the adverse effect of salt on the microbial flora. In choosing a suitable fungal species in the remediation of beaches, marshes and saline soils contaminated with PAH, it is important to assess the inhibitory effect of salinity on the biodegradation process. The transformation of pyrene in 15 days with different concentrations of sodium chloride 0, 24, 48 and 72 g L−1 reached 32, 42, 29 and 30%, while the biomass reached 4.1, 6.3, 9.5 and 8.9 g L−1, respectively, as shown in Fig. 5a. The best biodegradation of pyrene was reached when sodium chloride concentration was 24 g L−1; increasing the salinity concentration can enhance the growth rate but decrease the biodegradation. It was noticed that the biomass growth rate was slow at the beginning of the incubation period <7 days (data not shown) and a small amount of biomass was detected; however, it increased sharply after 7 days compared to zero salinity which has fixed biomass growth rate. This may be due to the adaptation ability of Candida sp. S1 to high salinity. This result was simmilar with previous result that some indigenous microbial communities have the ability to adapt to high salinity concentration and transfer PAH [25]. Mastuda et al. studied the response to sodium chloride variation among different fungal species and that growth of some fungal species was not affected by increasing salinity due to their tolerance [26]. These results indicate that Candida sp. S1 can be a good candidate for the bioremediation of PAH in water containing high salinity. The different concentrations of pyrene (10, 20, 30 and 40 mg L−1) showed the biodegradation of pyrene at 31, 35, 31 and 19% in 15 days, while the biomass reached 3, 3.5, 3.2 and 1.8 g L−1, respectively, as shown in Fig. 5b. Increasing the concentration of pyrene can cause the reduction of biodegradation rate and biomass growth due to toxicity.
Analysis of metabolites
The metabolite has a R f value of 0.29 and UV characteristics (λ max, 226 and 275 nm), simmilar with the benzoic acid standard. The retention time (t R) of metabolite was at 8.2 min, had M+ at m/z 282 and fragmentation ions at m/z 267 (M+-15), sequential loss of methyl (–CH3), and 193 (M+-89), sequential loss of –OSi (CH3)3, as well as the expected fragment ions at 147, 209, and 73 [(CH3)3Si] (Table 6). Based on the result obtained above, we determined the metabolite of pyrene by Candida sp. S1 as shown in Fig. 6. Candida sp. S1 is grown on pyrene as the source of carbon and energy with contribution of additional nutrient at the initial degradation; the initial attack occurs at the fourth and fifth position, by dioxygenase, to form a hydrodiol, which is further converted to 4,5-dihydroxyphenanthrene. 4,5-Dihydroxyphenanthrene can be further degraded into a major product, 1-hydroxy-2-naphthoic acid. 1-Hydroxy-2-naphthoic acid can be further transformed to form benzoic acid.
Conclusions
Candida sp. S1, screened from the tropical forest in Malaysia, was selected based on its ability to decolorize RB5. It was used to transfer the pyrene, a four-ring PAH. Through this research, parameters such as temperature, agitation, pH, glucose concentration, salinity and initial pyrene concentration were studied to investigate their influence on pyrene biodegradation. The optimum temperature for biodegradation and growth was 28 °C, whereas agitation at 80 rpm had a positive influence on the biodegradation and growth. The pyrene biodegradation and Candida sp. S1 growth were enhanced under acidic conditions. Candida sp. S1 could not utilize pyrene as a sole source of carbon and energy; thus, adding glucose would enhance the growth and biodegradation rate. Candida sp. S1 growth increased with increase in the salinity, while the maximum pyrene biodegradation reached 24 g L−1. In final conclusion, Candida sp. S1 is a potential candidate for pyrene bioremediation under saline and acidic environment. The metabolites showed that the initial attack occurred at the fourth and fifth positions of pyrene by dioxygenase to form a hydrodiol and it was further converted to dihydroxypyrene. Dihydroxypyrene can be further degraded into a major product, 1-hydroxy-2-naphthoic acid. 1-Hydroxy-2-naphthoic acid can be further transformed to form benzoic acid.
References
Gallego E, Roca FJ, Perales JF, Guardino X, Berenguer MJ (2008) VOCs and PAHs emissions from creosote-treated wood in a field storage area. Sci Total Environ 402:130–138
Wilhelm M, Hardt J, Schulz C, Angerer J (2008) New reference value and the background exposure for the PAH metabolites 1-hydroxypyrene and 1- and 2-naphthol in urine of the general population in Germany: basis for validation of human biomonitoring data in environmental medicine. Int J Hyg Environ Heal 211:447–453
Bishnoi K, Kumar R, Bishnoi NR (2008) Biodegradation of polycyclic aromatic hydrocarbons by white rot fungi Phanerochaete chrysosporium in sterile and unsterile soil. J Sci Ind Res 67:538–554
Moradi SE (2013) Naphthalene removal from water by novel mesoporous carbon nitride adsorbent. Chem Biochem Eng Q 27:365–372
Vela N, Martínez-Menchón M, Navarro G, Pérez-Lucas G, Navarro S (2012) Removal of polycyclic aromatic hydrocarbons (PAHs) from groundwater by heterogeneous photocatalysis under natural sunlight. J Photochem Photobiol A 232:32–40
Cheema SA, Khan MI, Tang X, Zhang C, Shen C, Malik Z, Ali S, Yang J, Shen K, Chen X, Chen Y (2009) Enhancement of phenanthrene and pyrene degradation in rhizosphere of tall fescue (Festuca arundinacea). J Hazard Mater 166:1226–1231
Wirasnita R, Hadibarata T, Yusoff ARM, Yusop Z (2014) Removal of bisphenol A from aqueous solution by activated carbon derived from oil palm empty fruit bunch. Water Air Soil Pollut 225(2148):1–12
Lei AP, Hu ZL, Wong YS, Tam NFY (2007) Removal of fluoranthene and pyrene by different microalgal species. Bioresour Technol 98:273–280
Xu SY, Chen YX, Lin KF, Chen XC, Lin Q, Li F, Wang ZW (2009) Removal of pyrene from contaminated soils by white clover. Pedosphere 19:265–272
Hadibarata T, Tachibana S, Itoh K (2007) Biodegradation of phenanthrene by fungi screened from nature. Pak J Biol Sci 10:2535–2543
Wang X, Gong Z, Li P, Zhang L (2007) Degradation of pyrene in soils by free and immobilized yeasts, Candida tropicals. Bull Environ Contam Toxicol 78:522–526
Sood N, Patle S, Lal B (2010) Bioremediation of acidic oily sludge-contaminated soil by the novel yeast strain Candida digboiensis TERI ASN6. Environ Sci Pollut Res 17:603–610
Lefebre O, Moletta R (2006) Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res 40:3671–3682
Hadibarata T, Kristanti RA (2014) Potential of a white-rot fungus Pleurotus eryngii F032 for degradation and transformation of fluorene. Fungal Biol 118(2):222–227
Hadibarata T, Kristanti RA (2013) Biodegradation and metabolite transformation of pyrene by basidiomycetes fungal isolate Armillaria sp. F022. Bioprocess Biosyst Eng 36(4):461–468
Nor NM, Hadibarata T, Zubir MMFA, Lazim ZM, Adnan LA, Fulazzaky MA (2015) Mechanism of triphenylmethane Cresol Red degradation by Trichoderma harzianum M06. Bioprocess Biosyst Eng 38(11):2167–2175
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) Software Version 4.0. Mol Biol Evol 24:1596–1599
Liu X, Zhang J, Jiang J, Li R, Xie Z, Li S (2011) Biochemical degradation pathway of reactive blue 13 by Candida rugopelliculosa HXL-2. Int Biodeterior Biodegrad 65:135–141
Adav SS, Chen MY, Lee DJ, Ren NQ (2007) Degradation of phenol by aerobic granules and isolated yeast Candida tropicalis. Biotechnol Bioeng 96:844–852
Tiwari S, Gaur R, Singh S (2012) Decolorization of a recalcitrant organic compound (Melanoidin) by a novel thermotolerant yeast, Candida tropicalis RG-9. BMC Biotechnol 12:30
Ghoshal G, Banerjee UC, Shivhare US (2014) Xylanase production by Penicillium citrinum in laboratory-scale stirred tank reactor. Chem Biochem Eng Q 28:399–408
Wang J, Ma X, Liu S, Sun P, Fan P, Xia C (2012) Biodegradation of phenol and 4-chlorophenol by Candida tropicalis W1. Proc Environ Sci 16:299–303
Peng RH, Xiong AS, Xue Y, Fu XY, Gao F, Zhao W, Tian YS, Yao QH (2008) Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev 32:927–955
Wen J, Gao D, Zhang B, Liang H (2011) Co-metabolic degradation of pyrene by indigenous white-rot fungus Pseudotrametes gibbosa from the Northeast China. Int Biodeterior Biodegrad 65:600–604
Kerr RP, Capone DG (1988) The effect of salinity on the microbial mineralization of two polycyclic aromatic hydrocarbons in estuarine sediments. Mar Environ Res 26:181–198
Matsuda Y, Sugiyama F, Nakanishi K, Ito S (2006) Effects of sodium chloride on growth of ectomycorrhizal fungal isolates in culture. Mycoscience 47:212–217
Acknowledgements
This research was financially supported by a Fundamental Research Grant Scheme, Ministry of Education, Malaysia (No. 4F813) and Research University Grant, Universiti Teknologi Malaysia (No. 13H93), which are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hadibarata, T., Khudhair, A.B., Kristanti, R.A. et al. Biodegradation of pyrene by Candida sp. S1 under high salinity conditions. Bioprocess Biosyst Eng 40, 1411–1418 (2017). https://doi.org/10.1007/s00449-017-1798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1798-7