Abstract
In this study, the effects of organic loading rate (OLR) and the addition of powdered activated carbon (PAC) on the performance and membrane fouling of MBR were conducted to treat real pharmaceutical process wastewater. Over 145 days of operation, the MBR system was operated at OLRs ranging from 1 to 2 kg COD m−3 day−1 without sludge wasting. The addition of PAC provided an improvement in the flux, despite an increase in the OLR:PAC ratio. The results demonstrated that the hybrid PAC-MBR system maintained a reduced amount of membrane fouling and steadily increased the removal performance of etodolac. PAC addition reduced the deposition of extracellular polymeric substance and organic matter on the membrane surface and resulted an increase in COD removal even at higher OLRs with low PAC addition. Membrane fouling mechanisms were investigated using combined adsorption fouling models. Modified fouling index values and normalized mass transfer coefficient values indicated that predominant fouling mechanism was cake adsorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater from the pharmaceutical manufacturing industry contains residuals from synthesized pharmaceutical active compound (PhAC) solvents and their intermediates [1]. Many of these compounds cannot be completely removed due to their non-biodegradable properties and their toxic effects on biological wastewater treatment plants. In addition, the compounds can enter the environmental ecological cycle. It is important to effectively remove these contaminants to protect the environment, drinking water resources and human health [2]. In the recent years, the impact of some PhACs on public health has been investigated. However, the health effects of a mixture of PhACs are still unknown. Because conventional water and wastewater treatment processes alone cannot serve as a safe guard toward PhACs in pharmaceutical wastewaters, it is essential to apply additional advanced treatment technologies [3, 4].
Membrane bioreactors (MBRs) are increasingly being used as alternatives to the conventional activated sludge treatment process. The distinctive characteristics of MBRs are better quality treated effluent that is suspended solids free with low organic matter concentrations, and less excess sludge production due to a high sludge retention time (SRT). Also, MBRs improve the removal of low biodegradable pollutants in the wastewater due to high sludge-substrate contact times. Various studies have indicated that the removal of trace organic matters by MBRs is higher and or more consistent compared to the activated sludge process [3, 5, 6]. Many PhACs are highly hydrophilic. Consequently, the adsorption of PhACs onto the sludge is limited, which limits the degradation of these compounds by bacteria in the biological treatment process [7]. Previous studies have indicated considerable variations in the removal of PhACs by MBR. The removal of certain PhACs (e.g., ibuprofen, bezafibrate, ketoprofen, acetaminophen and paroxetine) was achieved by adsorption or degradation in the MBR. However, the removal of some PhACs (e.g., carbamazepine, diclofenac) was incomplete [5, 8]. As a result, the activated sludge process, or the MBR, is not sufficient for the complete removal of all of the PhACs. These findings have pushed scientists to research the applicability of hybrid treatment systems, including the advanced oxidation and adsorption processes.
Hybrid MBR processes, which include the advanced oxidation (UV/O3/H2O2) and adsorption processes (powdered activated carbon—PAC), are used to prevent the spread of PhACs into the environment due to the insufficient treatment processes [3]. The use of advanced oxidation processes should be evaluated because of the by-products formed after oxidation. The hybrid PAC-MBR process increases the removal efficiency by adsorbing non-biodegradable, toxic and inhibitory organic matters [9]. Fouling of submerged membranes decreases because almost all of the organics are removed by PAC. The role of the membrane is only to retain the PAC and other suspended solids. The energy requirement is notably low and there is no major sludge problem [10]. In addition, membrane fouling decreases because some of the high-molecular-weight organic matters are adsorbed onto the macropores in the PAC [11]. Li et al. [12] indicated a reduction in membrane fouling using the hybrid PAC-MBR system over long-term operation. These researchers determined that the operating intervals were extended by approximately 1.8-fold compared to that without the PAC. Membrane fouling is a restricting factor in operating MBR systems. The mathematical modeling is used to clarify membrane fouling mechanism. In the literature, there is a lot of work about flux decline modeling in the MBR systems [13]. To our knowledge, flux decline modeling for MBR-PAC systems was not researched in detail.
The aim of this study was to introduce the treatment of real pharmaceutical process wastewater from PhAC (etodolac) production by the direct addition of PAC into a MBR (hybrid PAC-MBR system). The effects of organic loading rate (OLR) and PAC addition on MBR performance and membrane fouling were also investigated at infinite SRT. Membrane fouling was analyzed with Scanning Electron Microscope (SEM), Atomic Force Microscope (AFM) and Fourier Transform Infrared spectroscopy (FT–IR). Levels of chemical oxygen demand (COD) and etodolac were measured to evaluate the performance of the hybrid PAC-MBR system. Measurements of extracellular polymeric substances (EPSs), soluble microbial products (SMPs), zeta potential, and contact angle were also performed to explain their variation with OLR. The flux decline was evaluated using combined adsorption models with various pore blocking models in hybrid MBR-PAC system. So, the main fouling mechanism was overviewed.
Materials and methods
Wastewater
The wastewater used in this study was obtained from a pharmaceutical company involved in the chemical synthesis and manufacturing of PhACs. The wastewater was generated from equipment cleaning during the production of etodolac active matter. The characteristics of the wastewater are given in Table 1.
Seed sludge was obtained from the aeration tank in the wastewater treatment plant from the same company. The sludge was acclimatized and adapted to the MBR. The wastewater was stored at 4 °C. To maintain a COD/N/P ratio of 100/5/1, nitrogen and phosphate sources were added as NH4Cl and KH2PO4, respectively. 0.19, 0.29, 0.38 g L−1 NH4Cl and 0.03, 0.04, 0.06 g L−1 KH2PO4 were fed into the MBR for the OLR values of 1.0 kg COD m−3 day−1, 1.5 kg COD m−3 day−1 and 2.0 kg COD m−3 day−1, respectively.
Powdered activated carbon (PAC)
Throughout the experiments, a commercial activated carbon (WAC i600 M200), purchased from Eurocarb Products Ltd, Bristol, UK was used. WAC i600 M200 is a powdered wood-based activated carbon and is produced from specially selected feedstock [14]. The activated carbon was first rinsed with pure water, and then boiled for 3 h. Then, it was dried at 120 °C for 4 h and stored in a desiccator. The adsorption capacity of PAC was determined with batch adsorption tests before its addition to the MBR. Adsorption tests were performed at 25 ± 2 °C in a thermostated orbital shaker with an agitation speed of 200 rpm with 100 mL of a known initial concentration (1500 mg COD L−1). For the batch adsorption tests, the concentrations of the used PAC ranged between 0.5 and 10 g L−1. The adsorption capacity of the PAC was determined to be 720 mg COD per g of PAC at an equilibrium time of 72 h and an optimum adsorbent dosage of 2 g L−1.
Laboratory scale MBR system and experimental conditions
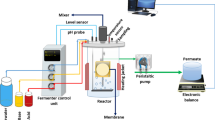
The laboratory scale MBR system used in this study is schematically shown in Fig. 1. The system is 34 cm high and has a 16 cm inner diameter. The system is made of a cylindrical plexiglass; with a working volume of 7 L. A submerged kestamid frame module was used. The polyethersulfone (PES) microfiltration membrane (FM MP005) was purchased from Microdyn-Nadir GmbH and was used in this study. The nominal pore size of the membrane is 0.05 µm, and the membrane module has an effective surface area of 74 cm2. The transmembrane pressure was kept constant at 200 mbar throughout the experiments. Compressed air was supplied by a membrane diffuser at a rate of 8–10 L min−1. The reactor was aerated from the bottom to supply oxygen for the microorganisms and to scour the membrane surface. The dissolved oxygen (DO) concentration in the bioreactor was 4.6 ± 1.1 mg L−1. A constant system temperature was maintained (25 ± 1 °C) using an adjustable heater. pH was automatically adjusted (7.3 ± 0.2) by acid and/or base dosing pumps (DMI208, Grundfos-Alldos).
The system required the use of two peristaltic pumps (Watson Marlow 323 E). Wastewater was continuously fed to the reactor, and permeate was continuously withdrawn from the plate module with these pumps. The permeate flow rate was measured every minute by an electronic balance and recorded by a computer via a PCI card and an RS 232 line. The system was backwashed by air. Automatic feeding of the wastewater to the reactor and the backwashing of the membrane module with air, were carried out by a control unit (Hach-Lange SC1000). The wastewater feeding time was chosen as 4 h and the backwashing duration was 5 min. The permeate flux is described by Darcy’s law:
where J is the permeate flux, A is the membrane filtration area, V is the total volume of permeate and t is the filtration time.
During the 145 days of operating the MBR system, OLRs were varied from 1 to 2 kg COD m−3 day−1 at an infinite SRT. No sludge was withdrawn from the system during the experimental periods except for sampling. The MBR system was studied under constant pressure mode (200 mbar). Therefore, hydraulic retention time (HRT) values in the MBR system varied depending on the flux values. The MBR was operated without PAC at OLR = 1 kg COD m−3 day−1 for the first 20 days. On the 20th day, 2 g L−1 of PAC was added directly to the reactor considering the optimum PAC dosage obtained from the batch studies. One of the aims of the study is to operate the system up to 50–55 % value of the initial flux. Also, 2 g L−1 of PAC addition was repeated each time with increasing OLR to investigate the effect of organic load. Considering the adsorption capacity of the PAC and flux decline, it was assumed that PAC in the system was saturated. Also, the OLR:PAC ratio was calculated with 2 g L−1 of PAC. Consequently, three OLR:PAC ratios of 0.5, 0.75, and 1 were studied during the operation of the MBR-PAC hybrid system.
Flux decline modeling
In this section, it was aimed to clarify the effective fouling mechanisms causing flux decline using modeling studies. J was modeled using the following Eq. (2) [15]:
Equation (2) was expressed as \(J = \left( {a + b \cdot t} \right)^{ - (1/2)} .\) a and b were determined by means of non-linear curve fitting of J values using Eq. (2). The SigmaPlot 9.01 (Systat Software Inc.) model program was used for the regression calculations. Compatibility between experiment and model results were evaluated in terms of (r 2) and root mean squared error (RMSE) values.
Modified fouling index (MFI) parameter was also used to evaluate the membrane fouling. MFI was determined from a plot of filtration time (t) per total permeate volume (t/V) versus total permeate volume (V) and its definition as follows:
According to Eq. (2), MFI was calculated as one-fourth of b model constant.
In addition, combined adsorption models as cake-adsorption, intermediate-adsorption and complete—adsorption developed by Bolton et al. [16] were used to investigate membrane fouling mechanisms. The equations of the models were given Table 2.
Analysis and experimental methods
Mixed liquor suspended solids (MLSS), mixed liquor volatile suspended solids (MLVSS), and COD analyses were performed using Standard Methods [17]. Etodolac was determined using the HPLC–MS–MS method in an accredited laboratory (ALS—Czech Republic). The API 4000 LC/MS/MS (AB Sciex, UK) unit, in the MRM-mode with 2 MRM—transitions, was used for each analyte. The detection limit of method was 0.05 µg L−1.
The EPS extraction process was carried out according to Frølund et al. [18]. The extractable portions of the EPS were extracted using a strong cation exchange resin (DOWEX Marathon C). These samples were stored at 4 °C prior to analysis for protein and polysaccharide fractions. The concentrations of both protein and polysaccharide fractions of the SMPs and EPSs were determined using methods proposed by Lowry et al. [19] and Dubois et al. [20], respectively. All samples were analyzed using a UV–vis spectrophotometer (PG Instruments T60U, UK) at a wavelength of 750 nm for proteins and 480 nm for polysaccharides.
The zeta potential of the sludge was determined using the Laser Doppler and Phase Doppler techniques by Malvern Zetasizer Nano ZS90 (UK).
The contact angles of the clean and fouled membranes were measured using the ‘sessile drop’ method with a goniometer (KSV Instruments, CAM 101, Finland) based on the procedure as defined by Kaya et al. [21]. The functional group characteristics of the membrane specimens were characterized using an FT–IR spectrometer (Perkin–Elmer Spectrum One FT–IR, USA).
The AFM was used to analyze the surface morphology and roughness of the clean and fouled membranes (NanoScope IV, Digital Instruments, US). The mean roughness (R a) and the root mean square (R rms) of the average height of membrane surface peaks were calculated to elaborately compare the roughness of clean and fouled membranes. The mean roughness (R a) is the mean value of surface relative to the center plane and is calculated as follows:
where z av is the average of the z values within the given area; z cu, the current z value; and p, the number of points within a given area. The root mean square of z values (R rms) is calculated by using Eq. (5):
Also, the mean difference in the height between the five highest peaks and the five lowest valleys (R z) was determined as relative to the mean plane, about which the image data has a minimum variance [21].
The cross-sectional images of the clean and fouled membranes were taken by SEM (JEOL/JSM-6335F-INCA, Japan).
Results and discussion
MBR–PAC performance at different OLR:PAC ratios
Flux
The variations in the permeate flux of the MBR system at different OLRs over the 145-day period is presented in Fig. 2. The flux decreased from 9 to 5 L m−2 h−1 in the first 20 days of operation without PAC addition (OLR = 1 kg COD m−3 day−1). After the first addition of PAC (20th day of operation (OLR = 1 kg COD m−3 day−1, OLR:PAC = 0.5), the membrane filterability improved within 5 days. The flux increased from 5 to 14 L m−2 h−1. Although the flux increased 2.8-fold due to PAC addition, a continuous decrease was observed for the following 30 days of operation. The second PAC addition of 2 g L−1 was applied on the 60th day of operation, and the OLR was increased to 1.5 kg COD m−3 day−1 (OLR:PAC = 0.75), With the addition of PAC, the flux improved by 1.5-fold, but the overall decreasing trend in flux could not be stopped. When the flux reached 7 L m−2 h−1, a PAC addition of 2 g L−1 was applied. Simultaneously, the OLR was increased to 2 kg COD m−3 day−1 (OLR:PAC = 1). As clearly seen in the Fig. 2, the improvement in the flux was less than the prior two additions. In the subsequent 23 days (80th–103rd days of reactor operation), a flux decline from 10.1 to 5.5 L m−2 h−1 was observed. On the 118th day, the flux reached 5.5 L m−2 h−1, and over the next 27 days, the flux remained constant. It should be noted that the system was operated without any physical or chemical cleaning procedures. The only procedure used was to scour the membrane surface continuously with air. Also, the same membrane was used over the 145-day operation period.
The role of PAC in upgrading flux depends on the amount of PAC, and the type and concentration of organic matters [22]. The data obtained from this work showed that PAC addition provided an improvement in the flux despite an increase in OLR, without membrane change or physical/chemical cleaning during the 145 days of operation. With a ratio of OLR:PAC = 0.5 an instantly but temporary improvement of app. threefolds in the flux of the system was obtained. The flux of 14 L m−2 h−1 was the highest value for the hybrid PAC-MBR system. As discussed in detail in “Flux decline modeling”, the cake-adsorption fouling mechanism was predominant in consequence of gel layer formation on the membrane surface in the MBR system without PAC. With the first addition PAC to the system (OLR:PAC = 0.5), the gel layer formation was decreased and the highest flux was obtained. Intermediate-adsorption mechanism was effective for this time interval. As indicated in another research [11], it could be said that the presence of PAC particles on the membrane surface reduces the boundary layer thickness, hence resulting in an increase in the flux. EPS concentration was found as a major factor causing membrane fouling by formation a barrier to the permeate flow. The improvement in flux with PAC addition could be attributed also to the decrease in the concentrations of EPS and SMP (detailed in “EPS and SMP”). In addition, pore blocking before the PAC addition could be seen from SEM images (in detail in “Analytical measurements”). Also, the SEM images supported to experimental flux decline results and estimated flux modeling coefficients. With PAC addition the shrinkage of the pores was decreased and caused increase in flux. The results obtained from AFM measurements (detailed in “Analytical measurements”) indicated also the difference between the two systems. Surface enrichment of EPS causing flux decline was decreased with PAC addition. It can be said that hybrid PAC-MBR system showed improvement in flux as a result of adsorption of EPS and other organics onto PAC. So, the positive effect of PAC on the flux could be attributed to it acting as a filter to reduce foulants in the bulk solution by adsorption, and by causing flocculation of the dissolved organics, fine particles and colloids [12]. This behavior decreases membrane fouling. However, this enhancement was only temporary. It was assumed that the added PAC was gradually saturated with the dissolved organics. Also, 2 g L−1 of PAC addition was repeated when the flux reached up to 50–55 % value of the initial flux. The observed flux decline after repeated PAC addition could be also attributed to the increase in the OLR:PAC ratio. Although the contribution of PAC to flux was diminished due to limited adsorption capacity, saturated PAC has a positive effect on filterability as a preventive factor [23]. As a result, a ratio of OLR:PAC = 0.5 is required to prevent fouling of the membranes by cake adsorption.
HRT values in the MBR system varied depending on the flux values. In the first 20 days of operation without PAC addition (OLR = 1 kg COD m−3 day−1) HRT increased from 4.6 to 7.9 h. After the first addition of PAC (OLR:PAC = 0.5), HRT dropped instantly to 2.7 h and increased in 38 days to 4.9 h. With OLR:PAC ratio of 0.75, HRT decreased to 3.2 h. However, at the end the of the OLR:PAC = 0.75 operation, HRT increased to 7.2 h. HRT dropped to 3.9 h with the PAC addition at OLR:PAC = 1. Consequently, it increased gradually to 7.9 h with decrease in flux. The results clearly indicated that HRT increased with decrease in flux at all the OLR:PAC ratios. Also, the effect of HRT on COD removal was explained in “Removal of COD and etodolac at different OLR:PAC ratios”.
Flux decline modeling
The results obtained from combined adsorption models are given in the Table 3. According the Table 3, all model results showed good correlations for r 2 and RMSE in all days except for 104–145 days. In the first 20 days of operation without PAC addition, the gel layer formation caused the cake-adsorption fouling mechanism based on the normalized mass transfer coefficients. After PAC addition to system, while intermediate-adsorption was effective between 20 and 59 days (OLR: 1 kg COD m−3 day−1, OLR:PAC = 0.5) gel layer formation were determined as predominant mechanism during 60–145 days (OLR:PAC = 0.75 and OLR:PAC = 1).
MFI is mainly used to clarify the cake formation caused by particles in the feed water. But when organic fouling was also occurred, pore blocking and pore adsorption can be occurred in the membrane [24]. According to MFI values given in the Table 3, more fouling was observed in the first 20 days without PAC addition. This indicates that cake formation on the membrane was the predominant fouling mechanism. Normalized mass transfer coefficients and MFI values were also supported each other. After the first PAC addition, MFI value was sharply decreased from 15.75 to 3.70 depending on the decreasing of gel layer formation. However, cake-adsorption mechanism was again predominant mechanism with increasing OLR:PAC ratio. But, both MFI values and normalized mass transfer coefficient values caused by cake-adsorption formation with PAC addition were not ever higher than values obtained by PAC addition.
Removal of COD and etodolac at different OLR:PAC ratios
The COD removal profile in the MBR is given in Fig. 3. The system was operated at OLRs of 1, 1.5 and 2.0 kg COD m−3 day−1 without PAC and with OLR:PAC values of 0.5–1. Table 4 shows the performance of the MBR system at various OLR:PAC ratios. Initially, the removal of COD in the MBR system without PAC addition was 81 %. The removal rate subsequently increased and reached steady-state at 91 %. After the first addition of 2 g L−1 PAC (20th day of the operation, OLR = 1 kg COD m−3 day−1, OLR:PAC = 0.5), the increasing trend for the COD removal rate reached further to 98 %. However, after a period of 15–17 days, the COD removal efficiency decreased to 85 %. On the 60th day of the operation, the OLR was increased to 1.5 kg COD m−3 day−1, and 2 g L−1 of PAC addition to the system was repeated (OLR:PAC = 0.75). COD removal initially increased to 95 %, and then decreased to 89 %. With the third PAC addition (at an OLR value of 2.0 kg COD m−3 day−1 and OLR:PAC = 1), COD removal increased to 97 %, and after 15 days, dropped to 90 %. The decreasing trend in the COD removal rate after approximately 15 days of PAC addition could be attributed to increase in residual COD. With increase in OLR:PAC rate, the amount of PAC was unable to adsorb all the residual COD and reached equilibrium. The OLR:PAC ratio was increased from 0.5 to 1 resulting in a decrease in the PAC amount per organic load. Even so, PAC addition to the MBR system provided an improved removal performance. A steady-state COD removal of 90 % was obtained at the end of the PAC-MBR operation. Considering the variation of the HRT values with change in the flux, the results clearly indicated that COD removal increased with increase in HRT during operation of the system. At the same HRT value of 7.9 h, the PAC-MBR system showed a 6 % higher COD removal than MBR system without PAC. It can be said that PAC addition had a direct effect on the COD removal efficiency of the system.
The performance of the MBR system for etodolac removal, at different OLRs and in the presence of different amounts of PAC, was evaluated (Table 4). In the absence of PAC, and at an OLR of 1 kg COD m−3 day−1, an etodolac removal of 73 % was obtained. In batch adsorption tests, the adsorption of etodolac onto PAC was found to be 98 % (data not shown). After adding PAC directly to the MBR, a sharp increase in etodolac removal efficiency was observed. At OLR:PAC = 0.5, the etodolac removal performance of the MBR increased to 99 %. The rapid increase in the removal efficiency of etodolac (after PAC addition to MBR) showed that the biological sludge had a limited capacity to eliminate etodolac by biodegration and/or biosorption and the hybrid PAC-MBR system provided a better removal performance. Nguyen et al. [6] obtained similar results for several trace organic contaminants including PhACs. In general, it was found fluctuations in the MLSS and/or MLVSS and SMP (detailed in “MLSS and MLVSS” and “EPS and SMP”, respectively) in the hybrid PAC-MBR system with increase of the OLR:PAC ratio. However, only 1–2 % decrease was observed in the removal of etodolac. Also, it could be said that etodolac removal was independent from MLSS and/or MLVSS and SMP. So, it can be said that the increase in the etodolac removal could be attained to the adsorption by PAC. PAC addition to the system (OLR:PAC = 0.5–1) was found effective for etodolac removal with 97–99 %.
It is clear that the addition of PAC improved the COD removal performance for a period of approximately 15 days. The improvement in the system was temporary, and after 15 days, the performance of this system was almost equal to that of the system operated without PAC addition. Considering the increase in OLR, it can be said that a reduction in COD removal (due to high organic loading) was prevented by the addition of PAC despite of the increase in OLR:PAC ratio from 0.5 to 1. In the case of etodolac removal, the improvement in the system remained stable. The increase in the OLR:PAC ratio had a limited effect of 1–2 % for etodolac removal. As a result, even a high OLR:PAC ratio of 1 with low PAC amount per organic load was found sufficient for etodolac removal. This means that the PAC dosing provided stability to the effluent quality in terms of PhAC (etodolac) concentration. Also, at the same HRT value the hybrid PAC-MBR system (PAC:OLR = 1) showed higher etodolac removal rate (97 %) than MBR system without PAC (73 %). Whereas only 6 % of COD removal improvement was with PAC addition. The improvement of etodolac removal could be attributed to the direct contribution of PAC. Various studies have reported that the PAC-MBR hybrid processes provided sustainable and improved system performances for the micropollutants [25, 26].
MLSS and MLVSS
The MLSS and MLVSS profiles are shown in Fig. 4. The MLSS concentration at the beginning of the MBR operation (without PAC addition) was 8.2 g L−1. The value stabilized at 6.5–14.5 g L−1 between 21 and 145 days of operation (OLR:PAC = 0.5–1). The addition of PAC at OLR = 1 kg COD m−3 day−1 (20th day) caused an increase in the MLSS concentration. The MLVSS concentration was 6.7 g L−1 in the seed sludge and varied between 3.9 and 5.9 g L−1 in the first 55 days. An appreciable increase in MLVSS concentration was observed at the end of the operation at OLR:PAC = 0.5. A gradual increase in the MLVSS concentration, from 5.9 to 12.5 g L−1, was observed at the start of operation with OLR:PAC = 0.75. Over the entire MBR operation (145 days of with and without PAC addition) the MLVSS/MLSS ratio ranged between 0.62 and 0.90. In the first 60 days (at OLR = 1 kg COD m−3 day−1, with and without PAC addition) the ratio was between 0.62 and 0.87. Over the last 85 days, the ratio observed was between 0.75 and 0.90. These results demonstrated that the PAC addition had a positive effect on the MLVSS/MLSS ratio. As known, PAC is a volatile solid with MLVSSPAC/MLSSPAC ratio of 75 %. The contribution of PAC to MLSS and MLVSS should be expected. Considering the total 6 g of PAC addition during the study, the additional increase of MLSS, MLVSS and MLVSS/MLSS ratio could be attributed to the positive effect of PAC addition.
EPS and SMP
The protein and carbohydrate fractions of the EPS and SMP were monitored along the entire operation of MBR. These were measured for both the bioreactor and permeate (Fig. 5). EPSp and EPSc refer to the protein and carbohydrate fractions of the extracellular polymeric substances, respectively. The amounts and characteristics of the EPS and SMP in the aerobic sludge are affected by many parameters, including substrate compositions, loading rates, aeration, SRT, and extraction methods [27, 28].
Generally, the EPS content in MBRs had been found ranging from 20 to 250 g kg−1 VSS [29]. It is also difficult to determine the relative contribution of PAC adsorption and microorganisms to the presence of EPS [26]. Several studies have showed the advantages of adding PAC in controlling membrane fouling in the MBR system due to adsorption effects that cause a reduction in EPS, SMP, TOC, fine colloids, and trace organics [12, 30–32]. As seen in Fig. 5, the concentrations of EPS and SMP in the bioreactor with PAC addition were found to be lower compared to without PAC addition. EPSc was found to be greater than EPSp during the overall operation time in the bioreactor. However, SMPp was found to be greater than SMPc in the bioreactor and permeate. Various research results on protein and carbohydrate fractions of the extracellular polymeric substances have also been reported. Ying and Ping [11] reported that the protein fraction of the EPS was greater than the carbohydrate fraction. An increase in PAC addition increased both fractions. In contrast, Chu et al. [28] and Kim et al. [30] found higher carbohydrate content compared to protein content. A sharp decrease in the concentrations of EPSp and EPSc in the bioreactor was observed with the first addition of PAC. This result could be attributed to the adsorption of most of the organic matter and produced EPS onto PAC. However, increase in OLR and OLR:PAC ratio- was resulted in a gradual increase in EPS. EPS concentration was increased because of the decreased PAC amount per organic load. At the end of the OLR = 2 kg COD m−3 day−1 operation, the EPSp and EPSc concentrations in the bioreactor were 85 and 65 % of the original concentrations at the start of the operation. This finding could be attributed to the increase in the OLR:PAC ratio. Also, Meng et al. [33] and Johir et al. [34] have reported that higher values of OLR could lead to an increase in EPS production.
The addition of PAC resulted in a decrease in both SMPp and SMPc concentrations in the bioreactor. This result is consistent with the studies by Munz et al. [25], Meng et al. [33] and Jamal Khan et al. [35]. A decrease in SMP could be partly due to either direct adsorption onto PAC, or the biodegradation by activated carbon [25, 35]. Unlike EPS production, an increase in the SMP production was not observed with increase in OLR:PAC ratio. The protein and carbohydrate fractions of the SMP from the MBR permeate were measured at different OLRs. The concentrations of SMPp were relatively higher than those of SMPc at all OLRs. The SMPp and SMPc fractions of the MBR permeate decreased with increasing OLR and PAC addition, which was similar to the SMP fractions in the bioreactor.
As a result, for all OLRs, the EPS concentration of the bioreactor with PAC was lower than that obtained without PAC. It can be concluded that the variation in the EPS concentration was affected by PAC addition and changes in the OLR:PAC ratio. The first PAC addition of 2 g L−1 (OLR:PAC = 0.5) resulted in a decrease of 88 % in the EPS concentration. An increase in OLR resulted in an increase in the EPS concentration because of decrease in the PAC amount per organic load. However, at the end of the operation even with a higher OLR:PAC ratio of 1, the EPS concentration was still 28 % less than the EPS concentration that was measured at the start without PAC addition. Meng et al. [33] reported that a higher OLR could lead to an increase in the production of EPS. Also, increased EPS production causes membrane fouling and decreased flux. In literature, high EPS concentrations were found to be responsible for the formation of a considerable barrier (cake layer) to the permeate flow due to membrane fouling [36]. As detailed in “Flux decline modeling”, flux modeling supported also the formation of cake layer at higher OLR:PAC ratios with high EPS concentrations. SEM images of the MBR system and hybrid PAC-MBR system could be seen as an evidence of less fouling because of reduced EPS concentrations by PAC adsorption (detailed in “Analytical measurements”). Also, our results demonstrated that EPS production in the bioreactor could be limited and controlled with PAC addition. PAC helped to slow down the membrane fouling rate by reducing the deposition of EPS and organic matter on the membrane surface. This showed that PAC may have scouring effect on the membrane surface. Consequently, PAC addition could be used as an EPS production control method to mitigate membrane fouling.
Analytical measurements
The surface morphologies of clean and fouled membranes were characterized using AFM. Fouled membrane was taken from the MBR system at the end of the operation. AFM images, and SEM images of the clean and fouled membranes, both with and without PAC addition, are shown in Fig. 6. The roughness parameters of the clean and fouled membranes (obtained from AFM measurements) are presented in Table 5. According to Rrms values, the clean membrane had the smoothest surface. The R rms value of the membrane sample from the PAC-MBR system was found to be close to the R rms value of the clean membrane. The highest R rms value was obtained from the fouled membrane that was used in the MBR without PAC addition. This finding confirmed that the membrane surface in the MBR without PAC addition was more fouled compared to the hybrid PAC-MBR. As seen from Table 5, The highest R a, R rms and R z values were obtained for the fouled membrane without addition PAC and the values varied 3.1–3.9 times according to clean membrane. However, the R a, R rms and R z values of the membrane sample from the PAC-MBR system was found to be close to the values of the clean membrane. This finding confirmed that the membrane surface in the MBR without PAC addition was more fouled compared to the hybrid PAC-MBR. In addition, the AFM results are supported by the MFI values obtained from flux modeling results. The highest R a, R rms and R z values obtained from fouled membrane without PAC addition, at which the highest value for MFI were also obtained.
The EPS concentration of the bioreactor without PAC addition was found to be 1.5-fold greater than the EPS concentration with OLR:PAC = 1.0 ratio. This increase of membrane surface roughness could be attributed to the surface enrichment of EPS due to fouling by adsorption. This result is consistent with the study of Tian et al. [35]. Changes in the hydrophilic/hydrophobic structures on the membranes were evaluated through contact angle measurements. The contact angles of the fouled membranes that were used in the MBR without and with PAC addition were measured as 61° and 58.5°, respectively. The increased contact angle values of fouled membranes compared to clean membrane (48.5°) demonstrated that the membranes became hydrophobic as a consequence of fouling. However, the slight difference in the contact angles values of the fouled membranes indicates PAC addition has not important effect on hydrophobicity of both fouled membranes.
The fouling of the membrane pores was characterized using SEM. The images of the clean and fouled membranes indicate that the pore structures of the membrane were altered. The structure of the fouled membrane from the hybrid PAC-MBR system changed less than the fouled membrane from the MBR system without PAC addition. It should be noted that the fouled membrane from the hybrid PAC-MBR system was taken at the end of operation (145th day and OLR:PAC = 1.0), whereas the fouled membrane sample of the MBR was taken at the end of the operation without PAC addition (19th day and OLR = 1 kg COD m−3 day−1). This result is meaningful because the pores of the fouled membrane from the hybrid PAC-MBR were less clogged despite longer operation time and increased OLR. The possible reason for the difference in the amount of clogged pores could be attributed to decreased fouling due to the adsorption of organics and EPSs onto the PAC.
The FT–IR peak positions and assignments of the clean and fouled membranes are shown in Table 6. In the spectrum of the clean FM MP005 membrane, a number of typical polysaccharide absorption peaks from the membrane material were observed. The broad absorption band between 3000 and 3600 cm−1 (centered at 3294 cm−1) is attributed to O–H stretching. In this region, the O–H stretching band might be defined as hydrogen bonded to S=O groups [37]. There is no obvious band in the spectrum ranging from 2500 to 4000 cm−1 for the fouled membrane of MBR without PAC addition. Since organic matter always has bands in this frequency range, their absence in this membrane suggests that the fouling layer on the membrane surface of the wastewater consisted of a large amount of inorganic matter [38]. The change on the fouled membrane from the hybrid PAC-MBR system was less. The band was only shifted to 3280 cm−1. Bands at 2933 and 2880 cm−1 are usually due to the stretching vibration of the C–H bond [37]. These bands disappeared for both fouled membranes. On the clean membrane surface, the C=O stretching band was observed at 1653 cm−1. This represents aromatic bands characteristics of the PES membrane [39]. The shifting of this band was observed for both fouled membranes. The C–O bond order increased and the band at 1653 cm−1 shifted to a higher wavenumber of 1661 cm−1 for the membrane of MBR without PAC. However, the band decreased and intensified due to the coordination through the oxygen atom. This finding was observed in the FT-IR spectra for the membrane of the hybrid PAC-MBR (1637 cm−1). The aromatic bands at 1578 and 1486 cm−1 are characteristic of the PES membrane. The band at 1241 cm−1 denotes C–C–O stretching. The clean membrane exhibited bands at 1151 and 1105 cm−1, which were not altered. However, the band at 1034 cm−1, which represents the sulfone group (S=O) stretching in the clean membrane, shifted to a higher waveband (1069 cm−1) for both fouled membranes. This band is in the frequency range of 1040–1100 cm−1, which is characteristic of SO4 2− and/or CO3 2− ions [38].
Membrane fouling, sludge sedimentation properties and biomass size distributions were evaluated through zeta potential and particle size distribution analysis. The results are given in Table 7.
Particle size distribution of the sludge in the MBR system provides information about the characteristics of the cake layer that influences filtration process by fouling. The particle size of the added PAC was 75 µm [13] whereas the particle size of the sludge was 70.6 µm. A decrease in sludge size from 70.6 to 53.5 µm was observed with the first PAC addition of 2 g L−1 in the bioreactor (OLR:PAC = 0.5). With the second PAC addition and increased OLR, the sludge size increased to 86.5 µm with decreasing the contribution of PAC (OLR:PAC = 0.75). At the end of the OLR:PAC = 1 operation, the sludge size in the bioreactor was found to be 84.7 µm. Park et al. [40] revealed that the final particle size of sludge shifts to a higher range in the case of smaller or comparable sludge particle size to the added PAC. However, particle size is not the only factor effecting the final size of the sludge. Also PAC concentration in the system has an impact. At lower PAC concentrations, the particle size of the sludge increases because of domination of suspended growth. Conversely, at higher PAC concentrations particle size of the sludge decrease to lower values due to domination of attached growth [40]. It can be said that the smaller sludge size after the first PAC addition was due to higher PAC concentration at OLR:PAC = 0.5 The increase in sludge size at higher OLR:PAC values with high OLR values could be attributed to the adsorption and flocculation of most of the dissolved organic, fine particles and colloids onto PAC [35, 41]. The relatively large flocs in the hybrid PAC-MBR system caused low fouling and, therefore, improved the filtration performance. Meng et al. [33] explained the decrease in specific resistance and subsequent improvement in the flux using the Carman-Cozeny equation.
Zeta potential measurements were carried out to quantify the surface charges of the materials in the wastewater. The zeta potential of the wastewater in the MBR without PAC was found to be −13.9. The zeta potential values of wastewaters from the hybrid PAC-MBR systems ranged from −10.6 to −11.4 (Table 7). Higher zeta potential values were measured in the hybrid PAC-MBR system compared to MBR without PAC. This result showed that negative surface charge of microbial flocs neutralized or decreased with PAC addition and Deng et al. [42] reported similar results with bioflocculant addition.
Conclusions
The following conclusions can be drawn from this study:
-
The flux temporarily improved 1.5–2.8-fold due to the addition of PAC despite increasing the OLR during 145 days of operation. Stability in the improved flux could not be observed because the added PAC became saturated with dissolved organics. With increase in OLR:PAC ratio as a result of decreased PAC amount per organic load the improvement in flux was lowered. The positive effect of PAC was temporary. Refreshing of PAC is necessary to improve the flux.
-
The flux modeling results based on the normalized mass transfer coefficients showed that the predomination of cake-adsorption fouling mechanism due to gel layer formation was eliminated with PAC addition at OLR:PAC = 0.5. However, at OLR:PAC values of 0.75 and 1 the gel layer formation was increased resulting more fouling. As a positive evidence of PAC addition, both MFI values and normalized mass transfer coefficient values caused by cake-adsorption formation with PAC addition were not never higher than values obtained by PAC addition.
-
The COD and etodolac removal improved with PAC addition to the MBR system. Considering the removal rates and OLR:PAC ratios, it can be said that PAC addition had a direct effect on the COD removal efficiency of the system. High and stable etodolac removal performance was observed with increasing OLR. However, similar to the flux, the improvement in the COD removal was temporary due to saturation of PAC.
-
EPS production in the bioreactor could be limited and controlled with PAC addition. Regardless of increased OLR:PAC ratio, PAC addition reduced EPS and the amount of organic matter deposited on the membrane surface. Also, EPS results were supported by AFM images. The highest R rms value was obtained from the fouled membrane that used in the MBR without PAC addition. In addition, the addition of PAC resulted in less clogging of the pores. Also relatively large flocs in the hybrid PAC-MBR system resulted in lower fouling and improved filtration performance. According to these results, it is clear that PAC addition decreased pore and surface membrane fouling.
As a result, the advantages of the hybrid PAC-MBR system compared to the MBR without PAC were reduced membrane fouling and a constant increase in PhAC removal performance. However, PAC addition did not consistently improve the permeate quality in terms of organic matter (e.g., COD).
Abbreviations
- a :
-
Flux model constant (s2 m−2)
- A:
-
The membrane filtration area (m2)
- AFM:
-
Atomic force microscope
- ATR:
-
Attenuated total reflection
- b :
-
Flux model constant (s m−2)
- BSA:
-
Bovine serum albumin
- COD:
-
Chemical oxygen demand (mg L−1)
- DO:
-
Dissolved oxygen (mg L−1)
- EPS:
-
Extracellular polymeric substance
- FT–IR:
-
Fourier transform infrared
- HRT:
-
Hydraulic retention time (h)
- J :
-
Permeate flux (L m−2 h−1)
- J 0 :
-
Initial permeate flux (L m−2 h−1)
- K a :
-
Mass transfer coefficient for adsorptive fouling (h−1)
- Kb :
-
Mass transfer coefficient for complete pore blocking (h−1)
- K b/K a :
-
The normalized mass transfer coefficient for complete pore-adsorption fouling
- K c :
-
Mass transfer coefficient for cake fouling (h m−2)
- K c*J 2o /K a :
-
The normalized mass transfer coefficient for cake-adsorption fouling
- K i :
-
Mass transfer coefficient for intermediate fouling (m−1)
- K i*J o/K a :
-
The normalized mass transfer coefficient for intermediate-adsorption fouling
- MBR:
-
Membrane bioreactor
- MLSS:
-
Mixed liquor suspended solid (mg L−1)
- mV:
-
Millivolt
- MLVSS:
-
Mixed liquor volatile suspended solid (mg L−1)
- OLR:
-
Organic loading rate (kg COD m−3 day−1)
- p :
-
Number of points within a given membrane surface area
- PAC:
-
Powdered activated carbon
- PhAC:
-
Pharmaceutical active compound
- PES:
-
Polyethersulfone
- r 2 :
-
Correlation coefficient
- R a :
-
The mean roughness on membrane surface (nm)
- R rms :
-
The root mean square of average height of membrane surface peaks (nm)
- R z :
-
The mean difference between five highest peaks and lowest valleys (nm)
- RMSE:
-
Root mean squared error
- SEM:
-
Scanning electron microscope
- SMP:
-
Soluble microbial product
- SRT:
-
Sludge retention time (d)
- t:
-
Filtration time (min)
- V:
-
Total volume of permeate (m3)
- z av :
-
The average of the z values within a given membrane surface area (nm)
- z cu :
-
The current z value (nm)
- a:
-
Mean roughness
- av:
-
Average
- c:
-
Carbohydrate
- c:
-
Clean membrane
- cu:
-
Current
- d:
-
Day
- h:
-
Hour
- p :
-
Protein
- z :
-
Height
- µ :
-
Dynamic viscosity of the solution (mPas)
- α :
-
Specific cake resistance (m kg−1)
References
Mascolo G, Balest L, Cassano D, Laera G, Lopez A, Pollice A, Salerno C (2010) Biodegradability of pharmaceutical industrial wastewater and formation of recalcitrant organic compounds during aerobic biological treatment. Bioresour Technol 10:2585–2591
Sanderson H, Johnson DJ, Reitsma T, Brain RA, Wilson CJ, Solomon KR (2004) Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul Toxicol Pharm 39:158–183
Nguyen LN, Hai FI, Kang J, Price WE, Nghiem LD (2012) Removal of trace organic contaminants by a membrane bioreactor-granular activated carbon (MBR-PAC) system. Bioresour Technol 113:169–173
Fr SH, Tambosi JL, Sena RF, Moreira PM, José HJ, Pinnekamp J (2012) The removal and degradation of pharmaceutical compounds during membrane bioreactor treatment. Water Sci Technol 65(5):833–839
Shariati FP, Mehrnia MZ, Salmasi BM, Heran M, Wisniewski C, Sarrafzadeh MH (2010) Membrane bioreactor for treatment of pharmaceutical wastewater containing acetaminophen. Desalination 250:798–800
Nguyen LN, Hai FI, Nghiem LD, Kang J, Price WE, Park C, Yamamoto K (2014) Enhancement of removal of trace organic contaminants by powdered activated carbon dosing into membrane bioreactors. J Taiwan Inst Chem E 45:571–578
Tambosi JL, Sena RF, Favier M, Gebhardt W, José HJ, Schröder HF, Moreira RFPM (2010) Removal of pharmaceutical compounds in membrane bioreactors (MBR) applying submerged membranes. Desalination 261:148–156
Radjenovic J, Petrovic M, Barcelo D (2007) Analysis of pharmaceuticals in wastewater and removal using membrane bioreactor. Anal Bioanal Chem 387:1365–1377
Orozco Ferro AM, Contreras EM, Zaritzky NE (2010) Dynamic response of combined activated sludge-powdered activated carbon batch systems. Chem Eng J 157:331–338
Xiang-Juan G, Han-Seung K (2008) The role of powdered activated carbon in enhancing the performance of membrane systems for water treatment. Desalination 225:288–300
Ying Z, Ping G (2006) Effect of powdered activated carbon dosage on retarding membrane fouling in MBR. Sep Purif Technol 52:154–160
Li YZ, He YL, Liu YH, Yang SC, Zhang GJ (2005) Comparison of the filtration characteristics between biological powdered activated carbon sludge and activated sludge in submerged membrane bioreactors. Desalination 174(3):305–314
Drews A (2010) Membrane fouling in membrane bioreactors: characterization, contradictions, cause and cures. J Memb Sci 63:1–28
Eurocarb Activated Carbon Products and Technology (2009) WAC i600 M200 Product specification, Bristol, UK
Turano E, Curcio S, De Paola MG, Calabrò IG (2002) An integrated centrifugation-ultrafiltration system in the treatment of olive mill wastewater. J Memb Sci 209:519–531
Bolton GR, Boesh AW, Lazzara MJ (2006) The effects of flow rate on membrane capacity: development and application of adsorptive membrane fouling models. J Memb Sci 279:625–634
APHA, AWWA, WEF (1995) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Frølund B, Palmgren R, Keiding K, Nielsen PH (1996) Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res 30(8):1749–1758
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Memb Sci 193:265–275
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Calorimetric method for determination of sugar and related substances. Anal Chem 28:350
Kaya Y, Barlas H, Arayici S (2009) Nanofiltration of Cleaning-in-Place (CIP) wastewater in a detergent plant: effects of pH, temperature and transmembrane pressure on flux behavior. Sep Purif Technol 65(2):117–129
Akram A, Stuckey DC (2008) Flux and performance improvement in a submerged anaerobic membrane bioreactor (SAMBR) using powdered activated carbon (PAC). Process Biochem 43:93–102
Remy M, Potier V, Temmink H, Rulkens W (2010) Why low powdered activated carbon addition reduces membrane fouling in MBRs. Water Res 44:861–867
Park C, Kim H, Hong S, Lee S, Choi S-I (2007) Evaluation of organic matter fouling potential by membrane fouling index. Water Sci Technol 7:27–33
Munz G, Gori R, Mori G, Lubello C (2007) Powdered activated carbon and membrane bioreactors (MBRPAC) for tannery wastewater treatment: long term effect on biological and filtration process performances. Desalination 207(1–3):349–360
Ma C, Yu S, Shi W, Tian W, Heijman SGJ, Rietveld LC (2012) High concentration powdered activated carbon-membrane bioreactor (PAC-MBR) for slightly polluted surface water treatment at low temperature. Bioresour Technol 113:136–142
Le-Clech P, Chen V, Fane TAG (2006) Fouling in membrane bioreactors used in wastewater treatment. J Memb Sci 284:17–53
Chu H, Zhang Y, Zhou X, Dong B (2013) Bio-enhanced powder-activated carbon dynamic membrane reactor for municipal wastewater treatment. J Memb Sci 433:126–134
Lin H, Zhang M, Wang F, Meng F, Liao B-Q, Hong H, Chen J, Gao W (2014) A critical review of extracellular polymeric substances (EPSs) in membranebioreactors: characteristics, roles in membrane fouling and control strategies. J Memb Sci 460:110–125
Kim JS, Lee CH, Chun HD (1998) Comparison of ultrafiltration characteristics between activated sludge and BAC sludge. Water Res 32:3443–3451
Dosoretz CG, Böddeker KW (2004) Removal of trace organics from water using a pumped bed-membrane bioreactor with powdered activated carbon. J Memb Sci 239:81–90
Ng CA, Sun D, Bashir MJK, Wai SH, Wonga LY, Nisar H, Wub B, Fane AG (2013) Optimization of membrane bioreactors by the addition of powdered activated carbon. Bioresour Technol 138:38–47
Meng F, Shi B, Yang F, Zhang H (2007) Effect of hydraulic retention time on membrane fouling and biomass characteristics in submerged membrane bioreactors. Bioprocess Biosyst Eng 30:359–367
Johir MAH, Vigneswaran S, Sathasivan A, Kandasamy J, Chang CY (2012) Effect of organic loading rate on organic matter and foulant characteristics in membrane bio-reactor. Bioresour Technol 113:154–160
Jamal Khan S, Visvanathan C, Jegatheesan V (2012) Effect of powdered activated carbon (PAC) and cationic polymer on biofouling mitigation in hybrid MBRs. Bioresour Technol 113:165–168
Tian Y, Chen L, Zhang S, Cao C, Zhang S (2011) Correlating membrane fouling with sludge characteristics in membrane bioreactors: an especial interest in EPS and sludge morphology analysis. Bioresour Technol 102:8820–8827
Stuart B (2004) Infrared spectroscopy: fundamentals and applications. Wiley, West Sussex
Dean JA (1999) Lange’s handbook of chemistry. McGraw-Hill, New York
Belfer S, Fainchtain R, Purinson Y, Kedem O (2000) Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J Memb Sci 172:113–124
Park H, Choo KH, Lee CH (1999) Flux enhancement with powdered activated carbon addition in the membrane anaerobic bioreactor. Sep Purif Technol 34:2781–2792
Satyawali Y, Balakrishnan M (2009) Effect of PAC addition on sludge properties in an MBR treating high strength wastewater. Water Res 43:1577–1588
Deng L, Guo W, Ngo HH, Zuthi FR, Zhang J, Liang S, Li J, Wang J, Zhang X (2015) Membrane fouling reduction and improvement of sludge characteristics by bioflocculant addition in submerged membrane bioreactor. Sep Purif Technol 156:450–458
Acknowledgments
This work was supported by the Research Fund of the Istanbul University (Project Number 4239).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaya, Y., Bacaksiz, A.M., Golebatmaz, U. et al. Improving the performance of an aerobic membrane bioreactor (MBR) treating pharmaceutical wastewater with powdered activated carbon (PAC) addition. Bioprocess Biosyst Eng 39, 661–676 (2016). https://doi.org/10.1007/s00449-016-1547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1547-3