Abstract
Enhancing functional gene expression is key to high-level production of active chitinases. For this purpose, the effects of culture cell density, inducer concentration, post-induction time and induction temperatures on the functional expression of two different chitinases (HsChiA1p, a family 18 archaeal chitinase and PtChi19p, a family 19 bacterial chitinase) were comparatively investigated. Results showed that the effect of each parameter on the activity of both chitinases was specific to each enzyme. In addition, different Escherichia coli host strains compatible with the expression in pET systems were assayed for active protein overexpression. When using BL21 Star (DE3), a significant increase of 60 % in expression was observed for the active archaeal chitinase HsChiA1p as compared to that found when using BL21 (DE3), indicating that the rne131 gene mutation efficiently stabilizes the mRNA for HsChiA1p. Using the Codon Adaptation Index value, rare codon analysis of the archaeal HschiA1 and bacterial Ptchi19 genes revealed that both DNA sequences were not optimal for maximal expression in E. coli. Different E. coli host strains possess extra copies of some of the tRNA genes for rare codons. For the Rosetta 2 (DE3) and the BL21 RP (DE3) strains, a significant increase of 40 % was reached for the activity of HsChiA1p and PtChi19p. Finally, as part of the protein still remained insoluble, the best conditions for recovering biologically active protein from inclusion bodies were established for each enzyme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitinases are glycosyl hydrolases that break down chitin (the second most abundant polymer in nature) to mono- and chito-oligosaccharides by hydrolysing the 1-4 β-glycoside bonds between N-acetyl d-glucosaminides. Chitinolytic enzymes belong to families 18 and 19 of the glycosyl hydrolases. Families 18 and 19 show different molecular structure and hydrolysis mechanism [1].
Several transcendent applications are traditionally related to chitinases, such as that for obtaining bioactive oligosaccharides of different sizes of interest to the pharmaceutical industry, the preparation of single-cell proteins, the treatment of chitosan waste or the fungicide, insecticide and nematicide properties [2, 3]. New important uses of chitinases have recently been reported, including their use for bioethanol production [4] and their addition to aquafeed for promoting a greater growth of fish and for enhancing the non-specific immunity function, giving protection against different pathogens [5].
It has been shown that chitinolytic enzymes can be synthesized by a large number of microbial species, and that each chitinolytic enzyme presents its own characteristics that make it useful for a specific function. In this context, many recent studies have reported numerous chitinases with different properties [1, 2]. However, the regulated synthesis and low activity of these enzymes in wild-type strains make them unsuitable for industrial production and, at present, very few chitinases are commercially available.
Therefore, the successful use of chitinases for real applications depends on increasing their production by generating strains and optimizing culture conditions that allow efficient protein production. Escherichia coli is the best known and one of the most commonly used hosts for high-level production of heterologous proteins, because of its rapid growth rate, ease of high cell density fermentation, low cost and the availability of excellent genetic tools [6–8]. Attempts to improve recombinant protein expression in E. coli have been widely carried out by the development of different strategies principally related with the expression vector design, gene dosage, promoter strength, mRNA stability, host design considerations, codon usage and fermentation conditions [7–9]. The main objective of these studies has usually been to obtain a high degree of accumulation of soluble product in the bacterial cell [10], even though it cannot be predicted if a recombinant protein will be obtained in E. coli in high amounts and in a soluble active form [7]. Indeed, expression of recombinant proteins in E. coli often leads to their accumulation as insoluble aggregates, forming inclusion bodies [10, 11]. Several methods have been described for the redirection of proteins from inclusion bodies into the soluble cytoplasmic fraction, but these strategies mainly relate to protein refolding or modifications of the expression conditions [10, 12].
Escherichia coli has been commonly and successfully used to express and demonstrate the functionality of different chitinases, as well as to study their biochemical characteristics [2]. However, the optimization of expression parameters and the host strain used for protein production have received little or no attention.
In previous studies, we purified and characterized two different chitinases: HsChiA1p family 18 chitinase from the marine extreme halophilic archaeon Halobacterium salinarum [13] and PtChi19p family 19 chitinase from the marine moderate halophilic bacterium Pseudoalteromonas tunicata [14]. As conditions for a high level of active protein production of recombinant HsChiA1p and PtChi19p are of relevant interest, we determined them by testing different expression parameters and, for the first time, different host expression strains, giving data for chitinases from both families 18 and 19 of the glycosyl hydrolases.
Materials and methods
Strains, growth conditions and plasmids
Strains and plasmids used in this study are shown in Table 1.
Escherichia coli strains were cultured in Luria–Bertani (LB) medium and incubated at 37 °C with shaking, when necessary. For E. coli BL21 RP (DE3) and Rosetta 2 (DE3) strains, the LB medium was supplemented with chloramphenicol (25 µg/mL). In addition, each E. coli-Hs-ChiA1 strain and each E. coli-Pt-Chi19 strain culture media were supplemented with ampicillin (100 µg/mL) and kanamycin (30 µg/mL), respectively.
Colloidal chitin preparation
Colloidal chitin was prepared following a modified version of the method of Roberts and Selitrennikoff [15]. Five grams of crab shell chitin (Sigma, C7170) was mixed and stirred with 90 mL of 37 % HCl at room temperature for 2 h. Then, 0.5 L of 95 % frozen ethanol was added and stirred for 30 min and, subsequently, centrifuged for 20 min at 6000 rpm at 4 °C. The pellet was washed with sterile water until a neutral pH was reached. Colloidal chitin was stored at −20 °C until needed and used at a proportion of 1 % (w/v) in distilled water.
Strategies followed to enhance expression of HsChiA1p and PtChi19p
The different E. coli-Hs-ChiA1 and E. coli-Pt-Chi19 recombinant strains were obtained by the transformation of pET-BM-Hs-ChiA1-ps and pET-Pt-Chi19 vectors, respectively, into several E. coli expression strains (Table 1). They were grown in LB medium supplemented with ampicillin (100 µg/mL) and kanamycin (30 µg/mL), respectively. In addition, BL21 RP (DE3) and Rosetta 2 (DE3) recombinant E. coli strains were supplemented with chloramphenicol (25 µg/mL).
All recombinant E. coli strains were cultured in 50 mL of appropriately supplemented LB at 37 °C, with shaking until the culture densities corresponded to an OD600 of 0.7. Subsequently, 0.5 mM of isopropyl β-d-1-thiogalactopyranoside (IPTG) was added and the cultures were incubated with shaking for 4 h at 37 °C. Moreover, BL21-Hs-ChiA1 and BL21-Pt-Chi19 were cultured in different expression conditions to find out those that provided the largest amount of soluble active protein. Different incubation temperatures (25–40 °C), optical densities at 600 nm (0.7–1.3) prior induction, IPTG concentrations (0.25–1.5 mM) and post-induction time periods (1–24 h) were tested using the above-mentioned strains. Samples were taken each hour after induction, centrifuged at 8000 rpm for 10 min and the pellets used for SDS-PAGE analysis. Following induction, the cultures were centrifuged under the same previously described conditions and the pellets were broken on ice, for 30 min, with a lysis buffer [50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 0.05 % (v/v) Tween®20, lysozyme (10 mg/mL); pH 8]. After being centrifuged for 30 min at 9500 rpm, the cell-free supernatant was dialysed overnight at 4 °C against dialysis buffer (20 mM Tris–HCl, 1.5 M NaCl; pH 7.3 for Hs-ChiA1, and 20 mM Tris–HCl, 2 M NaCl; pH 7.5 for Pt-Chi19) and the lysed-cell pellet stored at −20 °C. The dialysed product was collected as crude enzyme.
SDS-PAGE analysis
Samples were analysed by SDS-PAGE (10 % acrylamide/bis-acrylamide) according to Sambrook and Russell [16]. The gel was stained with EZBlue™ Gel Staining Reagent (Sigma). New England Biolabs molecular weight Broad Range marker was used as standard.
Protein concentration was determined following Bradford’s method [17].
Rare codon DNA sequence analysis
The Genscript Rare Codon Analysis Tool (http://www.genscript.com/cgi-bin/tools/rare_codon_analysis) was used to analyse the distribution of the codon usage frequency and the GC content (%) throughout the length of the genes HschiA1 and Ptchi19 that encode for HsChiA1p and PtChi19p proteins, respectively.
Solubilisation of inclusion bodies
The cell lysates from BL21 Star-Hs-ChiA1 and BL21 RP-Pt-Chi19 pellets, obtained after induction of protein expression, were solubilised in 400 µL of denaturing buffer (20 mM Tris–HCl, 8 M urea, 50 mM DTT, 2 mM EDTA; pH 8) at 37 °C, with shaking for 2 h [18]. Subsequently, refolding of the proteins was carried out by their rapid dilution [18] in a buffer composed of 20 mM Tris–HCl, 2 M NaCl, 2 mM CaCl2, 3.25 mM EDTA; pH 7.5. Various dilutions were prepared in different proportions 1:16, 1:8, 1:4, 1:2, 3:4 (solubilised cells:refolding buffer) and the protein refolding was detected by the capacity to hydrolyse colloidal chitin. The effect of refolding time on chitinase activity was also measured after 4, 8, 20 and 24 h.
Enzyme assays
Analysis of chitinase production using pNP-(GlcNAc) as substrate
The reaction mixtures consisted of 500 μL of 0.5 mM 4-nitrophenyl-N-acetyl-β-d-glucosaminide [pNP-(GlcNAc)] in distilled water and 500 µL of the crude enzyme. A final NaCl concentration of 1.5 and 2 M was present in the reaction for HsChiA1p and PtChi19p, respectively. Reactions with HsChiA1p and PtChi19p were incubated for 1 h at 40 and 43 °C, respectively, and stopped by the addition of 3 M Na2CO3. 4-Nitrophenol liberated during the reaction was determined spectrophotometrically at 410 nm. One unit (U) of enzyme activity was defined as the amount of enzyme releasing 1 μmol of 4-nitrophenol in 1 h. Assays were carried out in triplicate and the results were expressed as mean ± SD.
Recovery of chitinase activity from inclusion bodies using colloidal chitin as substrate
The reaction mixtures consisted of 40 µL of 1 % colloidal chitin and 40 µL of refolded protein from solubilised inclusion bodies (detailed in the inclusion bodies section). The concentration of reducing sugars was determined after 20 h of incubation at 35 °C by the Somogyi–Nelson method [19], using GlcNAc as the standard. One unit (U) of activity was defined as the amount of enzyme releasing 1 μmol of GlcNAc in the assay conditions. Assays were carried out in triplicate and the results were expressed as mean ± SD.
Statistical analysis
Firstly, assumption of normality (Kolmogorov–Smirnov Z test) and homogeneity of variances (Levene’s test) were tested. The effect of different expression conditions, E. coli host strains, as well as the dilution and time of refolding of the protein on the production of active chitinases, were analysed with a one-way analysis of variance (ANOVA) with Tukey post hoc comparisons for each dependent variable. All statistical analyses were performed with a 95 % of confidence interval and they were conducted with SPSS v.19.0 for Windows.
Results and discussion
Effect of different parameters on the production of the active recombinant chitinases HsChiA1p and PtChi19p
Chitinases are becoming more important due to their diverse biotechnological applications. Successful use of these enzymes for real applications depends on the possibility of having strains and culture/expression conditions that allow the production of these proteins in active form and large quantities at competitive costs.
Two different chitinases from H. salinarum (HsChiA1p belonging to GH18 family) and P. tunicata (PtChi19p belonging to GH19 family) had previously been expressed in E. coli BL21 (DE3) and characterized for exhibiting potentially important applications [13, 14].
Some parameters are known to have a considerable impact on the obtaining of soluble and active protein in heterologous expression [20]. In this context, the effect of the following factors was investigated to establish the conditions that would lead to higher amounts of soluble active HsChiA1p and PtChi19p: cell density; inducer concentration (IPTG); time of induction; post-induction temperature; and E. coli host strains.
Initial cell density
The biomass concentration upon induction should be considered a key parameter, because inducer uptake may be affected by the number of cells capable of incorporating the inducer within the cytoplasm [21], while it is also important to ensure that these cells are sufficiently active at the time of induction to reduce protein aggregation [7].
The effects on the production of HsChiA1p and PtChi19p of BL21-Hs-ChiA1 and BL21-Pt-Chi19 culture density, respectively, were assayed just before induction with 0.5 mM IPTG at 37 °C. Significant differences in the activities of HsChiA1p and PtChi19p according to culture’s density were obtained and results are shown in Fig. 1a. The highest levels of active protein were obtained at cell concentrations corresponding to optical densities (600 nm), of 1 for HsChiA1p (141.51 ± 3.38 mU/mL) and 0.7 for PtChi19p (80.57 ± 3.94 mU/mL), while a significant decrease in enzymatic activity was observed as the cell concentration of the cultures increased, particularly for PtChi19p (35 %). This is in agreement with the results of various studies, which have indicated that high cellular densities can reduce the availability of dissolved oxygen, produce high levels of carbon dioxide, decrease nutrient concentration, form acetate, reduce mixing or vary temperature in the culture media; thereby, giving rise to a decline of the metabolic activity of the cells and, therefore, of gene expression [22–24]. Nevertheless, shorter fermentations are of interest because the cost of the process would be reduced. All these reasons make optimization of the initial cell density of cultures a key parameter for optimal active protein production.
Effect of different expression conditions on the production of active HsChiA1p and PtChi19p enzymes. a Effect of the culture cell densities just before induction with IPTG. b Effect of the inducer concentration. c Effect of the post-induction time. d Effect of the induction temperature. Dark grey bar represents the data for the HsChiA1p protein. Light grey bar represents the data for the PtChi19p protein. For each parameter the highest activity was defined as 100 %. Bold lower case letters above the columns (a–c) denote significant differences for the chitinase HsChiA1p. Lower case letters above the columns (a–c) denote significant differences for the chitinase PtChi19p
Inducer concentration and post-induction time
The magnitude of inducer and length of induction period also affect recombinant protein production as well as cell growth [20]. Moreover, both parameters can influence protein folding and solubility because high levels of IPTG can give rise to the induction of the protein synthesis in a very fast way that may lead to its accumulation in inclusion bodies. This occurs when rates of protein synthesis overwhelm the folding machinery [25, 26]. Because of this, the finding of the optimum IPTG concentration that decreases physiological stress of the cells and increases specific productivity is of great interest.
Regarding the effect caused by the amount of inducer, different IPTG concentrations, from 0.25 to 1.5 mM, were used. Significant differences in the activities of HsChiA1p and PtChi19p according to inducer concentration were found (Fig. 1b). Although low inducer concentrations may result in an inefficient induction, it can be seen that the lower IPTG concentrations (0.5 and 0.25 mM) gave rise to the highest enzymatic activity for HsChiA1p (149.85 ± 5.61 mU/mL) and PtChi19p (110.46 ± 5.09 mU/mL), respectively, whereas larger quantities of the inducer led to a significant decrease in the activity of the proteins, particularly for PtChi19p (40 %). Reduction in production of the recombinant protein has previously been thought to be caused by, among other things, the toxic impact of the extracellular inducer concentration. This would lead to a reduction in cell metabolic activity and, therefore, in a decrease of gene expression [23, 24]. Moreover, it has been shown that reduction in the inducer concentration can sometimes lead to a better folding of recombinant proteins [26], which could result in an increase of enzyme activity. This result is also of interest from an economic perspective, because inducers are very expensive [20].
Post-induction incubation time was another important factor for the production of the active recombinant proteins HsChiA1p and PtChi19p. Induction was carried out by adding 0.5 mM IPTG and, subsequently, incubating the cultures at 37 °C from 1 to 24 h. Significant differences in the activities of HsChiA1p and PtChi19p according to post-induction incubation time were obtained and results are shown in Fig. 1c. As can be seen from this chart, the time of incubation after induction with IPTG has an important effect on the level of soluble active proteins, with each enzyme following a clearly different pattern. Yet, the highest activity for HsChiA1p was found 5 h after induction began (155.98 ± 4.28 mU/mL), decreasing significantly using shorter and longer times, although more than 60 % of enzymatic activity could be detected even after 10 h. It is known that longer induction times increase protein expression, but they may also endanger protein stability and/or induce proteolysis and, therefore, a reduction in the yield of recombinant protein [25]. Nevertheless, the highest levels of active PtChi19p were found 1 h after induction began (308.87 ± 25.78 mU/mL). However, it could be seen that this protein was less stable over time or degraded, because a significant decrease of 80 % of activity was detected after 5 h of induction. It has previously been established that proteolytic degradation is intimately linked with protein folding [8]. After 24 h, an important increase of enzyme activity was again observed for PtChi19p. This result could be explained due to the accumulation of protein over time or even, as previously mentioned, because of the effect of lower concentrations of IPTG on protein folding [26].
Induction temperature
Induction temperature during the expression of the HsChiA1p and PtChi19p in BL21 (DE3) was also analysed, because it has a pronounced effect on protein folding and stability [9]. A common strategy for increasing protein solubility is to reduce culture temperature after induction [25, 27]. It is known that this strategy has a pronounced effect on protein folding (modulation of chaperone activity) and stability [9], causing a reduction in the rate of protein synthesis, but allowing sufficient time for the new proteins to fold properly [28].
Temperatures within a range of 25–40 °C were studied after induction of the culture with 0.5 mM IPTG. Further lowering of the temperature to/under 20 °C is not of interest from an industrial perspective, because production costs would increase as artificial cooling would be required [29]. Significant differences in the activities of HsChiA1p and PtChi19p according to induction temperature were observed (Fig. 1d). Two different temperatures were optimized for achieving the highest levels of active HsChiA1p and PtChi19p enzymes. For HsChiA1p, the optimal temperature was between 30 and 37 °C (152.49 ± 8.87/149.72 ± 5.61 mU/mL), whereas the highest protein activity after incubation for PtChi19p was obtained at 25 °C (114.06 ± 1.65 mU/mL). Significantly, a reduction between 20 and 30 % in enzymatic activity was observed at suboptimal temperatures for both HsChiA1p and PtChi19p.
E. coli host strains and rare codon interference
In recent years, different host strains have been developed to enhance recombinant protein production in E. coli [7, 8]. Some of these E. coli strains, which are compatible with the expression in pET systems, were used for the overexpression of HsChiA1p and PtChi19p. Five E. coli host strains with different characteristics (Table 1) were transformed with recombinant vectors. The expression was induced with 0.5 mM IPTG for 4 h at 37 °C and the results obtained (Fig. 2) were compared to those using BL21 (DE3) strain, which is by far the most commonly used E. coli strain [8]. Significant differences in the activities of HsChiA1p and PtChi19p according to E. coli host strain were found.
Effect of the host expression E. coli strain on the procurement of active chitinases. Dark grey bar represents the data for the HsChiA1p protein. Light grey bar represents the data for the PtChi19p protein. For each experiment the highest activity was defined as 100 %. Bold lower case letters above the columns (a–e) denote significant differences for the chitinase HsChiA1p. Lower case letters above the columns (a–c) denote significant differences for the chitinase PtChi19p
When using the BL21 Star (DE3) host strain, a significant increase of 60 % in the active archaeal chitinase HsChiA1p was observed, whereas no significant differences were detected for bacterial chitinase PtChi19p. These results strongly suggest that the rne gene mutation (rne131), which the BL21 Star (DE3) strain presents, improves in a very effective way the mRNA stability of archaeal HsChiA1p. However, not all mRNAs are stabilized equally by the rne131 mutation, and those that better improve their stabilization are those more susceptible to RNase E [30].
Regarding E. coli Tuner (DE3) strain, a small but significant loss of active protein for PtChi19p and for HsChiA1p were found when compared to the results for the corresponding BL21 (DE3) recombinant strains although clearly, a more intense band corresponding to the recombinant protein was observed on SDS-PAGE (Results not shown). It could be thought that, because of the lacY deletion [7], a uniform IPTG entry in all the population cells benefits the expression of recombinant HsChiA1p and PtChi19p. However, these factors did not have an important effect on the activity of HsChiA1p and PtChi19p, probably due to the enhancement of protein production with incorrect folding. The synthesis of fewer polypeptides per cell could result in more correctly folded proteins, because the cell machinery would work more effectively. In this way, higher transcriptional levels do not automatically correspond to higher yields of functional protein [31].
One of the factors that can affect the recombinant protein expression levels in E. coli and/or its activity is an unfavourable codon usage of heterologous genes [7]. It is known that insufficient tRNA pools can produce translational stalling, premature translation termination, translation frameshifting and amino acid misincorporation [7, 32].
Rare codon analysis of the genes that encode for HsChiA1p and PtChi19p, selecting E. coli as the host for protein expression, indicated that both DNA sequences were not optimized for maximal expression in this species. The possibility of high protein expression level is correlated to the value of the Codon Adaptation Index (CAI). The CAI values obtained for the archaeal HschiA1 and bacterial Ptchi19 genes were 0.7 and 0.74, respectively, both lower than the CAI value of 0.8, which is rated as good for expression in the desired organism (E. coli).
One of the most common strategies for solving codon usage bias is to increase the availability of underrepresented tRNAs by host modification [7, 10]. For example, an important increase in the yield and fidelity of heterologous proteins has been reported when using, among others, E. coli strains with enhanced argU and proL [32], even though sometimes these strains can cause a decrease in protein solubility [7].
Analysis of the codons present in the genes that encode for HsChiA1p and PtChi19p showed that they have a high percentage of several rare codons for E. coli, which could be one of the reasons why there was only a low detection of activity for these proteins when using some of the E. coli strains. The percentage of low-frequency codons based on an E. coli expression host was 6 and 5 % for the genes that encode for HsChiA1p and PtChi19p, respectively. Moreover, the location of these rare codons in the sequences seems to be important. It has been said that the presence of rare codons near the 5′ end of a transcript affects translational efficiency [6]. This occurs with the gene that encodes for HsChiA1p, as it presents a CCC in the fourth codon starting from that end. In addition, the GC content of the 5′ coding region of certain genes appears to influence recombinant protein expression [6]. The average GC content for the genes that encode for HsChiA1p and PtChi19p was 65.69 and 46.25 %, respectively, both within the ideal percentage range of GC content (30–70 %) for efficient protein expression. However, several peaks outside this range were detected for the archaeal HschiA1 gene and these could adversely affect transcriptional and translational efficiency.
The E. coli Rosetta 2 (DE3) and BL21 RP (DE3) strains possess extra copies of some of the tRNA genes for rare codons to facilitate the expression of genes from other microorganisms with a different use of codons. The frequencies of some of the rare codons in E. coli genes are: AGA (Arg), 0.24 %; AGG (Arg), 0.21 %; CCC (Pro), 0.24 %; ATA (Ile), 0.5 %; CTA (Leu), 0.34 %; GGA (Gly), 0.82 % and CGG (Arg), 0.5 % [33]. The first three codons listed above are overexpressed in E. coli BL21 RP (DE3) and all codons on the above list are overexpressed in E. coli Rosetta 2 (DE3).
When using Rosetta 2 (DE3) as host strain, a significant increase of 40 % was observed for the activity of HsChiA1p enzyme. On the other hand, using BL21 RP (DE3) as host strain, a significant increase of 40 % in the activity of PtChi19p was found. These results are consistent with the fact that the gene that encodes for HsChiA1p presents CCC and CGG codons in a higher proportion than they are found in E. coli (1.27 and 0.73 %, respectively), while the gene that encodes for PtChi19p presents a higher frequency of CCC and ATA codons (0.62 % for both).
As it can be seen from these results, the recombinant strains BL21Star-Hs-ChiA1 and BL21RP-Pt-Chi19 were the ones that showed the highest activity for the chitinases HsChiA1p (278.60 ± 25.20 mU/mL) and PtChi19p (139.40 ± 6.95 mU/mL), respectively.
Recovery of chitinase activity from inactive inclusion bodies
It is well known that even when conditions are optimized for a high level of active protein expression, recombinant proteins in E. coli often appear as accumulated insoluble aggregates in inclusion bodies. These proteins lack activity and need to be solubilised and refolded to recover part of their own biological activity [11, 12].
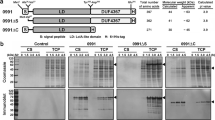
As BL21Star-Hs-ChiA1 and BL21RP-Pt-Chi19 were the strains that showed the highest activity for HsChiA1p and PtChi19p chitinases, respectively, they were chosen for further study. An analysis on SDS-PAGE was carried out with these strains to compare the amounts of recombinant protein present in the soluble and insoluble fractions (Fig. 3). An important amount of the recombinant protein remained accumulated in inclusion bodies in both cases, but particularly for PtChi19p (Fig. 3b), where the overexpressed insoluble and inactive protein is very often exclusively contained [12]. Taking advantage of the high expression levels of inclusion body proteins, by being able to convert inactive and misfolded inclusion body proteins into soluble bioactive products, could be a great challenge [34]. Therefore, with the goal of turning inactive chitinases from inclusion bodies into active HsChiA1p and PtChi19p proteins, one attempt at solubilising them from BL21Star-Hs-ChiA1p and BL21RP-Pt-Chi19p recombinant strains was carried out. For this purpose, the pellets obtained after cell lysis were solubilised according to the method previously described by Díaz et al. [18]. The concentrations of the insoluble proteins obtained from BL21Star-Hs-ChiA1 and BL21RP-Pt-Chi19 were 0.75 and 1.09 mg/mL, respectively. Subsequently, a refolding step was carried out by mixing the sample with different proportions of refolding buffer. The refolding buffer used had 2 M NaCl in its composition, because the presence of high salt concentrations is considered to be one of the most important factors for the correct folding of halophilic enzymes [18]. Significant differences in the activities of HsChiA1p and PtChi19p according to dilution proportion of solubilised proteins were obtained. Results of the recovery of soluble and active protein from inclusion bodies are shown in Fig. 4a, and it can be seen that refolding of the solubilised proteins at a dilution proportion of 1:2 (0.38 mg/mL of protein) and 1:4 (0.27 mg/mL of protein) gave rise to the highest recovery of activity for HsChiA1p (16.19 ± 0.20 U/mg) and PtChi19p (12.07 ± 0.37 U/mg), respectively.
a SDS-PAGE analysis of the soluble and insoluble fractions obtained from the BL21Star-Hs-ChiA1 culture induced with IPTG. b SDS-PAGE analysis of the soluble and insoluble fractions obtained from the BL21RP-Pt-Chi19 culture induced with IPTG. Lane M broad range (Biolabs) protein marker, Lane 1 soluble fraction, Lane 2 insoluble fraction. The arrows show the recombinant proteins HsChiA1p (a) and PtChi19p (b)
a Effect of the refolding dilution on the recovery of soluble and active HsChiA1p and PtChi19p chitinases from solubilised inclusion bodies. b Effect of the refolding time on the recovery of active HsChiA1p and PtChi19p chitinases from solubilised inclusion bodies diluted 1:2 and 1:4, respectively. Lines with closed circles represent the data for the HsChiA1p protein. Lines with opened circles represent the data for the PtChi19p protein. For each experiment the highest activity was defined as 100 %. Bold lower case letters above the columns (a–e) denote significant differences for the chitinase HsChiA1p. Lower case letters above the columns (a–e) denote significant differences for the chitinase PtChi19p
Once the best dilution proportion to recover active protein was established for each enzyme, the effect of the folding time was also determined, as it is another important parameter to be taken into account to improve protein activity [18, 34]. Significant differences in the activities of HsChiA1p and PtChi19p according to folding time of solubilised proteins were observed. As shown in Fig. 4b, the refolding of these enzymes was also time dependent in that HsChiA1p and PtChi19p recovered their highest activities when 8 h (18.35 ± 0.55 U/mg) and 20 h (12.13 ± 0.30 U/mg) of refolding were completed, respectively.
The recovery of bioactive proteins from inclusion bodies is a complex process and the optimum conditions have to be determined on a case-by-case basis [34]. Indeed, although both HsChiA1p and PtChi19p are chitinases, they required different conditions to refold from an aggregated state in inclusion bodies to soluble and active proteins.
Conclusions
The effect of different expression parameters on the production of active recombinant chitinases HsChiA1p and PtChi19p from the marine microorganisms H. salinarum and P. tunicata, respectively, was set up in E. coli. The expression of active enzymes was significantly improved by changes in temperature and post-induction time of incubation, in the amount of inducer, in the density of the cultures prior to induction and in the E. coli host expression strains used. The effects that each parameter caused on the activity of the two studied chitinases were specific to each enzyme. The recovery of enzyme activity from inclusion bodies, generated during the induction of cultures, was achieved by their solubilisation and subsequent refolding. Reaching the maximal amount of active recombinant enzymes from the heterologous expression of both archaeal family 18 and bacterial family 19 chitinases in E. coli is of interest for their scaling up and more cost-effective future production for biotechnological applications.
References
Adrangi S, Faramarzi MA (2013) From bacteria to human: a journey into the world of chitinases. Biotechnol Adv 31:1786–1795
Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, Javed S (2013) Chitinases: an update. J Pharm Bioallied Sci 5:21–29
Yang J, Liang L, Li J, Zhang KQ (2013) Nematicidal enzymes from microorganisms and their applications. Appl Microbiol Biotechnol 97:7081–7095
Inokuma K, Takano M, Hoshino K (2013) Direct ethanol production from N-acetylglucosamine and chitin substrates by Mucor species. Biochem Eng J 72:24–32
Zhang Y, Zhou Z, Liu Y, Cao Y, He S, Huo F, Qin C, Yao B, Ringo E (2014) High-yield production of a chitinase from Aeromonas veronii B565 as a potential feed supplement for warm-water aquaculture. Appl Microbiol Biotechnol 98:1651–1662
Hannig G, Makrides SC (1998) Strategies for optimizing heterologous protein expression in Escherichia coli. Trends Biotechnol 16:54–60
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:1–17
Waegeman H, Soetaert W (2011) Increasing recombinant protein production in Escherichia coli through metabolic and genetic engineering. J Ind Microbiol Biotechnol 38:1891–1910
Jana S, Deb JK (2005) Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol 67:289–298
Sørensen HP, Mortensen KK (2005) Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Fact 4:1
Lee GH, Cooney D, Middelberg APJ, Choe WS (2006) The economics of inclusion body processing. Bioprocess Biosyst Eng 29:73–90
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99:303–310
García-Fraga B, da Silva AF, López-Seijas J, Sieiro C (2014) Functional expression and characterization of a chitinase from the marine archaeon Halobacterium salinarum CECT 395 in Escherichia coli. Appl Microbiol Biotechnol 98:2133–2143
García-Fraga B, da Silva AF, López-Seijas J, Sieiro C (2015) A novel family 19 chitinase from the marine-derived Pseudoalteromonas tunicata CCUG 44952T: heterologous expression, characterization and antifungal activity. Biochem Eng J 93:84–93
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol 134:169–176
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. CSHLP, New York
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Díaz S, Pérez-Pomares F, Pire C, Ferrer J, Bonete MJ (2006) Gene cloning, heterologous overexpression and optimized refolding of the NAD-glutamate dehydrogenase from Haloferax mediterranei. Extremophiles 10:105–115
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Palomares LA, Estrada-Mondaca S, Ramírez OT (2004) In: Balbás P, Lorence A (eds) Methods in molecular biology. Recombinant gene expression: reviews and protocols. Humana Press, Totowa
Fernández-Castané A, Caminal G, López Santín J (2012) Direct measurements of IPTG enable analysis of the induction behavior of E. coli in high cell density cultures. Microb Cell Fact 11:58. doi:10.1186/1475-2859-11-58
Balbás P (2001) Understanding the art of producing protein and nonprotein molecules in Escherichia coli. Mol Biotechnol 19:251–267
Hu JH, Wang F, Liu CZ (2015) Development of an efficient process intensification strategy for enhancing Pfu DNA polymerase production in recombinant Escherichia coli. Bioprocess Biosyst Eng 38:651–659
Olaofe OA, Burton SG, Cowan DA, Harrison STL (2010) Improving the production of a thermostable amidase through optimising IPTG induction in a highly dense culture of recombinant Escherichia coli. Biochem Eng J 52:19–24
Tolia NH, Joshua-Tor L (2006) Strategies for protein coexpression in Escherichia coli. Nat Methods 3:55–64
Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol 22:1399–1408
Zhang J-D, Li A-T, Xu J-H (2010) Improved expression of recombinant cytochrome P450 monooxygenase in Escherichia coli for asymmetric oxidation of sulfides. Bioprocess Biosyst Eng 33:1043–1049
Gupta P, Ghosalkar A, Mishra S, Chaudhuri TK (2009) Enhancement of over expression and chaperone assisted yield of folded recombinant aconitase in Escherichia coli in bioreactor cultures. J Biosci Bioeng 107:102–107
Volontè F, Marinelli F, Gastaldo L, Sacchi S, Pilone MS, Pollegioni L, Molla G (2008) Optimization of glutaryl-7-aminocephalosporanic acid acylase expression in E. coli. Protein Expr Purif 61:131–137
López PJ, Marchand I, Joyce SA, Dreyfus M (1999) The C-terminal half of RNase E, which organizes the Escherichia coli degradosome, participates in mRNA degradation but not rRNA processing in vivo. Mol Microbiol 33:188–199
De Marco A (2013) Recombinant polypeptide production in E. coli: towards a rational approach to improve the yields of functional proteins. Microb Cell Fact 12:101
Novy R, Drott D, Yaeger K, Mierendorf R (2001) Overcoming the codon bias of E. coli for enhanced protein expression. Innovations 12:1–3
Nakamura Y, Gojobori T, Ikemura T (2000) Codon usage tabulated from international DNA sequence databases: status for the year 2000. Nucleic Acids Res 28:292
De Bernardez ClarkE (1998) Refolding of recombinant proteins. Curr Opin Biotechnol 9:157–163
Acknowledgments
G.-F., B. is grateful to Dr. Martha Clokie and Dr. Didier Philippe for allowing her to make a working visit to the Department of Infection, Immunity and Inflammation of the University of Leicester (United Kingdom) where she improved her knowledge and skills about protein expression. This work was supported by the Xunta de Galicia (Grant Number 10PXIB310278PR). G.-F., B. has a predoctoral fellowship from the Xunta de Galicia-Campus do Mar. L.-S., J. has a predoctoral fellowship from the University of Vigo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Fraga, B., da Silva, A.F., López-Seijas, J. et al. Optimized expression conditions for enhancing production of two recombinant chitinolytic enzymes from different prokaryote domains. Bioprocess Biosyst Eng 38, 2477–2486 (2015). https://doi.org/10.1007/s00449-015-1485-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1485-5