Abstract
Organic carbon, nitrogen, and sulfur are highly concentrated in municipal solid waste (MSW) landfill leachate, which usually frustrates conventional leachate treatment technologies from the perspective of energy costs. Therefore, the possibility of converting leachate to a new energy source via microbial fuel cell (MFC) technology has been examined recently. This paper summarizes the power output and energy recovery efficiency of the leachate-fed MFCs according to different feeding patterns, cell structures, and loading rates. Also, we assess potential energy-generating chemicals in leachate like nitrogen and sulfur compounds and propose alternative pathways, which may lift strict ratios between organic carbon and nitrogen content in conventional denitrification of leachate and are expected to achieve a higher voltage than traditional organic-oxygen based cells. Although currently power output of leachate-fed MFCs is limited, it seems well possible that dynamic characteristics of MSW leachates and microbial physiologies underlying some bio-electrochemically efficient activities (e.g., direct interspecies electron transfer) could be stimulated in MFC systems to improve the present status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill leachate is the percolate of excessive rainwater and moisture of wastes. Although its quantity is influenced by precipitation, most (>70 %) of the liquid derives from the degradation of organics and the release of moisture (Sao Mateus Mdo et al. 2012; Zhang et al. 2010). Organic substances constitute around 60 % of municipal solid waste (MSW) in landfills and have a moisture content of 40 % or more, which means that copious amounts of leachate are generated (Zakir Hossain et al. 2014). Although composting and incineration of solid waste are preferred (EC 1999), landfill sites still receive the largest amount of MSW worldwide (350 million tons) regardless of countries’ development levels (Hoornweg and Bhada-Tata 2012). It is estimated that, depending on the climate, the volume of leachate generated over the lifespan of a landfill is equivalent to 15 to 50 % of the total volume of MSWs deposited (Canziani and Cossu 1989). The concentrations of typical contaminants in MSWs landfill leachate such as biodegradable organic matter, inorganic macro-components (e.g., hydrogen sulfide, ammonia), and xenobiotic organic compounds (XOCs) are 100 times higher than in domestic wastewater (Kjeldsen et al. 2002; Koshy et al. 2007).
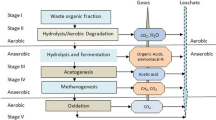
Considering its high generation rates and strength, many technologies have been applied to leachate treatment including advanced oxidation processes (Deng and Englehardt 2006), membrane separation (Chan et al. 2007), passive aeration in bio-filters (Xie et al. 2010), and anaerobic digestion (Zairi et al. 2013). All these technologies, except for anaerobic digestion, consume energy (Fig. 1). Microbial fuel cell (MFC) technology is thought to be a promising treatment alternative to reduce contaminants and simultaneously recover energy from MSW landfill leachate (Ganesh and Jambeck 2013). Although MFC technology has been frequently reviewed for domestic and industrial wastewater treatment ( Gil-Carrera et al. 2013a; Kim et al. 2010), its consideration towards MSW landfill leachate treatment is rarely reported. Hence, this review summarizes studies pertinent to the interface between landfill leachate characteristics and MFC technologies including energy potentials of chemicals in leachate, key factors in system efficiency enhancement, and functional microbial communities. We attempt to inspire potential researchers to explore more energy harnessing pathways from MSW leachates to convert MSW landfilling to a greener technology.
Energy costs of different treatment technologies, including aerobic systems (AS1 and AS2 denote low and conventional aeration rates, respectively), anaerobic digestion (AD), and hybrid process (AD + AS) by Christgen et al. (2015); microbial electrolysis cells (MEC) by Gil-Carrera et al. 2013b); membrane bioreactor (MBR) by Jabornig and Podmirseg (2015); advanced oxidation process (AOP) by Kohler et al. (2012); and leachate-fed MFC by Zhang and He (2013)
Characteristics and energy potential of MSW landfill leachates
Characteristics and composition of MSW landfill leachate
Landfills are very dynamic systems, and leachate characteristics vary as a function of landfilling procedure. MSW leachate usually falls into three categories, fresh, intermediate, and mature, according to the composition of the landfills and the degradation stages of the waste (Fig. 2). In detail, leachates drained from hydrolysis and fermentation stages are considered fresh and intermediate, respectively; organics present include volatile fatty acids (VFAs), aromatic hydrocarbons, phenols, and chlorinated aliphatic (Kjeldsen et al. 2002). Furthermore, 85 % of the organics are present as dissolved organic matter (DOM), while high molecular weight compounds account for only ~1.3 % (He et al. 2006). This readily biodegradable leachate is generated over a period of 3 to 5 years, which is relatively short compared to the overall life time of the landfill (typically 30 to 50 years). Field studies show that this organic mixture exhibits a chemical oxygen demand (COD) in the range of 4,000 to 40,000 mg/L (Tchobanoglous et al. 1993); 60~70 % of this COD is biodegradable and can be further converted into short chain VFAs in the end of the first two stages (Fig. 2). Therefore, microbiological methods can be productively applied to the treatment of fresh and intermediate leachates (Fig. 1).
COD balance of the organic fraction (redrawn from Lema et al. (1988)) and chemicals’ redox potential in landfill leachate at different stages
However, the next stage is rather time consuming and may take 15 to 30 years. This lengthy degradation period can be attributed to the low activity and reproduction rates of methanogens under initial acidic conditions (Kim 2003), while another landfill specific reason is the presence of elevated levels of toxicants (heavy metals, XOCs, and ammonia) during the period of methanogenesis (Bernard et al. 1997). The content of heavy metals like cadmium (Cd2+), chromium (Cr3+), copper (Cu2+), lead (Pb2+), nickel (Ni2+), and zinc (Zn2+) is highly variable in leachate; their average content is typically <1 mg/L, an amount equivalent to ~0.02 % of heavy metals received in total (Flyhammar et al. 1998; Kjeldsen et al. 2002). However, the effects of these heavy metals on methanogenesis and bioelectricity generation are generally minor compared to the effects of ammonia (Ariunbaatar et al. 2015; Choi et al. 2014). Our own previous work has showed that NH3-N reaches levels of ~1,000 mg/L in mature leachate with few volatile acids, amines, or alcohols detected (Xie et al. 2010). Among these “organic leftovers,” the proportion of high molecular weight DOM increases to 32 %, and 60 % of these are detected as fulvic and humic-like compounds, which can neither be directly utilized in nitrogen reduction nor as biofuels (Puig et al. 2011; Wu et al. 2015). Indeed, their low biodegradability has largely frustrated conventional bio-treatment methods; therefore, physiochemical technologies are often used (Fig. 1).
Status quo of energy generation from organics in leachate
Acknowledging that organic carbon is abundant in leachate, particularly in fresh and intermediate ones (Fig. 2), Damiano et al. (2014) and You et al. (2006) have proven that landfill leachate can be used in MFCs, but at the same time, these authors argue that dynamic leachate should be pre-stabilized since a too low COD (<150 mg/L) concentration would be a limiting factor for bioelectrochemical reactions at the anode, while on the other hand, excessively high COD concentrations (>1,000 mg/L) may bring down coulombic efficiency (CE), especially in membraneless systems. Likewise, Ozkaya et al. (2013) found that a continuous increase in influent COD concentration was companied by an initial rise and subsequent sharp decrease in power density (Table 1); but in contrast, the CE value of consumed leachate showed a constant decline (57 to 1 %). Therefore, dilution of the carbon-rich stream to a proper COD and/or loading range is strongly recommended. Interesting in this context is also a study by Teng et al. (2010), who working with simulated leachate pointed out that at increasing proportions of butyrate and propionate power density and CE decreased from 1.9 to 1.0 W/m3 and from 34 to 20 %, respectively. This decline is partially consistent with the observation of Puig et al. (2011) that even though power density increased from 0.15 to 0.3 W/m3 with the addition of more raw leachate, CE finally dropped to ~2 %, whereas this value generally ranges from 20 to 30 % in pure culture (alcohol-fed) MFCs (Kim et al. 2007). It appears that high heterogeneity of carbon sources in landfill leachate could potentially put a dent in the energy and treatment efficiency of MFCs. This implies that more (bio)engineering is needed before this highly complex and carbon abundant mixture can serve as a suitable substrate for current generation in MFCs.
Apart from substrates, the effectiveness of a leachate-fed MFCs could also be affected by the reactor configuration and the operational conditions of the reactor. Table 1 shows that the dual chamber configuration slightly increases the systems’ CE but that this has no positive effect on power density, probably due to the correspondingly increased internal resistance. In single chamber MFCs, molecular oxygen can easily diffuse across the membrane to the anode and bring down the CE. In membraneless fuel cells, which are characterized by low internal resistances and high power densities, the system uses only 1.5 % of its consumed electrons for electricity generation (Zhang et al. 2008). In addition, compared with batch feeding, the continuous pattern is clearly at a disadvantage with respect to energy generation (Table 1), probably because the microbially produced mediators (as secondary metabolites) involved in shuttling electrons to the anode (Lovley 2006; Rabaey et al. 2004) are continuously removed from the system. Indeed, these setup constrains on electricity generation are not specific to leachate-fed systems. What characterizes these systems is their high susceptibility to electron losses given the high concentrations of potential electron acceptors (nitrate, nitrite, sulfate, etc.) and amenable substrates for methanogens in the anode compartment; commonly identified competing pathways therefore include denitrification and methanogenesis (You et al. 2006; Zhang and He 2013).
In summary, even though the high organic content in landfill leachate could presumably reduce the “side effects” of carbon sources and reactor setup on power density, systems mentioned above all additionally suffered from low CE issues. Thus, transforming carbon-diverse leachates to substrates (e.g., acetate) favored by exoelectrogens should be given priority in operation. This would suggest a multi-stage approach. Indeed, it has been well established that running systems in series (stacks) or implementing anaerobic pretreatment can both increase electrogenic potential of leachates and lower the competitiveness of competing electron acceptors like nitrate (Galvez et al. 2009; Tugtas et al. 2013). The reason why the use of metal-modified electrodes, which are routinely recommended as enhancing CE, is not advised is that young leachate is very corrosive while its high hydrogen sulfide content could lead to metal poisoning or sulfur precipitation problems. Even though Ozkaya et al. (2013) stated that their Ti-TiO2 electrodes enhanced power by 15 times and sustained for 20 days, the CE problem in the high leachate loading rate scenario is yet to be resolved (Table 1).
Energy generation from inorganics in leachate and prospected pathways
Using nitrate as electron acceptor in MFCs is promising since (i) its redox potential is comparable to that of oxygen (nitrate to N2, Fig. 2) and (ii) it alleviates the concern about oxygen diffusion into the anode compartment. The first published designs based the use of bio-cathodes on the argument that exoelectrogens can reduce nitrate/nitrite with an electrode as electron donor (Gregory et al. 2004). However, this concept had always been realized in a potentiostat-poised half cell (external current supply is necessary), until Clauwaert et al. (2007) developed a system where a biocathode catalyzed denitrification by utilizing electrons from microorganisms oxidizing acetate in the anode.
As shown in Fig. 2, the inorganic nitrogen content starts to increase with age in intermediate and mature leachates, mainly in the form of NH3-N, and typically reaches values of 700 mg/L (Kjeldsen et al. 2002). Harnessing this energy source necessitates a pre-nitrification step. For example, Lee et al. (2013) used an external aeration column to oxidize leachates and then fed the highly concentrated N-ions (mostly nitrate) to the cathode as potential electron acceptors. Their system achieved a peak power density of 12 mW/m2 (Table 1), lower than obtained with oxygen biocathode (Zhang and He 2013). This is not surprising because the redox potential of nitrate/nitrogen is theoretically inferior to the O2/H2O’s (0.7 vs. 0.8 V). However, Zhang and He (2013) further showed that the CE based on leachate-organic/nitrate (8.4 %) is ten times higher than on leachate-organic/oxygen (0.6 %). Thus, we may infer from these studies that the presence of highly concentrated inorganic N can turn the leachate into a suitable MFC substrate.
Nevertheless, bio-cathodes as mentioned above still need external energy to maintain a negative potential and implement nitrification (Gregory et al. 2004; Lee et al. 2013). Therefore, it would be intriguing to know if energy can be harnessed from the ammonia oxidation aiming to offset, if any, the aeration costs. Kim et al. (2008) detected no electricity generated with the addition of ammonia at first, but Fig. 3a shows that both aerobic and anaerobic ammonia oxidation (anammox) are exergonic and may theoretically indicate a spontaneous electron flow. Thus, the pending issue is whether the corresponding bio-catalysts (electrogenic species/enzymes) exist. Fortunately, recent studies concerning N-based MFCs suggested that not only ammonia but also intermediate products (e.g., nitrate and hydroxylamine) could all be utilized as fuels at the anode by typical nitrifying bacteria Nitrosomonas and Nitrospira (Chen et al. 2014). Lee et al. (2013) observed that an anammox biocathode generated slightly higher power density than a denitrifying biocathode (~30 vs. ~8 W/m2) in a leachate-fed system, although the exact pathways were not unequivocally confirmed. Initiatives to integrate anammox in leachate-fed MFCs are based on the premises that the configuration of bioelectrogenic systems (a combination of anoxic and aerobic physiologies) and the low carbon/nitrogen ratio in mature leachate can both facilitate this autotrophic process. However, these drivers may lead to the misuse or overgeneralization of anammox-MFC concept. For example, Li et al. (2014a) just took advantage of anoxic condition in anodic chamber for implementing anammox but did not explain its role in electricity generation. From the perspective of energy generation, future research hence may need to explore the possibility of anammox (or similar functional) bacteria in electrode respiration, which in principle has been proven possible by Jadhav and Ghangrekar (2015).
Mechanisms by which nitrogen (a; −335 and −272 kJ/mol indicate energy released of anaerobic and aerobic ammonia oxidation, respectively, under standard conditions) and sulfur metabolites (b, c) contribute to electricity generation in microbial fuel cells. Dashed line and solid line represent biotic and abiotic pathways, respectively. SO, SRB, AOB, and NRB denote sulfide-oxidizing bacteria (e.g., Desulfobulbus propionicus), sulfate-reducing bacteria, ammonia-oxidizing bacteria, and nitrate-reducing bacteria, respectively. DSR represents the denitrification sulfide removal process, where one of the currently identified functional microorganism is Pseudomonas sp. C27 (Cai et al. 2013). Those electrons released by sulfide oxidation but not accepted by nitrate were finally transferred to the anode electrode; the exact electron numbers cannot be confirmed at our current knowledge
The sulfate content in leachate generally ranges from 500 to 2,000 mg/L and is usually readily reduced by sulfate-reducing bacteria (SRB) to sulfide in stage 1 (~1 year after landfilling) ( El-Fadela et al. 2002; Kjeldsen et al. 2002). Sulfur-based MFCs have revealed thatSO 2 −4 , S2 − and S0 can act as electron mediators rather than as substrates (Ieropoulos et al. 2013; Rabaey et al. 2006). The high organics and sulfur content in landfill leachate therefore can be viewed as wastewater mixed with electron mediators. Ieropoulos et al. (2013) and Lee et al. (2014) reported that oxidation of this mixture achieved a higher specific power of 80 and 60 mW/m2, respectively, in comparison to other leachate-fed MFCs (Table 1), and that the oxidation of sulfide involved two electrons transferring to the anode with the formation of S0 nanoparticles (Fig. 3b). However, Holmes et al. (2004) and Gong et al. (2013) have reported that these two electrons were harvested abiotically (at a potential no less than −0.27 V) and another six were harvested biotically, possibly by Desulfobulbus propionicus, on an electrode poised at a potential above 0.3 V (Fig. 3c). Interestingly, a higher anode voltage is thus required for complete sulfur oxidation and consequently for harnessing all electrons and eliminating S0 deposition induced electrode fouling issues, while on the other hand, an elevated anode potential lowers the output energy, which is a function of the difference between anode and cathode potential. Tailored research will be needed to determine the optimum for specific leachates.
Stepping away from these existing organic/sulfur systems, Cai et al. (2013) have constructed a new system that was expected to use sulfide and nitrate as electron donor and acceptor, respectively. This system achieved a constant current density of ~150 mA/m2, where >90 % sulfide and nitrate was removed. This research is inspiring considering the large amounts of sulfide and inorganic N coexisting in mature leachate (Fig 2). Also, sulfide/nitrate-based cells could theoretically gain 1.87 V (Eq. 1, by Cai et al. (2013); this value is considerably higher than the theoretical gain for the traditional organic C (acetate)/oxygen redox couple (~1.2 V).
It is unknown which portion of the energy/voltage electrogenic microorganisms retain for their own metabolism. More research is needed to explore in which pathways and with what efficiencies functional microorganism transfer electrons to or gain electrons in bioelectrochemical systems treating mature leachate with high S and N content.
Specific microbial accesses in MSW leachate to energy in the MFC
As part of the stepwise degradation process depicted in Fig. 1, many anaerobes in landfill leachate indeed are capable of intracellular electron transfer to nitrate and sulfate for anaerobic respiration (Wang et al. 2014). What distinguishes electrogenic bacteria is their ability to transport electrons outside their membrane. In their special respiration chains, electrodes are used as terminal electron acceptors, while the protons generated are transferred to and consumed at the cathode. Commonly detected electrogenic bacteria belong to three genera, Shewanella (Shewanella oneidensis, Shewanella putrefaciens), Geobacter (Geobacter sulfurreducens, Geobacter metallireducens), and Rhodoferax (Rhodoferax ferrireducens), which can all gain energy from the use of insoluble metal as an electron acceptor in nature (Chaudhuri and Lovley 2003; Szollosi et al. 2015). It is notable that these microorganisms are all classified as Proteobacteria, organisms that are abundant in both fresh and mature MSW landfill leachate (Kobayashi et al. 2013; Liu et al. 2011). This may imply that landfill leachate possesses a wealth of organisms that can gain energy in MFCs. In effect, rarely does one single species or genus dominate in the bacterial communities attached on the anode, especially when fed with complex substrates such as landfill leachate (Logan and Regan 2006).
Recent metagenomic analysis has revealed a clear disparity in microbial communities between acetate-fed and leachate-fed scenarios, where the relative abundance of Geobacter declined tenfold upon addition of leachate (Zhang et al. 2014). One possible reason is that complex organics cannot be efficiently or directly used by the electrogenic microorganisms (detailed in next section). Another reason may be that heavy metals present in leachate may adversely affect the viability of exoelectrogens. Abourached et al. (2014) reported that maximum tolerable concentrations (MTCs) of the ions Cd (VI) and Zn (II) for the electrochemically active microorganisms are 0.2 and 0.4 mg/L; a higher concentration (>0.5 mg/L) could significantly inhibit this reaction, as indicated by a 70 % reduction in voltage. As summarized by Kjeldsen et al. (2002), total zinc in MSW landfill leachate (0.2 to 5.3 mg/L) exceeded the MTC in most cases. However, heavy metal ions can readily form complexes with inorganic and organic ligands, which would safeguard MFCs against “heavy metal poisoning.” Thus, further study on heavy metals’ toxicity in leachate to electrogenic microorganisms is urgently needed.
Fermentation and interspecies electron transfer in leachate
Landfill leachate degradation processes are distinct from those studied in single substrate incubations (e.g., studies with acetate in microbial fuel cells) or typical wastewater treatment pathways: they feature an intricate combination of sequential and parallel processing of substrates, dictated by distinct and stepwise microbial activities (Renou et al. 2008). Therefore, enhancing energy recovery efficiency from leachate is difficult to achieve, if the abundance of exoelectrogens is truly the key factor in electricity production. However, the “food chain” mentioned above may imply alternative potentialities for electricity generation (Dolfing 2014). Firstly, fermentation is a prerequisite for the effective oxidation of complex organic matter, such as aromatic compounds and long-chain fatty acids (McInerney et al. 2009). Pre-fermentation of leachate (acetate and succinate as major products) increased the electron recovery rates in a fuel cell by 20 % (Mahmoud et al. 2014). This is because the currently known exoelectrogens cannot metabolize complex substrates (Chaudhuri and Lovley 2003). Thus, MFCs fed with complex substrates, which generally have a lower redox potential than acetate (e.g., glucose vs. acetate, Fig. 2), only recovered 2–6 % of the theoretical voltage (Lee et al. 2008; Logan 2004). It appears that abundant energy, stored in complex substrates, for example carbohydrates, in waste streams is harnessed limitedly in MFCs as a substantial part of this energy is released during fermentation to the substrates that are “edible to exoelectrogens.”
However, there is evidence that electrons could be shuttled to electrodes directly from fermentative microorganisms (Fig. 4a), such as Geothrix fermentans, Clostridium butyricum, and Pseudomonas chlororaphis via self-produced electron mediators, Fe (III) reduction, or abiotic oxidation of their products (e.g., hydrogen) at the anode (Hernandez et al. 2004; Lovley 2006; Park et al. 2001). Nevertheless, the role and function of fermentative microorganisms in leachate-fed MFCs are still controversial. Hydrogen-oxidizing methanogens (responsible for hydrogen transfer in leachate fermentation) can outcompete exoelectrogens (Lee et al. 2008), and pragmatically the methanogens are sometimes allowed to take control (in anode) to enhance proton recover rates (Chae et al. 2010). But, importantly, coexistence of Hydrogenophaga (hydrogen-gas consuming exoelectrogens) and fermentative bacteria significantly increased energy output (Kimura and Okabe 2013). Thus, further research is warranted to evaluate whether fermentation should be combined with bioelectricity generation.
Mechanisms of electrons transferred to anode by fermentative bacteria (a) and proposed pathways involving exoelectrogens and fermentative bacteria (b). Dashed line and solid line represent substrate and electron pathways, respectively, and FB and EB denote fermentative bacteria and exoelectrogenic bacteria, respectively. Red circles denote self-produced electron mediators
The occurrence of syntrophy in landfill sites based on hydrogen-mediated interspecies electrons transfer is well established (Jakobsen et al. 1998). Direct interspecies electron transfer (DIET) on the other hand is favored thermodynamically because it is more energy efficient: (i) hydrogen inhibits fermentation of organics and (ii) the hydrogen gradients needed for interspecies hydrogen transfer dissipate energy (Dolfing 1992; Fukuzaki et al. 1990; Summers et al. 2010). One prerequisite for DIET is physical contact. The existence of DIET was first observed by Gorby et al. (2006), who observed that fermentative microbes (Pelotomaculum thermopropionicum) were wired to methanogens (Methanothermobacter thermoautotropicus) by conductive filaments, which were previously thought to be exclusive to exoelectrogens transferring electrons to metals and electrodes. Subsequently, Summers et al. (2010) observed that biomass aggregates are another form of physical contact where DIET can occur.
It has been suggested by Lovley (2006) that transfer of electrons to natural extracellular material is an adaptive evolution process selecting for the most effective strategies for energy production. We speculate that large organic polymers could trigger similar processes at leachate-fed anodes. It therefore would be interesting to know whether electrons can be directly transferred from fermentative organisms (complex organics) via exoelectrogens (presumably via NAD+/NADH) to an anode (Fig. 4b). These physical contacts allowing DIET would overcome the energy-harvesting barriers of non-degradable substrate (by exoelectrogens) in fresh and intermediate leachate. Recently, Li et al. (2014b) have successfully used nanoFe3O4, analogous to nanowires, to connect syntrophic microorganisms in an engineered form of DIET. It is illuminating that the addition of nanomaterial may facilitate this energy efficient electron pathway. Apart from direct electron transfer, mediated electron transfer (MET) in bioelectrochemical systems is also frequently observed (Schröder 2007). In MET, electron mediators wire microbial metabolism to a fuel cell anode via the shifts of their redox potentials. Actually, the high content of accumulated humic substances in mature leachate (60 % of the DOM) compared to <6 % in most other types of wastewater, specifically favor MET. Regular addition of exogenous mediators would be technologically unfeasible and economically questionable (Kjeldsen et al. 2002). For this reason, mature leachate has been mixed with waste and/or wastewater in order to streamline the electron transfer chain in substrate oxidation and fermentation. For example, Capodici et al. (2014) reported that the electron transfer efficiency (respiration rate) increased by 40 % with addition mature leachate. Also, research by Ferraz et al. (2014) has shown that humic substances at a low leachate mix ratio (~2 %) enhanced the systems’ bioactivity and indicated that this refractory organic material underwent self-degradation. From these observations, we conclude that mature leachate can be theoretically converted to energy harnessing facilitators.
Conclusions
Landfill leachate-based MFCs provide us a new research platform, which straddles engineering and the underpinning science. This review shows that fresh and intermediate leachate can in principle be used for bioelectricity generation, but that efficient energy recovery will require integrated decomposition of a highly diverse waste stream, for example by operating fuel cells in series and/or coupled to anaerobic pre-digestion. Metal modification of electrodes can be applied to enhance output energy if the material is cost-efficient, and Ti-TiO2 is a good option compared to platinum. But this approach with respect to leachate treatment is clouded by issues like H2S-induced metal catalyst poisoning and sulfur precipitation. Most MFCs therefore opt for the metal-modified electrode as air cathode. The current application of leachate-fed bioelectrochemical systems is mainly aimed at contaminant removal and has circumvented the tension between organic carbon and nitrogen content, which plagues conventional denitrification in mature leachate, through anammox and simultaneous N and S removal. These designs do however suggest that the high concentrations of inorganic N and S metabolites in leachate can also be used as novel biofuels; the energy “appropriation” by electrogenic microorganisms will be the key limit in their application. Also, it appears that the relationship between fermentative microorganisms and electrogenic bacteria is critical in energy output enhancement, particularly via the energy efficient DIET. This potential can be realized by the addition of nanomaterials and/or humic substance-rich mature leachate. The current generation of “off-the-shelf” MFC technology has already been shown to be applicable to the treatment of landfill leachate, but economics will eventually decide whether this technology is appropriate.
References
Abourached C, Catal T, Liu H (2014) Efficacy of single-chamber microbial fuel cells for removal of cadmium and zinc with simultaneous electricity production. Water Res 51:228–233. doi:10.1016/j.watres.2013.10.062
Ariunbaatar J, Di Perta ES, Panico A, Frunzo L, Esposito G, Lens PN, Pirozzi F (2015) Effect of ammoniacal nitrogen on one-stage and two-stage anaerobic digestion of food waste. Waste Manag 38:388–398. doi:10.1016/j.wasman.2014.12.001
Bernard C, Colin JR, Anne LD (1997) Estimation of the hazard of landfills through toxicity testing of leachates: 2. Comparison of physico-chemical characteristics of landfill leachates with their toxicity determined with a battery of tests. Chemosphere 35(11):2783–2796. doi:10.1016/S0045-6535(97)00332-9
Cai J, Zheng P, Zhang J, Xie Z, Li W, Sun P (2013) Simultaneous anaerobic sulfide and nitrate removal coupled with electricity generation in microbial fuel cell. Bioresour Technol 129:224–228. doi:10.1016/j.biortech.2012.11.008
Canziani R, Cossu R (1989) Landfill hydrology and leachate production. In: Christensen TH, Cossu R, Stegmann R (eds) Sanitary landfilling: process, technology and environmental impact. Academic, London
Capodici M, Di Trapani D, Viviani G (2014) Co-treatment of landfill leachate in laboratory-scale sequencing batch reactors: analysis of system performance and biomass activity by means of respirometric techniques. Water Sci Technol 69(6):1267–1274. doi:10.2166/wst.2014.005
Chae KJ, Choi MJ, Kim KY, Ajayi FF, Park W, Kim CW, Kim IS (2010) Methanogenesis control by employing various environmental stress conditions in two-chambered microbial fuel cells. Bioresour Technol 101(14):5350–5357. doi:10.1016/j.biortech.2010.02.035
Chan GYS, Chang J, Kurniawan TA, Fu C-X, Jiang H, Je Y (2007) Removal of non-biodegradable compounds from stabilized leachate using VSEPRO membrane filtration. Desalination 202(1-3):310–317. doi:10.1016/j.desal.2005.12.069
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21(10):1229–1232. doi:10.1038/nbt867
Chen H, Zheng P, Zhang J, Xie Z, Ji J, Ghulam A (2014) Substrates and pathway of electricity generation in a nitrification-based microbial fuel cell. Bioresour Technol 161:208–214. doi:10.1016/j.biortech.2014.02.081
Choi C, Hu N, Lim B (2014) Cadmium recovery by coupling double microbial fuel cells. Bioresour Technol 170:361–369. doi:10.1016/j.biortech.2014.07.087
Christgen B, Yang Y, Ahammad SZ, Li B, Rodriquez DC, Zhang T, Graham DW (2015) Metagenomics shows that low-energy anaerobic-aerobic treatment reactors reduce antibiotic resistance gene levels from domestic wastewater. Environ Sci Technol 49(4):2577–2584. doi:10.1021/es505521w
Clauwaert P, Rabaey K, Aelterman P, Schamphelaire LJD, Pham TH, Boeckx P, Boon N, Verstraete W (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360. doi:10.1021/es062580r
Damiano L, Jambeck JR, Ringelberg DB (2014) Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl Biochem Biotechnol 173(2):472–485. doi:10.1007/s12010-014-0854-x
Deng Y, Englehardt JD (2006) Treatment of landfill leachate by the Fenton process. Water Res 40(20):3683–3694. doi:10.1016/j.watres.2006.08.009
Dolfing J (1992) The energetic consequences of hydrogen gradients in methanogenic ecosystems. FEMS Microbiol Ecol 101:183–187. doi:10.1111/j.1574-6941.1992.tb01654.x
Dolfing J (2014) Syntrophy in microbial fuel cells. ISME J 8(1):4–5. doi:10.1038/ismej.2013.198
El-Fadela M, Bou-Zeida E, Chahineb W, Alaylic B (2002) Temporal variation of leachate quality from pre-sorted and baled municipal solid waste with high organic and moisture content. Waste Manag 22:269–282
European Council (1999) Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste. In: European Communities (ed) 1999/31/EC.
Ferraz FM, Povinelli J, Pozzi E, Vieira EM, Trofino JC (2014) Co-treatment of landfill leachate and domestic wastewater using a submerged aerobic biofilter. J Environ Manag 141:9–15. doi:10.1016/j.jenvman.2014.03.022
Flyhammar P, Tamaddon F, Bengtsson L (1998) Heavy metals in a municipal solid waste deposition cell. Waste Manag Res 16(5):403–410
Fukuzaki S, Nishio N, Shobayashi M, Nagai S (1990) Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl Environ Microbiol 56(3):719–723
Galvez A, Greenman J, Ieropoulos I (2009) Landfill leachate treatment with microbial fuel cells; scale-up through plurality. Bioresour Technol 100(21):5085–5091. doi:10.1016/j.biortech.2009.05.061
Ganesh K, Jambeck JR (2013) Treatment of landfill leachate using microbial fuel cells: alternative anodes and semi-continuous operation. Bioresour Technol 139:383–387. doi:10.1016/j.biortech.2013.04.013
Gil-Carrera L, Escapa A, Mehta P, Santoyo G, Guiot SR, Moran A, Tartakovsky B (2013a) Microbial electrolysis cell scale-up for combined wastewater treatment and hydrogen production. Bioresour Technol 130:584–591. doi:10.1016/j.biortech.2012.12.062
Gil-Carrera L, Escapa A, Moreno R, Moran A (2013b) Reduced energy consumption during low strength domestic wastewater treatment in a semi-pilot tubular microbial electrolysis cell. J Environ Manag 122:1–7. doi:10.1016/j.jenvman.2013.03.001
Gong Y, Ebrahim A, Feist AM, Embree M, Zhang T, Lovley D, Zengler K (2013) Sulfide-driven microbial electrosynthesis. Environ Sci Technol 47(1):568–573. doi:10.1021/es303837j
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Si I, Logan B, Nealson KH, Fredrickson JK (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. PNAS 106(23):11358–11363. doi:10.1073/pnas.0904454106
Greenman J, Gálvez A, Giusti L, Ieropoulos I (2009) Electricity from landfill leachate using microbial fuel cells: comparison with a biological aerated filter. Enzyme Microb Technol 44(2):112–119. doi:10.1016/j.enzmictec.2008.09.012
Gregory KB, Bond DR, Lovley DR (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6(6):596–604. doi:10.1111/j.1462-2920.2004.00593.x
He PJ, Xue JF, Shao LM, Li GJ, Lee DJ (2006) Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill. Water Res 40(7):1465–1473. doi:10.1016/j.watres.2006.01.048
Hernandez ME, Kappler A, Newman DK (2004) Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl Environ Microbiol 70(2):921–928. doi:10.1128/aem.70.2.921-928.2004
Holmes DE, Bond DR, Lovley DR (2004) Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl Environ Microbiol 70(2):1234–1237. doi:10.1128/aem.70.2.1234-1237.2004
Hoornweg D, Bhada-Tata P (2012) What a waste: a global review of solid waste management urban development series. Word Bank, Washington, DC 20433 USA
Ieropoulos I, Galvez A, Greenman J (2013) Effects of sulphate addition and sulphide inhibition on microbial fuel cells. Enzyme Microb Technol 52(1):32–37. doi:10.1016/j.enzmictec.2012.10.002
Jabornig S, Podmirseg SM (2015) A novel fixed fibre biofilm membrane process for on-site greywater reclamation requiring no fouling control. Biotechnol Bioeng 112(3):484–493. doi:10.1002/bit.25449/abstract
Jadhav DA, Ghangrekar MM (2015) Effective ammonium removal by anaerobic oxidation in microbial fuel cells. Environ Technol 36(6):767–775. doi:10.1080/09593330.2014.960481
Jakobsen R, Albrechtsen HJ, Rasmussen M, Bay H, Bjerg PL, Christensen TH (1998) H2 concentrations in a landfill leachate plume (Grindsted, Denmark): in situ energetics of terminal electron acceptor processes. Environ Sci Technol 32:2142-2148
Kim I (2003) Effect of low pH on the activity of hydrogen utilizing methanogen in bio-hydrogen process. Int J Hydrogen Energ 29:1133–1140. doi:10.1016/j.ijhydene.2003.08.017
Kim JR, Jung SH, Regan JM, Logan BE (2007) Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour Technol 98(13):2568–2577. doi:10.1016/j.biortech.2006.09.036
Kim JR, Premier GC, Hawkes FR, Rodriguez J, Dinsdale RM, Guwy AJ (2010) Modular tubular microbial fuel cells for energy recovery during sucrose wastewater treatment at low organic loading rate. Bioresour Technol 101(4):1190–1198. doi:10.1016/j.biortech.2009.09.023
Kim JR, Zuo Y, Regan JM, Logan BE (2008) Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol Bioeng 99(5):1120–1127. doi:10.1002/bit.21687
Kimura Z, Okabe S (2013) Acetate oxidation by syntrophic association between Geobacter sulfurreducens and a hydrogen-utilizing exoelectrogen. ISME J 7(8):1472–1482. doi:10.1038/ismej.2013.40
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32(4):297–336. doi:10.1080/10643380290813462
Kobayashi H, Saito N, Fu Q, Kawaguchi H, Vilcaez J, Wakayama T, Maeda H, Sato K (2013) Bio-electrochemical property and phylogenetic diversity of microbial communities associated with bioelectrodes of an electromethanogenic reactor. J Biosci Bioeng 116(1):114–117. doi:10.1016/j.jbiosc.2013.01.001
Kohler C, Venditti S, Igos E, Klepiszewski K, Benetto E, Cornelissen A (2012) Elimination of pharmaceutical residues in biologically pre-treated hospital wastewater using advanced UV irradiation technology: a comparative assessment. J Hazard Mater 239–240:70–77. doi:10.1016/j.jhazmat.2012.06.006
Koshy L, Paris E, Ling S, Jones T, Berube K (2007) Bioreactivity of leachate from municipal solid waste landfills—assessment of toxicity. Sci Total Environ 384(1-3):171–181. doi:10.1016/j.scitotenv.2007.06.017
Lee DJ, Liu X, Weng HL (2014) Sulfate and organic carbon removal by microbial fuel cell with sulfate-reducing bacteria and sulfide-oxidising bacteria anodic biofilm. Bioresour Technol 156:14–19. doi:10.1016/j.biortech.2013.12.129
Lee HS, Parameswaran P, Kato-Marcus A, Torres CI, Rittmann BE (2008) Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrates. Water Res 42(6-7):1501–1510. doi:10.1016/j.watres.2007.10.036
Lee Y, Martin L, Grasel P, Tawfiq K, Chen G (2013) Power generation and nitrogen removal of landfill leachate using microbial fuel cell technology. Environ Technol 34(17-20):2727–2736. doi:10.1080/09593330.2013.788040
Lema JM, Mendez R, Blazquez R (1988) Characteristics of landfill leachates and alternatives for their treatment: a review. Water Air Soil Pollut 40:223–250
Li C, Ren H, Xu M, Cao J (2014a) Study on anaerobic ammonium oxidation process coupled with denitrification microbial fuel cells (MFCs) and its microbial community analysis. Bioresour Technol 175:545–552. doi:10.1016/j.biortech.2014.10.156
Li H, Chang J, Liu P, Fu L, Ding D, Lu Y (2014b) Direct interspecies electron transfer accelerates syntrophic oxidation of butyrate in paddy soil enrichments. Environ Microbiol 17(5):1522–1547. doi:10.1111/1462-2920.12576
Liu J, Wu W, Chen C, Sun F, Chen Y (2011) Prokaryotic diversity, composition structure, and phylogenetic analysis of microbial communities in leachate sediment ecosystems. Appl Microbiol Biotechnol 91(6):1659–1675. doi:10.1007/s00253-011-3354-8
Logan BE (2004) Extracting hydrogen and electricity from renewable resources. Environ Sci Technol 38(9):160A–167A
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(12):512–518. doi:10.1016/j.tim.2006.10.003
Lovley DR (2006) Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 4(7):497–508. doi:10.1038/nrmicro1442
Mahmoud M, Parameswaran P, Torres CI, Rittmann BE (2014) Fermentation pre-treatment of landfill leachate for enhanced electron recovery in a microbial electrolysis cell. Bioresour Technol 151:151–158. doi:10.1016/j.biortech.2013.10.053
McInerney MJ, Sieber JR, Gunsalus RP (2009) Syntrophy in anaerobic global carbon cycles. Curr Opin Biotechnol 20(6):623–632. doi:10.1016/j.copbio.2009.10.001
Ozkaya B, Cetinkaya AY, Cakmakci M, Karadag D, Sahinkaya E (2013) Electricity generation from young landfill leachate in a microbial fuel cell with a new electrode material. Bioprocess Biosyst Eng 36(4):399–405. doi:10.1007/s00449-012-0796-z
Park HS, Kim BH, Kim HS, Kim HJ, Kim GT, Kim M, Chang IS, Park YK, Chang HI (2001) A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7(6):297–306. doi:10.1006/anae.2001.0399
Puig S, Serra M, Coma M, Cabre M, Dolors Balaguer M, Colprim J (2011) Microbial fuel cell application in landfill leachate treatment. J Hazard Mater 185(2-3):763–767. doi:10.1016/j.jhazmat.2010.09.086
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70(9):5373–5382. doi:10.1128/AEM.70.9.5373-5382.2004
Rabaey K, Sompel KV, Maignien L, Boon N, Aelterman P, Clauwaert P, Schamphelaire LD, Pham HT, Vermeulen J, Verhaege M, Lens P, Verstraete W (2006) Microbial fuel cells for sulfide removal. Environ Sci Technol 40(17):5218–5224. doi:10.1021/es060382u
Renou S, Givaudan JG, Poulain S, Dirassouyan F, Moulin P (2008) Landfill leachate treatment: review and opportunity. J Hazard Mater 150(3):468–493. doi:10.1016/j.jhazmat.2007.09.077
Sao Mateus Mdo S, Machado SL, Barbosa MC (2012) An attempt to perform water balance in a Brazilian municipal solid waste landfill. Waste Manag 32(3):471–481. doi:10.1016/j.wasman.2011.11.009
Schröder U (2007) Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys Chem Chem Phys 9(21):2619–2629. doi:10.1039/b703627m
Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR (2010) Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. doi:10.1126/science.1196526
Szollosi A, Rezessy-Szabo JM, Hoschke A, Nguyen QD (2015) Novel method for screening microbes for application in microbial fuel cell. Bioresour Technol 179:123–127. doi:10.1016/j.biortech.2014.12.004
Tchobanoglous G, Theisen H, Vigil S (1993) Integrated solid waste management, engineering principles and management issues. Irwin McGraw Hill, Boston
Teng SX, Tong ZH, Li WW, Wang SG, Sheng GP, Shi XY, Liu XW, Yu HQ (2010) Electricity generation from mixed volatile fatty acids using microbial fuel cells. Appl Microbiol Biotechnol 87(6):2365–2372. doi:10.1007/s00253-010-2746-5
Tugtas AE, Cavdar P, Calli B (2013) Bio-electrochemical post-treatment of anaerobically treated landfill leachate. Bioresour Technol 128:266–272. doi:10.1016/j.biortech.2012.10.035
Wang C, Zhao Y, Xie B, Peng Q, Hassan M, Wang X (2014) Nitrogen removal pathway of anaerobic ammonium oxidation in on-site aged refuse bioreactor. Bioresour Technol 159:266–271. doi:10.1016/j.biortech.2014.02.093
Wu D, Wang C, Dolfing J, Xie B (2015) Short tests to couple N2O emission mitigation and nitrogen removal strategies for landfill leachate recirculation. Sci Total Environ 512–513:19–25. doi:10.1016/j.scitotenv.2015.01.021
Xie B, Lv BY, Hu C, Liang SB, Tang Y, Lu J (2010) Landfill leachate pollutant removal performance of a novel biofilter packed with mixture medium. Bioresour Technol 101(20):7754–7760. doi:10.1016/j.biortech.2010.04.103
You SJ, Zhao QL, Jiang JQ, Zhang JN, Zhao SQ (2006) Sustainable approach for leachate treatment: electricity generation in microbial fuel cell. J Environ Sci Health A 41(12):2721–2734. doi:10.1080/10934520600966284
Zairi M, Aydi A, Dhia HB (2013) Leachate generation and biogas energy recovery in the Jebel Chakir municipal solid waste landfill, Tunisia. J Mater Cycles Waste Manag 16(1):141–150. doi:10.1007/s10163-013-0164-3
Zakir Hossain HM, Hasna Hossain Q, Uddin Monir MM, Ahmed MT (2014) Municipal solid waste (MSW) as a source of renewable energy in Bangladesh: revisited. Renew Sust Energ Rev 39:35–41. doi:10.1016/j.rser.2014.07.007
Zhang DQ, Tan SK, Gersberg RM (2010) Municipal solid waste management in China: status, problems and challenges. J Environ Manag 91(8):1623–1633. doi:10.1016/j.jenvman.2010.03.012
Zhang F, He Z (2013) A cooperative microbial fuel cell system for waste treatment and energy recovery. Environ Technol 34(13-16):1905–1913. doi:10.1080/09593330.2013.770540
Zhang H, Chen X, Braithwaite D, He Z (2014) Phylogenetic and metagenomic analyses of substrate-dependent bacterial temporal dynamics in microbial fuel cells. PLoS ONE 9(9):1–8. doi:10.1371/journal.pone.0107460.g001
Zhang JN, Zhao QL, You SJ, Jiang JQ, Ren NQ (2008) Continuous electricity production from leachate in a novel upflow air-cathode membrane-free microbial fuel cell. Water Sci Technol 57(7):1017–1021. doi:10.2166/wst.2008.063
Acknowledgments
This work was supported by the Natural Science Foundation of China (31370510, 313111197) and Shanghai Project of International Cooperation of Science and Technology (13520720600) and the Royal Society (Grant IE131283).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, D., Wang, T., Huang, X. et al. Perspective of harnessing energy from landfill leachate via microbial fuel cells: novel biofuels and electrogenic physiologies. Appl Microbiol Biotechnol 99, 7827–7836 (2015). https://doi.org/10.1007/s00253-015-6857-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6857-x