Abstract
Jatropha curcas has great potential as an oil crop for use in biodiesel applications, and the outer shell is rich in lignocellulose that may be converted to ethanol, giving rise to the concept of a biorefinery. In this study, two dilute pretreatments of 0.5% H2SO4 and 1.0% NaOH were performed on Jatropha shells with subsequent simultaneous saccharification and fermentation (SSF) of the pretreated water-insoluble solids (WIS) to evaluate the effect of inhibitors in the pretreatment slurry. A cellulase loading of 15 FPU/g WIS, complimented with an excess of cellobiase (19.25 U/g), was used for SSF of either the washed WIS or the original slurry to determine the effect of inhibitors. Ethanol and glucose were monitored during SSF of 20 g of pretreated biomass. The unwashed slurry showed to have a positive effect on SSF efficiency for the NaOH-pretreated biomass. Maximum efficiencies of glucan conversion to ethanol in the WIS were 40.43% and 41.03% for the H2SO4- and NaOH-pretreated biomasses, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for biofuels has increased in recent years due to the rising prices of oil and concern for the environment and carbon dioxide emissions [1]. This has made countries such as Brazil, with a predominantly tropical climate and large cultivatable area, one of the exporters of biodiesel and ethanol to countries looking to decrease their dependence on foreign oil and meet national legislature for biofuel usage. In 2009, the production of biodiesel in Brazil was slightly more than 1.6 million cubic meters per year [2, 3], much of which is produced from soybeans. Ethanol production in the same year was roughly 26 million cubic meters per year but is also rapidly expanding. Currently, all ethanol produced in Brazil is from sugarcane [3].
Estimates show that the use of agricultural residues from around the world have the potential to produce up to 442 GL (109 L) of ethanol from lignocellulosic per year, equivalent to 29% of global gasoline consumption [4]. In Brazil alone, large quantities of agricultural residues are produced from food and ethanol production as well as when extracting oil for biodiesel. Various agricultural residues including corn stover [5], wheat straw [6], rice straw and hulls [7, 8], barley straw [9], cotton stalks [10] and sugarcane bagasse [11] have all been studied for their potential as sources of cellulose for bioethanol production. As new sources of vegetable oil are being applied for the production of biodiesel, with efficiencies and yields much greater than those of soybeans, new sources of biomass will be available for the production of bioethanol.
Jatropha curcas is one of the oil plants considered to have the greatest potential for biodiesel production in various countries around the world, including Brazil. Its high oil yield (nearly four times that of soybeans) and resistant growing conditions (capable of growing diverse tropical and subtropical climates) make it appealing for biodiesel production. However, the amount water available to the plant plays a significant role in the productivity of the shrub [12].
The complete process of bioethanol production is dependent on the characteristics of the biomass; however, it is the cost-to-effectiveness ratio that finally determines form of pretreatment to be used [13, 14]. Pretreatment of lignocellulosic biomass produces a hydrolysate containing large quantities of inhibitors to both saccharification and fermentation [15]. These inhibitors include sugar degradation products, lignin degradation products, acetic acid and other compounds [16].
In order to prevent the accumulation of glucose, which inhibits cellulase activity, simultaneous saccharification and fermentation (SSF) is often performed. Pretreated solids are often washed with hot water to remove inhibitors produced during pretreatment; however, at low concentrations, these inhibitors may actually have a positive effect on ethanol production due to yeast stressing [9, 17, 18]. This would therefore eliminate the need for washing of the biomass and allow for direct co-fermentation if the appropriate yeasts are available.
The objective of this study was to evaluate the production of bioethanol from shells of the Jatropha fruit via the enzymatic hydrolysis of cellulose found in the shells. The effects of two pretreatment techniques and use of either washed or unwashed water-insoluble solids (WIS) on saccharification and fermentation for the production of ethanol were studied in this work.
Materials and methods
Raw material
Jatropha curcas shells were obtained from a local producer in the region of Viçosa, Minas Gerias, Brazil. The shells were delivered to the Federal University of Viçosa directly after being cracked to obtain the oil seed. Moisture content was determined by placing the samples in a drying oven at 80 °C and comparing the initial weight and the weight after 24 h in the drying oven. The biomass was ground to approximately 1 mm in a bench-top hammer mill (Marconi TE 040, Piracicaba, SP, Brazil).
Pretreatment
Percent solid recovery determination
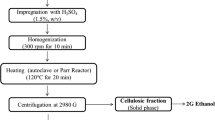
Three pretreatment repetitions using both sulfuric acid and sodium hydroxide were performed to calculate the percent solids required. Twenty grams of ground biomass was placed in a 500-mL flask, and 0.5% H2SO4 or 1.0% NaOH was added to reach a solid/liquid concentration of 10%. The flasks were then heated in an autoclave to 121 °C for 1 h before being cooled. Samples were then washed with distilled water, filtered and dried at 80 °C. After drying, the samples were weighed to calculate the percentage of solids recovered after pretreatment, and the chemical composition of the pretreated biomass was also determined in order to verify pretreatment effectiveness for concentrating cellulose.
Pretreatment for SSF
Pretreatment for SSF included the same process as was used for the percent solid recovery determination; however, the initial amount of biomass to be pretreated was altered in order to compensate for the material lost as soluble and produce exactly 20 g of pretreated solids. This calculated biomass was then subjected to the same conditions of 0.5% H2SO4 or 1.0% NaOH in an autoclave for 1 h at 121 °C.
The pretreated biomass slurry was filtered and washed with distilled water, or the entire slurry was used for SSF. The pretreated biomass was washed and filtered using a vacuum filter assembly, coupled with a vacuum pump and a glass fiber filter. Samples were then stored in a refrigerator at 4 °C until utilized for SSF. It is also important to note that the samples were not completely dried in an effort to avoid cell wall collapse.
Yeast cultivation
The Saccharomyces cerevisiae strain M70 [19] was produced in order to ferment glucose generated during enzymatic hydrolysis. The yeast was inoculated in 5 mL of an YPD medium and placed in a shaker at 30 °C and 200 rpm for 24 h. After this time, the medium was transferred to 50 mL of YPD medium and after another 24 h transferred to 500 mL of YPD medium, maintaining the same cultivation conditions. After cultivation, the yeast suspension was centrifuged for 5 min at 1,500×g, and the supernatant was discarded [5]. The yeast cells were resuspended in approximately 75 mL of distilled water and quickly transferred to the appropriate SSF flask. Lapse time between resuspension and application of the yeast to the SSF was less than 1 h. From this solution, an aliquot of 1 mL was injected into a 1.5-mL microtube and dried in a furnace at 50 °C in order to calculate the concentration of dry cell mass in the suspension.
SSF
The SSF experiments were performed in 500-mL glass Erlenmeyer-flasks with a 275-mL working volume. In the unwashed samples, the slurry was neutralized to a pH of 5.0 and 50 mM sodium citrate buffer with a pH of 5.0 was added to maintain a WIS concentration of 7.5% (w/v). The same sodium citrate buffer was also added to the washed and filtered samples to each the same WIS concentration of 7.5% (w/v).
Cellulase from Trichoderma reesei ATCC 26921 and Cellobiase from Aspergillus niger were purchased from Sigma–Aldrich Brasil (São Paulo, Brazil). All samples were subjected to a cellulase enzyme loading of 15 FPU/g biomass and supplemented with cellobiase consisting of 125% of the volume of cellulase added (19.25 U/g pretreated biomass).
Directly after addition of the enzyme mixtures, the produced S. cerevisiae cell suspension was added to the concentration of 0.8 g/L (dry yeast cells). The SSF experiments were performed in a shaker at 35 °C and 150 rpm. Samples of 1.5 mL were removed at 0, 2, 4, 8, 12, 24, 48, 72, 96 and 120 h using sterilized equipment to avoid contamination and frozen for posterior analysis.
Efficiency of the cellulose conversion process was determined using the theoretical values for glucan conversion to glucose (1.11 g/g) and fermentation of glucose to ethanol (0.51 g/g) [20]. This relationship is shown in Eq. 1:
where EtOH is the concentration of ethanol produced (g/L), BioS is the concentration of biomass in the slurry (g/L), Cel% is the concentration of cellulose in the biomass (g/g), ConvGlu is the glucan to glucose conversion coefficient (1.11 g/g) and ConvEtOH is the conversion coefficient of glucose to ethanol (0.51 g/g).
Analysis
High-performance liquid chromatography (HPLC)
Samples for HPLC analysis were prepared by filtering through a 0.45-μm filter (Milli-Q) and properly diluted with deionized water (Milli-Q). Ethanol, glucose, xylose and furfural concentrations, as well as the presence of citric and acetic acids, were determined by HPLC with a refractive index detector (Shimadzu Corporation, Kyoto, Japan) and a Shim-Pack SCR-101P column (Shimadzu) at 80 °C and flow rate of 0.5 mL/min with water as the eluent.
Enzyme activity
Total cellulase activity was assayed using Whatman’s number one filter paper as a cellulose source and expressed as FPU (filter paper units) [21]. The produced reducing sugars were determined at 540 nm with a spectrophotometer (Beckman model DU-65) using a 3,5-dinitrosalicylate reagent (DNS) [22].
Chemical composition
Composition data of the Jatropha shells was determined by HPLC carbohydrate analysis [23]. These HPLC analyses were performed in duplicate, and the average value was used. Protein concentration was calculated using the Kjeldahl nitrogen analysis [24] and, using nitrogen to protein conversion factor of 6.25, also performed in duplicate. The calculated average values were presented in all graphs and figures with their accompanying standard deviations.
Soluble lignin and insoluble lignin were determined separately according to standard methods, respectively [25, 26]. In this work, the total lignin content was expressed as the sum of both soluble lignin and insoluble lignin.
Results and discussion
Raw material
The composition of the raw material from Jatropha cultivated in the region of Viçosa, Minas Gerais, Brazil, can be found in Table 1. Little information is found in literature regarding the chemical composition of Jatropha meal and even less on the outer shells used in this study; however, data encountered on the chemical composition show large variations depending on the region and rainfall [12, 27, 28]. Despite Jatropha possessing an abundant vegetable oil source, lipid concentration in the outer shell is insignificant, and therefore, no oil extraction techniques were applied. The effect of pretreatment on the protein content of the shell was also of little interest since the initial protein content was relatively low.
The determined chemical composition of Jatropha shells was similar to the data presented by the Minas Gerais Center of Technology (CETEC), which found fiber, lignin and protein contents in dry-weight basis (d.b.) of 53.52, 36.72 and 7.80%, respectively [29]. Other components that were not quantified accounted for a large portion (roughly 30%) of the shell composition, which may include minerals, ash, other fibers, etc. but were not of interest in this study.
The experimental results obtained in this work showed a lower fiber content (approximately 40%). In the present study, fiber content was given as the sum of glucan, xylan, arabinan, galactan and mannan; however, other fiber sources may be present, which were not quantified in this work. Interestingly, the lignin content was reasonably less than that determined by CETEC [30]. This difference may be due to different quantification methods used but may also indicate the large variation between lignin contents in different varieties of Jatropha. This suggests that if Jatropha is used for biofuel production, especially ethanol, it is important to select varieties with low lignin concentrations.
Pretreatments
The dilute-acid pretreatment promoted higher recovery of WIS in slurry (72.9%) compared to alkaline pretreatment, for which 50.4% of solids were recovered. On the other hand, the glucan concentration in resultant WIS from alkaline pretreatment was significantly higher (50.0%) when compared to WIS glucan content from acid pretreatment (33.9%). This is likely a result of the high lignin solubilization in the alkaline pretreatment (63.1%) compared to the acid pretreatment (10.6%).
As stated in other works, the alkaline pretreatment was able to solubilize a significant portion of lignin present [10, 31]. In both cases, more than 93% of glucan was maintained. It is also interesting to note that more than 96% of xylan was maintained after acid pretreatment and slightly more than 99% after alkaline pretreatment, indicating nearly no acid hydrolysis of xylan. The greatest difference was found in arabinan, where 87.5% was recovered after acid pretreatment and only 23% recovered after alkaline pretreatment. When comparing results from the pretreatment of cotton stalks with both sulfuric acid and sodium hydroxide, Silverstein et al. [10] found that sulfuric acid had a greater effect on xylan, completely hydrolyzing all xylan in the biomass; however, the authors used 2% H2SO4 instead of the 0.5% used for this study. These authors also showed that alkaline treatments are generally specific to lignin solubilization, as observed in this study, where only 36.9% of lignin was recovered after alkaline pretreatment in comparison with 89.4% for the acid pretreatment. Their results for solids recovery from cotton stalks after pretreatment showed to be nearly identical as well in which 59.07 and 73.7% of solids were recovered after pretreatment with 1.0% NaOH and 0.5% H2SO4 at 121 °C for 1 h, respectively.
Simultaneous saccharification and fermentation
The SSF experiments were performed using 20 g of pretreated material, both from the acid and from the alkaline pretreatments. It should be emphasized that the alkaline pretreatment was much more effective for producing a more cellulose-rich material (Table 2). Comparison of the SSF results for the acid and alkaline pretreatments using washed WIS is shown in Fig. 1, both of which were subjected to the same cellulase loading. Repeatability of the process was verified by the standard deviation shown by the error bars in Fig. 1.
Although concentrations were lower than those published in other works, which ranged from 2 to 5 g/L of dry yeast cells [9], the yeast cell concentration of 0.8 g/L was sufficient to ferment all glucose present in the sample, proven by the minimal concentration of glucose detected after 4 h of SSF. After only 2 h, a rapid increase was observed in ethanol concentrations, while a drastic decrease in glucose was noticed, as observed in Figs. 1 and 2.
Glucose (filled circle), xylose (filled inverted triangle) and ethanol (filled square) concentrations during SSF a using acid-pretreated WIS and treated with cellulase + cellobiase enzymes and b using alkaline-pretreated WIS and treated with cellulase + cellobiase enzymes. The solid symbols represent the washed WIS, and hollow symbols represent the utilization of the entire slurry
Ethanol yield from SSF after 48 h was 6.07 and 7.68 g/L in the washed samples for the acid and alkaline pretreatments, respectively. For all SSF forms tested (washed or unwashed biomass), the samples treated with NaOH presented higher ethanol concentrations than those treated with H2SO4 due to the greater initial glucan content in the biomass samples treated with NaOH.
When comparing the two pretreatment methods, both pretreated biomass types presented similar curves for ethanol production as a function of time, reaching maximum concentrations after 12–24 h. In the majority of cases, ethanol concentration began to decrease from 24 to 48 h, suggesting ethanol respiration by the yeast because of an insufficient fermentable sugar content. When comparing efficiencies for cellulose (glucan) conversion to ethanol, at 24 h of SSF, the maximum concentration of ethanol produced from acid-pretreated biomass was encountered slurry containing washed biomass (5.82 g/L), while in the alkaline-pretreated biomass, the highest ethanol concentration was found in the unwashed slurry (8.71 g/L) (Fig. 2). These values correspond to cellulose (glucan) conversion efficiencies of 40.43 and 41.03% for the acid- and alkaline-pretreated biomasses, respectively.
Figure 2b shows ethanol and glucose concentrations for SSF of the alkaline-pretreated biomass with cellulase and cellobiase enzymes. In this figure, the ethanol concentration declined slightly after 24 h of SSF for the entire slurry subjected to cellulase + cellobiase enzymes. This was also observed for the washed biomass previously pretreated with the dilute acid (Fig. 2a). In both cases, high ethanol concentrations were achieved followed by decreases, which may suggest ethanol respiration by the yeast after consumption of all available glucose in the medium. This behavior was similar in most repetitions, indicating that in order to improve ethanol yields, glucose must be made available to the yeast at a faster rate or fermentation conditions must be modified to prevent the yeast from utilizing ethanol as an energy source.
Chromatography analyses showed large differences in the quantity of acetic acid (organic acids) for the washed WIS and unwashed slurry. Organic acids were encountered in the unwashed slurries, but no furfural was detected (data not shown). The fact that no furfural (byproduct of hemicellulose acid hydrolysis) was found signified that the sulfuric acid concentration was quite low or that the pretreatment time was short, limiting the acid hydrolysis of the hemicelluloses fractions. Although the objective was to pretreat the biomass without generating large quantities of inhibitors, the fact that no furfural was encountered may suggest that higher acid concentrations or longer pretreatment retention times might improve enzymatic hydrolysis without generation excessive quantities of inhibitors. Moreover, no xylose concentration was encountered after pretreatment, which is typically found due to the acid hydrolysis of xylan, reinforcing the fact that the acid pretreatment could be intensified.
The minimal difference observed in ethanol production between treatments with washed and unwashed WIS indicates that the presence of potential inhibitors (organic acids but no furfurals) had little effect on the saccharification and fermentation processes. After 48 h, the ethanol concentrations for the washed and unwashed WIS pretreated with NaOH and subjected to cellulase enzyme loading were 7.37 and 7.76 g/L, respectively (Fig. 2). These values corresponded to 34.72 and 36.55% conversion of glucan present in the pretreated biomass to ethanol. In another study, wheat straw pretreated with dilute acid gave only 49% conversion of total carbohydrates to sugars [6].
Conclusion
This work was the first of its kind to evaluate ethanol production from Jatropha curcas shells. Low overall SSF efficiencies were likely due to insufficient pretreatment methods, which should be intensified to better liberate cellulose for enzymatic hydrolysis. The S. cerevisiae strain used was adequate to ferment all glucose produced by the cellulase enzymes; however, the use microorganisms capable of fermenting pentose and hexose sugars could greatly improve the efficiency of the system if used in conjunction with hemicellulase enzymes. Subsequent studies should consider more intensive pretreatments to allow better enzymatic digestion and therefore greater ethanol concentrations.
References
Sims REH, Hastings A, Schlamadinger B, Taylor G, Smith P (2006) Energy crops: current status and future prospects. Glob Change Biol 12:2054–2076
Agencia Nacional de Petroleo (2011) Authorized capacity of biodiesel production plants. http://www.anp.gov.br/?pg=29048&m=&t1=&t2=&t3=&t4=&ar=&ps=&cachebust=1322657707167. Accessed May 2011
Brazilian Federal Government, Ministry of Mines and Energy (2010) Brazilian Energy Balance. Empresa de Pesquisa Energetica, Brazil
Kim S, Dale BE (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 26:361–375
Öhgren K, Bura R, Lesnicki G, Saddler J, Zacchi G (2007) A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pretreated corn stover. Process Biochem 42:834–839
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40:3693–3700
Karimi K, Emtiazi G, Taherzadeh MJ (2006) Ethanol production from dilute-acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae. Enzyme Microb Technol 40:138–144
Saha BC, Cotta MA (2008) Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy 32:971–977
Linde M, Galbe M, Zacchi G (2007) Simultaneous saccharification and fermentation of steam-pretreated barley straw at low enzyme loadings and low yeast concentration. Enzyme Microb Technol 40:1100–1107
Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne J (2007) A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol 98:3000–3011
Adsul MG, Ghule JE, Shaikh H, Singh R, Bastawde KB, Gokhale DV, Varma AJ (2005) Enzymatic hydrolysis of delignified bagasse polysaccharides. Carbohydr Polym 62:6–10
Abou Kheira AA, Atta NMM (2008) Response of Jatropha curcas L. to water deficit: yield, water use efficiency and oilseed characteristics. Biomass Bioenergy 33:1343–1350
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatment technologies. Bioresour Technol 96:2019–2025
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98:1947–1950
Tengborg C, Galbe M, Zacchi G (2001) Reduced inhibition of enzymatic hydrolysis of steam-pretreated softwood. Enzyme Microb Technol 28:835–844
Öhgren K, Vehmaanperä J, Siika-Aho M, Galbe M, Viikari L, Zacchi G (2007) High temperature enzymatic prehydrolysis prior to simultaneous saccharification and fermentation of steam pretreated corn stover for ethanol production. Enzyme Microb Technol 40:607–613
Oberoi HS, Vadlani PV, Brijwani K, Bhargav VK, Patil RT (2010) Enhanced ethanol production via fermentation of rice straw with hydrolysate-adapted Candida tropicalis ATCC 13803. Process Biochem 45:1299–1306
Vicente MdA, Fietto LG, Castro IdM, Santos ANGd, Coutrim MX, Brandão RL (2006) Isolation of Saccharomyces cerevisiae strains producing higher levels of flavoring compounds for production of “cachaça” the Brazilian sugarcane spirit. Int J Food Microbiol 108:51–59
US Department of Energy (2006) Theoretical ethanol yield calculator. http://www1.eere.energy.gov/biomass/ethanol_yield_calculator.html. Accessed May 2011
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Wallis AFA, Wearne RH, Wright PJ (1996) Chemical analysis of polysaccharides in plantation eucalypt woods and pulps. Appita J 49:258–262
Horwitz W, Senzel A, Park DL, Reynolds H (eds) (1975) Official methods of analysis of the Association of Official Analytical Chemists, 12th edn. Association of Official Analytical Chemists, Washington
Goldschmid O (1971) Ultraviolet spectra. In: Sarkaned KV, Ludwig CH (eds) Lignins: occurrence, formation, structure and reactions. Wiley, New York
Gomide JL, Demuner BJ (1986) Determinação do teor de lignina em material lenhoso: método Klason modificado. O Papel 47:36–38
Openshaw K (2000) A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenergy 19:1–15
Martínez-Herrera J, Siddhuraju P, Francis G, Dávila-Ortíz G, Becker K (2006) Chemical composition, toxic/antimetabolic constituents, and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chem 96:80–89
Fundaçao Centro Tecnológico de Minas Gerais (CETEC) (1983) Programa Energia—Produção de combustíveis líquidos a partir de óleos vegetais. p 152
CETEC (2006) Composição Química da Torta de Pinhão-Manso. www.PinhaoManso.com.br
Zhu S, Wu Y, Yu Z, Chen Q, Wu G, Yu F, Wang C, Jin S (2006) Microwave-assisted alkali pre-treatment of wheat straw and its enzymatic hydrolysis. Biosyst Eng 94:437–442
Acknowledgments
The authors would like to thank the Brazilian foundations CNPq and CAPES for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Visser, E.M., Filho, D.O., Tótola, M.R. et al. Simultaneous saccharification and fermentation (SSF) of Jatropha curcas shells: utilization of co-products from the biodiesel production process. Bioprocess Biosyst Eng 35, 801–807 (2012). https://doi.org/10.1007/s00449-011-0662-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0662-4