Abstract
A novel l-ascorbyl fatty acid ester, l-ascorbyl linoleate was successfully prepared by enzymatic esterification and transesterification in a non-aqueous medium using immobilized lipase as biocatalyst. Changes in enzymatic activity and product yield were studied for the following variable: the nature of the fatty acid, the fatty acid concentration and water content. The yield of synthesis for the C18 unsaturated fatty acids were higher than for the C18 saturated fatty acid. Initial enzyme concentration does not affect the equilibrium of the reaction. And the product yield (33.5%) in the transesterification was higher than that of the esterification (21.8%) at a high-substrate concentration 0.3 M. The medium water content was found to have a distinct influence on the l-ascorbyl linoleate synthesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipase (Ec 3.1.1.3) is now known to catalyze reverse reactions of hydrolysis in organic solvents by direct esterification with free acid, transesterification and interesterification (ester–ester interchange). Each lipase demonstrates its distinct substrate specificity and regioselectivity. This unique phenomenon is being exploited by lipid biotechnologists to modify existing fats and oil or to synthesize novel ester products with potential applications in the food, cosmetics, and pharmaceutical industries. One of the primary interests is the incorporation of fatty acids with potential health benefits into various starting materials such as sugar, derivatives of sugars, glycerides, and phospholipids [1]. Present-day demand for eco-friendly technologies has changed the scenario in favor of natural additives in place of synthetic compounds, especially in food and personal care products. l-Ascorbic acid and its derivatives have attracted considerable attention in recent years for preventing oxidative stress related diseases.
The l-ascorbic acid (Vitamin C) is widely used natural antioxidant [2]. Its solubility in water is good (200 g per 1 l water at 25°C). However, its highly hydrophilic behavior prevents its application in cosmetics or fats and oils [3, 4]. To alter the solubility of the l-ascorbic acid, it was converted into oil-soluble fatty acid ester. Therefore, the modification of l-ascorbic acid via esterification or transesterification with aliphatic molecules (such as fatty acids) can be used as a tool to alter solubility in oil-based formulae and emulsion. There are many reports on the lipase-catalyzed synthesis of l-ascorbyl esters of saturated fatty acids, in which more attention has been paid to the synthesis of l-ascorbyl palmitate. Humeau et al. [5] and Bradoo et al. [6] studied the synthesis of l-ascorbyl palmitate by Candida antarctica lipase and by Bacillus stearothermophilus SB1 lipase, respectively. l-Ascorbyl palmitate was used initially to preserve oil- and emulsified-carotene-based food colors. Now it is also used as a crumb-softening agent in bread. It can even inhibit the development of cancer [7].
Unsaturated fatty acids have more beneficial effects on human nutrition than saturated fatty acids (Shlomo et al. 2002). Especially, linoleic acid whose alkyl group contains 18 carbon atoms in a linear configuration and a pair of olefinic bonds, it is an essential fatty acid. As alternatives to a saturated fatty acid, l-ascorbyl linoleate and l-ascorbyl oleate were synthesized and it has been proved that they have important potential application [8, 9], l-ascorbyl oleate had a better protective effect on human umbilical cord vein endothelial cells treated with H2O2 than of l-ascorbyl palmitate. Viklund et al. studied the synthesis of l-ascorbyl oleate in t-amyl alcohol [10], and they got high yields (80%). However, the reactants concentration was very low (16 mmol oleic acid in 184 ml t-amyl alcohol) and the cycle of reaction was very long (65 h).
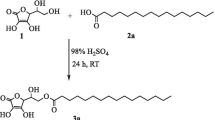
Our previous research has emphasized the synthesis of long-chain saturated fatty acid esters of l-ascorbic acid. However, there is considerable interest in developing scalable technologies, which are suitable for the biocatalytic synthesis of long-chain unsaturated fatty acid esters of l-ascorbic acid. We report for the first time the enzymatic synthesis of l-ascorbyl unsaturated fatty acid ester—l-ascorbyl linoleate by esterification and transesterification, and compare enzymatic esterification with transesterification for l-ascorbyl esters synthesis. The reaction is shown in Fig. 1.
In this article, the esterification of l-ascorbic acid with linoleic acid and the transesterification of l-ascorbic acid with methyl linoleate were investigated using Novozym 435 (Type B lipase from Candida antarctica, used to synthesize fatty acid ester) in 2-methyl-2-butanol.
Materials and methods
Materials
Novozym 435 (Type B lipase from Candida Antarctica, immobilized on a macroporous acrylic resin) was kindly provided by Novo Nordisk (Denmark). Stearic acid (98%) and oleic acid (90%) were obtained from Aldrich. Linoleic acid (90%) and methyl linoleate (95%) were purchased from Fluka. The purity of l-ascorbic acid was over 99%. All the solvents were of high-performance liquid chromatography (HPLC) grade and were stored over activated molecular sieves 4 Å (from Merck) prior to use.
l-Ascorbyl linoleate synthesis by esterification and transesterification
A mixture of l-ascorbic acid (1.0 mmole) and linoleic acid (1.5 mmole) or methyl linoleate (1.5 mmole) in 2-methyl-2-butanol (5 ml), and C. antarctic lipase Novozym 435 (100 mg) were agitated on a shaker at 200 rpm and 50°C. These conditions were used except when otherwise stated in the text.
In the experiment of water content effect, water was added directly to the enzyme reaction mixture and the reactions initiated by sonicating for 30 S, and water content was analyzed by Karl Fischer titration.
Quantitative measurement of substrates and products
l-Ascorbyl linoleate, l-ascorbic acid, linoleic acid, and methyl linoleate concentrations were quantified by HPLC (Shimadzu, Japan) chromatography on a reverse phase column (Hewlett Packard XDB-C18, 250 × 4 mm2, 5 μm), eluent 85/15 (v/v), methanol/water, flow rate 1 ml/min, temperature 40°C, injection volume 15 μL, detection UV detector at 210 nm.
All the experiments were carried out at least in fourfold sample and all results were expressed as means.
Results and discussion
Influence of the nature of the fatty acid
It was already known that the fatty acid chain length had a considerable influence on product formation [11, 12]. Few studies are known those describe l-ascorbyl esters synthesis with different chain length fatty acid. So fatty acid of various chain lengths were studies for l-ascorbyl fatty acid ester synthesis in sealed flasks containing 5 ml 2-methyl-2-butanol at 50°C. Results show that the initial rate and yield of l-ascorbyl stearate are higher than l-ascorbyl laurate or l-ascorbyl palmitate (Table 1). Interestingly, an increase in fatty acid chain length results in an improvement in initial rates and yields. Selmi et al. [11] have reported that the longer the acyl chain is, the higher the yield becomes for triglyceride synthesis by Rhizomucor miehei lipase. Generally, lipases showed reduced activity towards shorter chain fatty acids [13].
In order to assess the effect of the presence of double bond on the yield, C18 fatty acid was studied. In comparison, the experimental results show that the yield of synthesis for the C18 unsaturated fatty acids were higher than for the C18 saturated fatty acid (Table 1). Flores et al. [14] reported similar results for the esterification of fatty acids and butanol using C. antarctica lipase in solvent-free medium. There is no straightforward explanation for the fact that for the same chain length, a higher ester yield is found for the unsaturated fatty acid. This phenomenon is related with the interdiffusion of the fatty acid and immobilized enzyme in organic media.
Enzymatic synthesis of l-ascorbyl linoleate
To learn as much as possible about the enzymatic synthesis between l-ascorbic acid and linoleic acid, further studies have been done in our laboratory to optimize this reaction.
Figure 2 shows the time course for Novozym 435 lipase-catalyzed synthesis l-ascorbyl linoleate with linoleic acid as the substrate in 2-methyl-2-butanol. Maximum yield (21.8%) was obtained after 240–420 min of incubation, suggesting that equilibrium may have been reached in 240 min. Beyond this reaction time, there was little improvement on the synthesis of l-ascorbyl linoleate.
Time course for immobilized Novozym 435 lipase-catalyzed synthesis of l-ascorbyl linoleate with linoleic acid or methyl linoleate in 2-methyl-2-butanol All reactions were carried out at 50°C, with 1.0 mmole l-ascorbic acid and 1.5 mmole linoleic acid or 1.5 mmole methyl linoleate with 100 mg enzyme in 5 ml 2-methyl-2-butanol
Table 2 illustrates the effect of varying the enzyme concentration (Novozym 435) on the esterification of l-ascorbic acid and linoleic acid to produce l-ascorbyl linoleate in 2-methyl-2-butanol. The enzyme concentration was varied from 10 g/l to 120 g/l. Results show that initial enzyme concentration does not affect the equilibrium of the reaction, as long as the latter can be reached before the experimentation is stopped. The higher the enzyme concentration is, the shorter the equilibrium time of the reaction is, but increase process cost. Considering reaction time and cost of immobilized lipase, we chose a middle value (40~80 g/l Novozym 435 concentration) to study. Initial rates are almost constant to enzyme concentration in the range of 20–80 g/l (Fig. 3). For higher enzyme inputs, the initial rates decrease, this can probably be due to that all the active sites of the enzyme that can be utilized are not saturated with substrate, there would be a serious internal and external mass transfer limitation of substrate in the immobilized enzyme system.
For process optimization, it is important to study the effect of the initial fatty acid concentrations. Figure 4 shows that initial rates increased with linoleic acid concentration from 0 to 300 mM. And the product concentration increased with the increase of linoleic acid concentration within the experiment concentration range. As far as conversion are concerned, they decrease when the initial linoleic acid concentration is raised, for initial linoleic acid concentration of 6 and 400 mM, the corresponding conversion are equal to 86 and 16%, respectively.
Effect of water content in the transesterification of l-ascorbic acid with methyl linoleate
The medium water content was found to have a distinct influence on the l-ascorbyl linoleate synthesis during the transesterification of l-ascorbic acid with methyl linoleate. According to Mutua and Akoh [15], initial water in the reaction medium favors hydrolysis of the ester substrate to the detriment of synthesis of the ester product. In the case described here, reactions compete in the system initially hydrolysis, esterification and transesterification:
l-ascorbyl linoleate concentration is representative of the l-ascorbyl linoleate synthesis reaction. On the other hand, the hydrolysis of methyl linoleate and l-ascorbyl linoleate can be quantified by measuring the concentration of linoleic acid. The l-ascorbyl linoleate synthesis concentration and the hydrolysis concentration of methyl linoleate and l-ascorbyl linoleate can be compared at different concentration of water (Table 3). Results indicate that the hydrolysis is higher than that of the ester synthesis within 1 h at higher water contents. For example, at water content 3.0 g/l, initially, reaction ② was inhibited, the hydrolysis concentration, namely the methyl linoleate hydrolysis, is 1.7-fold than that of l-ascorbyl linoleate synthesis at reaction 1 h; after 8 h, the hydrolysis and synthesis yields are 32 and 10.7%, respectively. Moreover, this shows that the less water in the medium, the more the l-ascorbyl linoleate synthesis is favored. Elimination of water is, therefore, likely to result in an improvement in yields.
Conclusions
The present work describes l-ascorbyl linoleate synthesis by means of enzyme-catalyzed esterification l-ascorbic acid with linoleic acid and transesterification l-ascorbic acid with methyl linoleate in organic solvent using lipase B from Candida Antarctica. The test experiment of water and methanol removal demonstrated that the reaction equilibrium position was shifted towards synthesis of l-ascorbyl linoleate and the hydrolysis of ester was also eliminated.
References
Akoh CC, Mutua LN (1994) Synthesis of alkyl glycoside fatty acid esters effect of reaction parameters and the incorporation of n-3 polyunsaturated fatty acids. Enzyme Microb Technol 16:115–119
Wang XY, Seib PA, Ra KS (1992) l-ascorbic acid and its 2-phosphorylated derivatives in selected food: Vitamin C fortification and antioxidants properties. J Food Sci 60:1296–1299
Schuler P (1990) Natural antioxidants exploited commercially in food antioxidants, Hudson BJF (eds) Elsevier Science publishers, London, pp113–127
Han D, Yi OS, Shin HK (1990) Antioxidative effects of ascorbic acid solubilized in oils via reversed micelles. J Food Sci 55:247–249
Humeau C, Giarardin M, Coulon D (1995) Miclo a synthesis of 6-O-palmitoyl L-ascorbic acid catalysed by candida antarctica lipase. Biotechnol Lett 17:1091–1094
Bradoo S, Saxena RK, Gupta R (1999) High yields of ascorbyl palmitate by thermostable lipase-mediated esterification. J Am Oil Chem Soc 76:1291–1295
Tang LH, Zhang H, Shehate MM, Sun YF (2000) A kinetic study of the synthesis of ascorbate fatty acid esters catalysed by immobilized lipase in organic media. Biotechnol Appl Biochem 32:35–39
Song QX, Wei DZ (2002) Study of Vitamin C ester synthesis by immobilized lipase from Candida sp. J Molec Catal B: Enzymatic 18:261–266
Song QX, Wei DZ, Zhou WY, Xu WQ, Yang SL (2004) Enzymatic synthesis and biological effect of unsaturated fatty acid ester of l-ascorbic acid, Biotechnol Lett 26(23):1777–1780
Viklund F, Alander J, Hult K (2003) Antioxidative properties and enzymatic synthesis of ascorbyl FA esters, J Am Oil Chem Soc 80:795–799
Selmi B, Gontier E, Ergan F, Thomas D (1998) Effects of fatty acid chain length and unsaturation number on triglyceride synthesis catalyzed by immobilized lipase in solvent-free medium. Enzyme Microb Technol 23:182–186
Cao L, Fishcher A, Bornscheuer UT, Schmid RD (1997) Lipase-catalyzed solid phase preparation of sugar fatty acid esters. Biocatal Biotransform 14:269–283
Eigtred P (1992) Enzymes and lipid modification In: Advances in applied lipid research (Padley FB, Ed.) JAI press Ltd., Grenwich, CT. 1:1–64
Flores MV, Sewalt JJW, Janssen AEM, Van der padt A (1999) The nature of fatty acid modifies the equilibrium position in the esterification catalyzed by lipase. Biotechnol Bioeng 67:364–371
Mutua LN, Akoh CC (1993) Synthesis of alkyl glycoside fatty acid esters in non-aqueous media by candida sp lipase. J Am Oil Chem Soc 70:43–46
Acknowledgements
This work was supported by the Key Disciplinary Foundation of Shanghai.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to the article.
Rights and permissions
About this article
Cite this article
Song, Q., Zhao, Y., Xu, W. et al. Enzymatic synthesis of l-ascorbyl linoleate in organic media. Bioprocess Biosyst Eng 28, 211–215 (2006). https://doi.org/10.1007/s00449-005-0006-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-005-0006-3