Abstract
Lima bean plants (Phaseolus lunatus) exhibit compensatory growth responses to herbivory. Among the various factors that have been identified to affect plant compensatory growth are the extent and type of tissue damage, the herbivore’s feeding mode and the time of damage. Another factor that can greatly impact plant responses to herbivory, but has been largely ignored in previous studies, is the action of parasitoids. In most cases, parasitoids halt or slow down the development of herbivorous hosts, which, can result in decreased leaf damage, thereby affecting plant responses and ultimately plant fitness. Here, we investigated the effects of two koinobiont parasitoids on the amount of leaf damage inflicted by the Southern armyworm Spodoptera latifascia to wild lima bean, and the consequences of this for plant growth and seed production in the field. We specifically tested the hypothesis that the action of parasitoids will reduce plant damage and that this reduction will alter plant growth responses and seed production. Indeed, we found that in the presence of parasitoids plants suffered less damage than plants with only herbivores. As a consequence, compensatory growth was reduced and more and heavier seeds were produced earlier in the season, compared to plants exposed to only herbivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are attacked by a variety of herbivores. The consequences of these attacks for plant fitness will depend on the intensity and timing of damage (Crawley 1989; Boege and Marquis 2005), and on the plant’s defensive and physiological responses (Karban and Myers 1989; Karban and Baldwin 1997), which can act alone or in conjunction with the ecological environment (Strauss and Agrawal 1999). Among the different strategies to cope with tissue loss due to herbivore damage, resistance and tolerance are particularly well studied (Strauss and Agrawal 1999; Núñez-Farfán et al. 2007). Resistance is the ability of a plant to deter and minimize herbivore damage, whereas tolerance is a plastic response that allows a plant to endure or recover from damage through physiological mechanisms or compensatory growth (Agrawal 2000; Stowe et al. 2000). Tolerance response to herbivory has a genetic basis (Mauricio et al. 1997; Kessler and Baldwin 2002; Fornoni 2011), but is also affected by abiotic (Wise and Abrahamson 2005, 2007) and biotic factors such as competition with other plants and the action of herbivores (Edenius et al. 1993; Puettmann and Saunders 2001). For instance, a growing number of studies reveal that as a response to herbivore damage, plants compensate by increasing their photosynthetic capacity, reallocate photoassimilates to different tissues and eventually may produce more leaves, more branches, and flower early (reviewed in Núñez-Farfán et al. 2007). However, the extent to which these responses affect plant reproductive success is still a topic of debate (Dietrich et al. 2005; Heil 2010; Tito et al. 2016).
Among the various factors that have been identified to affect the type and quantity of compensatory growth are the extent (Martínková et al. 2008) and type of damage (Huhta et al. 2009), the herbivore’s feeding mode (Rosenheim et al. 1997; Kotanen and Rosenthal 2000; Tiffin 2000), and the timing and duration of damage (Boege et al. 2007). Another factor that can greatly impact plant responses to herbivory, but so far has received little attention, is the action of the natural enemies of herbivores (Kaplan et al. 2016).
Herbivores are frequently attacked by parasitoids and it is generally accepted that parasitoids can reduce the negative impact of herbivores on plants (Hoballah and Turlings 2001; Poelman et al. 2011; Gols et al. 2015).
Some parasitoids known as idiobionts will kill or paralyze the host immediately upon parasitization, halting host development and stopping it from further feeding (Mackauer and Sequeira 1993; Godfray 1994; Harvey 2005). Conversely, the hosts of koinobiont parasitoids continue to feed after oviposition (Hoballah and Turlings 2001; reviewed in Harvey 2005). Yet the nature and outcome of the interaction between hosts and koinobiont parasitoids is complex and will largely depend on whether the latter are solitary or gregarious, and on the manner in which they affect the growth and development of their host (Smilowitz and Iwantsch 1973; Harvey et al. 1994; Harvey 2005). In some cases parasitized hosts will feed at a slower rate and grow smaller than non-parasitized ones (Hoballah and Turlings 2001). In other cases parasitized herbivores can eat more (Coleman et al. 1999; Van der Meijden and Klinkhamer 2000) or for longer periods (Thorpe 1933; Beckage and Riddiford 1982), than unparasitized ones, in which case plants will sustain greater damaged and this may increase induced defensive responses (Ode et al. 2016). Yet, in virtually all known cases, parasitoids, idiobionts or koinobionts reduce the amount of damage that their hosts inflict to plants (van Loon et al. 2000).
Thus, it could be imagined that by altering the nature and amount of herbivore damage, parasitoids can affect plant growth and ultimately plant reproduction. As yet, to our knowledge, no study has examined the extent to which parasitoids can affect plant growth and compensatory responses. The implications of such effects are of great significance for the presumed positive effects of parasitoids on plant fitness, as to date, field evidence supporting this notion is still very scarce (Gomez and Zamora 1994; Hoballah and Turlings 2001; Gols et al. 2015).
Here, we investigated the effects of two koinobiont parasitoids, one solitary and one gregarious, on the amount of leaf damage inflicted by the lateral lined or velvet armyworm Spodoptera latifascia on wild lima bean (Phaseolus lunatus) and the consequences for plant growth and seed production. In two field experiments, each with a different parasitoid species, we compared leaf damage among control plants (without herbivory), plants exposed to Spodoptera larvae, and plants exposed to both larvae and parasitoids. We counted the number of trifolia per plant as a proxy of plant growth, number and time of appearance of flowers and pods, and measured several seed traits (number, mass and germination success). With these experiments, we tested the hypothesis that the presence of parasitoids will reduce plant damage and that this reduction will result in altered plant growth responses and seed production in the field.
Materials and methods
Study system
Wild lima bean (Phaseolus lunatus) plants occur naturally throughout Meso- and South America (Freytag and Debouck 2002). Plant phenology is synchronized with the regional weather. In our study site (see below), the first inflorescences appear in October–November and the seeds are produced at the end of December and early January (Heil 2004; Hernández-Cumplido et al. 2010). At this field site, lima bean plants are attacked by several herbivore species (Ballhorn et al. 2009; Moreira et al. 2015; Hernandez-Cumplido et al. 2016a, b). One of these herbivores is the polyphagous noctuid moth Spodoptera latifascia. Its larvae can cause significant leaf damage; one single larva can eat up to 60% of the leaf surface of an older plant or even entirely defoliate a young plant (Cuny, personal observation). Adult moths lay their eggs in batches on the upper surface on the leaves and upon hatching first instar larvae disperse and feed individually on all parts of the leaf (Pogue 2002; Cuny personal observation). Lima bean plants exhibit compensatory growth in response to varying degrees of herbivory (Moreira et al. 2015; Hernandez-Cumplido et al. 2016b). If and how these responses are influenced or altered by the action of the herbivores’ natural enemies is not known.
At the field site, larvae of S. latifascia are frequently attacked by two generalist parasitoids, Chelonus insularis (Hymenoptera: Braconidae) and Euplectrus platyhypenae (Hymenoptera: Eulophidae). The former is a solitary egg-larval parasitoid that parasitizes hosts at the egg or first instar stage. One female can parasitize several eggs from the same batch (Ables and Vinson 1981; Jourdie et al. 2010). Parasitized larvae continue to develop until late third instar when parasitoid larvae emerge to pupate and the host dies. E. platyhypenae is a gregarious ectoparasitoid that lays its eggs on the dorsum of third and fourth instar larvae of S. latifascia and other Lepidoptera (Capinera 2001; Coudron et al. 1990). The relative abundance of the two parasitoid species varies greatly between years (Cuny, personal observation) and the reasons for these fluctuations in abundance are not yet understood.
Plants
Plants used for our experiments originated from seeds collected the previous year in a natural population of wild lima bean plants close to our field site (15°55′14.3″N 97°07′35.1″W and 17°00′40.4″N 100°06′09.9″W) for the first and second experiment, respectively; for more information on these populations see Shlichta et al. (2014). The experiments took place in January 2014 (experiment 1) and from October 2014 to March 2015 (experiment 2) at the Experimental Campus of The Universidad del Mar, located 15 km northwest of the city of Puerto Escondido (Oaxaca, Mexico, 15°55′27.9″N 97°09′04.3″W).
Insects
Colonies of S. latifascia and the parasitoids C. insularis (first experiment) and E. platyhypenae (second experiment) were established early in the season with field-collected insects from wild lima bean plants found in the surroundings of the experimental campus of the Universidad del Mar. Larvae of S. latifascia were reared under natural light and temperature conditions on artificial diet (“beet armyworm diet”, BioServ, Flemington, NJ, USA). Both parasitoids were reared on larvae of Spodoptera frugiperda collected in maize fields close to the experimental field and fed with the same artificial diet. We chose to use this species for the rearing, because of its much higher abundance in the nearby maize fields compared to S. latifascia. Previous experiments showed that the two parasitoid species develop successfully on both Spodoptera species (Cuny et al. unpublished data). Caterpillars were reared in plastic containers (13 × 15 × 5 cm) with fabric mesh for aeration and adult parasitoids were kept in mating cages (Bugdorm insect rearing cages, 30 × 30 × 30 cm).
Experimental set-up
We conducted two field experiments to determine if parasitoids affected the amount of damage inflicted by S. latifascia and the consequences for plant growth. In both experiments lima bean plants were subjected to three herbivory treatments: (1) control (without insects), (2) unparasitized larvae of Spodoptera latifascia and (3) either S. latifascia larvae parasitized by C. insularis (first experiment) or S. latifascia larvae in the presence of female E. platyhypenae (second experiment). We recorded the amount of leaf damage (first and second experiment), and plant growth and seed production (second experiment).
Experiment 1: herbivory and parasitism by Chelonus insularis
The first experiment served to obtain preliminary information on the potential of parasitoids to reduce herbivory on lima bean plants. For this we compared the amount of damage inflicted by S. latifascia larvae that were either unparasitized or parasitized by C. insularis. Seeds were individually sown in 5-L pots filled with native soil and distributed among 18 field cages (two plants per cage, Bioquip Outdoor Cage 6 × 6 × 6′, 20 × 20 Mesh Lumite). Each pot was placed on a tray filled with water to prevent the experimental larvae from moving between plants and predatory ants from climbing on the plants. To obtain parasitized larvae, three batches of S. latifascia eggs were placed in a parasitoid cage with 10 C. insularis wasps (males and females) during 48 h. Batches were removed after females were observed parasitizing. Bioassays conducted before the experiment showed that using this method we obtained around 90% parasitism. Parasitized egg batches then were kept in separate containers until larvae emerged from the eggs, at which point they were transferred to artificial diet until the start of the experiment. Although rearing early instar larvae on artificial diet could potentially affect later physiological and behavioral responses, we chose to do this because of the significant mortality of earlier instars when reared on plant material. As larvae were randomly assigned to the different treatments, we can assume that any early effects on their development due to the artificial diet would be the same among the three treatments. Prior to the experiment, to habituate larvae to the switch from artificial diet to plant material, all larvae (unparasitized and parasitized) were fed with P. lunatus leaves for 10 h, followed by a 3-h starvation period. We randomly assigned three different treatments to cages (two plants per cage, one treatment per cage and 7–8 cages per treatment): (1) control plants (without S. latifascia larvae), (2) plants with twenty unparasitized early-third instars of S. latifascia and (3) plants with twenty early-third instar parasitized larvae of S. latifascia randomly selected from the containers of “parasitized egg batches”. Preliminary experiments revealed that using 20 larvae per plant we could obtain enough damage to quantify potential differences among treatments and at the same time, minimize larval crowding that could prompt dispersion to adjacent plants. Every day, trays were refilled with water and plants were checked for unwanted insects that could have entered the cages. Based on levels of damage found in natural plant populations, we estimated leaf damage on the whole plant using the following scale: (1) no damage, (2) less than 25% of leaf surface eaten, (3) between 25 and 50% of leaf surface eaten and (4) more than 50% of leaf surface eaten. This experiment was repeated twice. To avoid any bias during sampling, plants were coded such that treatments applied to the different plants were not known during the estimation of damage.
Experiment 2: herbivory and parasitism by Euplectrus platyhypenae
Based on the results found in the first field experiment, we improved the experimental design and conducted a second experiment the following year. This experiment differed from the first one in that (1) the parasitoid used was Euplectrus platyhypenae, because of its much higher abundance in the field during that season, and (2) for the third treatment, instead of using parasitized larvae, we released female parasitoids inside cages with healthy third instar larvae of S. latifascia. This was done because trial experiments showed that even with the greatest care, manipulation of parasitized larvae resulted in very high mortality. For this experiment, plants were followed for the whole season until seed production.
Forty-eight plants were grown in 5-L pots and distributed in 24 field cages (Bioquip, Outdoor Cage 6 × 6 × 6′, 20 × 20 Mesh Lumite). Twenty-five days later, we followed the same procedure as in the previous experiment and we randomly assigned the three different treatments to cages with two plants per cage, one treatment per cage and eight cages per treatment. The treatments were: (1) control (plants without S. latifascia larvae), (2) herbivore alone (15 third instar larvae of S. latifascia per plant), and (3) herbivore + parasitoid (15 third instar larvae of S. latifascia per plant, plus five mated Euplectrus platyhypenae females per cage) (Fig. 1). Prior to the experiment, to habituate larvae to the switch from artificial diet to plant material, larvae were fed with P. lunatus leaves for 10 h, followed by a 3-h starvation period. Insects were left in the cages for 9 days. For each plant, we took a picture of two randomly selected trifolia (between the 5th and the 8th trifolium), and used it to calculate the mean damaged area per trifolium with Adobe Photoshop (Xiao et al. 2005). Two months later, when most of the leaf herbivores were no longer present in the field, cages were removed to reduce humidity and to allow the plants to dry and mature their pods. Plants were watered twice per week, checked every other day and undesirable insects (beetles, grasshoppers) were removed. For each plant, we recorded the following traits: number of trifolia (three leaflets), flowering time, number of tendrils with at least one flower, time of appearance and number of green pods and total number of seeds produced after 4 and 8 weeks. A sample of ten seeds per plant was weighed with a micro-balance (Mettler Toledo XP6, Columbus, Ohio, USA) to the nearest 0.01 mg. The following field season the germination ability of these seeds was measured by sowing them in 5-L pots at the same experimental field site. We used 14–16 plants per treatment, ten seeds per plant (two pots with five seeds per pot).
Schematic illustration of the experimental protocol for the second experiment. The treatments were: (1) control (plants without Spodoptera latifascia larvae), (2) herbivore alone (fifteen third instar larvae of S. latifascia per plant), and (3) herbivore + parasitoid (fifteen third instar larvae of S. latifascia per plant, plus five mated Euplectrus platyhypenae females per cage)
Statistical analyses
Variables that met model assumptions, number of trifolia, time to flowering and to pod production, number of flowers and green pods, and seed mass, were analyzed with a mixed linear model (PROC MIXED). Conversely, variables that did not meet assumptions of normality were analyzed with a generalized linear mixed model (PROC GLIMMIX): following a gamma distribution for leaf damage in the first experiment, a Poisson distribution for leaf damage in the second experiment, the total number of seeds and for seeds produced early in the season. Finally, a binomial distribution was used for seed germination success (Littell et al. 2006; Moreira et al. 2015; Abdala-Roberts et al. 2016). Pearson correlations (PROC CORR) were used to test for the potential correlation between the trifolia damage area and the number of trifolia in the second experiment.
For leaf damage in the first experiment, cages as well as the two blocks (the experiment was repeated twice) were considered as random factors (to account for repeated measures taken on the same experimental unit), and the herbivory treatments as fixed factors. For all the variables measured in the second experiment, cages (or pots in the case of the germination test) were considered as random factors, and herbivory treatments as fixed factors.
During the second experiment, one control plant suffered severe damage from an herbivore accidentally entering the cage and was removed from the analysis, as well as two plants from the parasitoid treatment that were destroyed by the wind during seed collection. All statistical analyses were performed with Statistical Analysis System (SAS Institute, Cary, North Carolina, USA), using Kolmogorov–Smirnov (PROC UNIVARIATE) to test model assumptions. For each analysis, we provide means ± SE, with different letters that indicate a significant difference between herbivory treatments.
Results
Experiment 1: herbivory and parasitism by Chelonus insularis
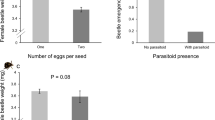
Lima bean plants with S. latifascia larvae parasitized by C. insularis suffered 35% less damage than plants attacked by non-parasitized larvae (Fig. 2a, F2,49 = 127.38, P < 0.001). Despite our efforts to maintain control plants without insect damage, some herbivores found their way inside the cages inflicting some damage (< 0.5 cm2 per trifolium), but far less than the introduced S. latifascia larvae (> 1.5 cm2 per trifolium). For herbivore-treated plants, unwanted herbivory could not be discerned from the one inflicted by the focal herbivores, but we can assume that it was similar among the three treatments.
a Estimation of total plant damage by larvae of Spodoptera latifascia during the first experiment, measured on a scale from 1 (no damage) to 4 (more than 50%). For this experiment, Chelonus insularis was used for the treatment herbivore + parasitoids. Generalized linear mixed model, P < 0.001, control: n = 24; herbivore alone: n = 22; herbivore + parasitoid: n = 10. b Damaged mean area per plant by larvae of Spodoptera latifascia measured in cm2. For the herbivore + parasitoid treatment, larvae were in presence of females of the parasitoid Euplectrus platyhypenae. We used a generalized linear mixed model. P = 0.039, control: n = 15; herbivore alone: n = 16; herbivore + parasitoid: n = 16. c Mean number of trifolia per plant (three leaflets) produced at the end of the season. Mixed linear model, P = 0.022, control: n = 15; herbivore alone: n = 16; herbivore + parasitoid: n = 16. Error bars show standard error of the mean, and different letters indicate statistically significant differences (P < 0.05)
Experiment 2: herbivory and parasitism by E. platyhypenae
Leaf damage
Plants from the two herbivore treatments (herbivore alone and herbivore + parasitoid) suffered significantly more damage than control plants (Fig. 2b, F2,23 = 3.75, P = 0.039). Although not significant, results show a trend on the presence of parasitoids and a reduction of the amount of damage, with plants from the herbivore + parasitoid treatment suffering almost 30% less damage than plants with only herbivores.
Plant growth, time to flowering and pod production
Control plants (without herbivores) produced on average a lower number of trifolia throughout the season, followed by plants from the herbivore + parasitoid treatment, and the highest number of trifolia was produced by plants from the herbivore alone treatment (Fig. 2c, F2,23 = 4.55, P = 0.022). When compared to control plants, plants from the treatment with only herbivores overcompensated as a result of herbivore damage (producing more trifolia than control plants), while plants with both herbivores and parasitoids produced the same number of new trifolia as the control plants.
The first flowers were produced in December, 8 weeks after sowing and the first green pods appeared after 9 weeks. Plants continued to produce flowers and green pods until the end of January. There were not significant differences in the time of flowering (F2,23 = 2.83, P = 0.079) and pod production (F2,23 = 0.2, P = 0.81) among plants from the three treatments. Likewise, no difference was found in total number of flowers (F2,23 = 0.38, P = 0.69) and pods (F2,21 = 0.71, P = 0.5) produced by plants from the different treatments (Online Resource 1a, b).
Seed output and seed traits
Although no differences were found in the total number of seeds produced by plants from the three herbivory treatments at the end of the season (Fig. 3a, F2,22 = 0.35, P = 0.71), highly significant differences were found in the mean number of seeds per plant produced early in the season (during the four first weeks of seed production) (Fig. 3b, F2,22 = 6.14, P = 0.008). During this period, plants with only S. latifascia larvae produced significantly fewer seeds than control plants and plants with herbivores and parasitoids (almost 80 and 40% fewer seeds, respectively).
Mean number of seeds produced per plant a for the whole season (P = 0.71), and b during the first 4 weeks of seed production (P = 0.008). Generalized linear mixed models were used for the two variables, and their sample size was the same: control: n = 15; herbivore alone: n = 16; herbivore + parasitoid: n = 14. Error bars indicate standard error of the mean. Different letters indicate statistically significant differences (P < 0.05)
Seeds produced by plants from the control treatment were on average significantly heavier than seeds from plants with only herbivores and plants with both herbivores and parasitoids (Fig. 4a, F2,445 = 5.44, P = 0.005), and no significant differences were found between the two latter treatments. No significant differences were found in germination success of seeds produced by plants from the three treatments (Fig. 4b, F2,92 = 0.23, P = 0.79). Because we detected great variation in the time of seed production among individual plants in all treatments, we performed an additional analysis to examine the relationship between time of seed production and seed mass within each treatment. Within each treatment, plants that produced seeds earlier, produced on average larger seeds than plants that produced seeds late in the season (control plants: F1,6 = 44.84, P < 0.001; herbivore alone: F1,8 = 13.14, P = 0.006 and herbivore + parasitoids: F1,6 = 7.21, P = 0.036, Online Resource 2).
a Mean mass, and b mean germination success of seeds produced by plants exposed to the three herbivory treatments. A linear mixed model was used for seed mass: P = 0.005, control: n = 149; herbivore alone: n = 160; herbivore + parasitoid: n = 140. For germination, a generalized linear mixed model was used: P = 0.79, control: n = 40; herbivore alone: n = 40; herbivore + parasitoid: n = 35. Error bars indicate standard error of the mean. Different letters indicate statistically significant differences (P < 0.05)
Discussion
The overall results from this study reveal that parasitoids can influence plant responses to herbivore damage in different ways. Independent of their life history strategy, solitary (C. insularis) or gregarious (E. platyhypenae), the two parasitoid species help to reduced leaf damage caused by Spodoptera latifascia, and as a result plant compensatory growth was attenuated. Plants exposed to herbivores only overcompensated for the loss of leaf tissue (more trifolia produced compared to control plants), whereas plants in the presence of parasitoids (second experiment) compensated for tissue damage (no difference in number of trifolia compared to control plants). In addition, we found that when plants were in the presence of parasitoids, more and heavier seeds were produced earlier in the season compared to plants that were attacked by caterpillars alone.
Parasitoid effects on herbivory
Koinobiont parasitoids, allow their hosts to continue to feed on the plant before they die (Askew and Shaw 1986; Harvey 2005) and, therefore, may not always have positive effects on plants. This is especially true for some gregarious parasitoids that sometimes even cause their host to feed more (Coleman et al. 1999; Van der Meijden and Klinkhamer 2000; Xi et al. 2015). Indeed, we found that reduction on herbivore damage was greater in the presence of the solitary C. insularis than of the gregarious E. platyhypenae (Fig. 2a). Similarly, Gols et al. (2015) found that plants of Sinapis arvensis suffered less damage when larvae were parasitized by either the gregarious Cotesia glomerata or the solitary parasitoid Hyposoter ebeninus.
Herbivore and parasitoid effects on plant compensatory growth
Herbivory by Spodoptera latifascia caused the lima bean plants to produce more trifolia, an overcompensation response that is commonly found in many plants (Strauss and Agrawal 1999). Compensatory growth in lima bean plants in response to natural herbivore damage (Moreira et al. 2015; Hernandez-Cumplido et al. 2016b) and mechanical damage (Blue et al. 2015) was known, but this is the first time that we observed overcompensation. These findings confirm that this response may be context-dependent such that it could vary depending on the amount of damage (Mauricio et al. 1993; Koptur et al. 1996) and the feeding mode of the herbivore (Manzaneda et al. 2010; Utsumi et al. 2013; Moreira et al. 2015).
Our results support this idea coined by Lucas-Barbosa et al. (2016) that plant tolerance responses following herbivory and mortality due to parasitoids are complementary factors that may benefit plants when faced with specialist herbivores. We show that the two are interlinked. One way in which parasitoids may influence the induced regrowth response is simply by reducing the amount of tissue damage. However, we did not find a clear correlation between the amount of plant damage and plant regrowth (Online Resource 3). It may be that this relationship is not linear and that at some level of damage plants can no longer compensate. For example, Blue et al. (2015) found that while a moderate amount of mechanical damage (33% of leaf area removed) inflicted on lima bean plants resulted in compensation, larger amounts of damage (66% of leaf area removed) significantly decrease fruit number and seed mass. Similarly, compensatory growth after artificial damage in the herbaceous biennial Gentianella campestris has been found to be the highest for plants that suffered intermediate levels of damage (Juenger et al. 2012).
Alternatively, parasitized larvae may induce a different response in plants than unparasitized larvae, both in terms of direct (Ode et al. 2016) and indirect defenses (Fatouros et al. 2005; Poelman et al. 2012). The oral secretions of the caterpillars may be affected by parasitization (Poelman et al. 2012). That factors in oral secretions are important for plant growth responses was shown by Korpita et al. (2014), who found that tomato plants that were mechanically damaged and then treated with regurgitate of Manduca sexta had more regrowth than plants damaged and treated with water. It is, therefore, well possible that the patterns of herbivore-induced plant regrowth that we observed are the result of the combined effect of loss of leaf tissue, and differential physiological reactions to attacks by unparasitized or parasitized larvae. Studies are underway to examine this hypothesis.
Herbivore and parasitoid effects on plant reproduction and seed traits
Even though control plants suffered considerably less damage than plants from the two herbivory treatments, at the end of the season the herbivore-treated plants had fully caught up and the total number of seeds that they produced was the same as for control plants. These findings agree with a large body of literature showing that moderate amount of damage allow plants to fully recover (Mauricio et al. 1993; Koptur et al. 1996; Blue et al. 2015; Moreira et al. 2015). Yet, early in the season control plants and plants from the herbivore + parasitoid treatments produced more seeds than plants from the herbivore alone treatment (Fig. 3b). We were surprised by these results as in other studies with this same system, but a different herbivore (adult beetles), we found that plants exposed to either herbivores or mechanical damage flowered and produced seeds earlier than control plants (Hernandez-Cumplido et al. 2016a, b). Variables such as the type, time and frequency of damage, but also considerable environmental variation among years, may all have contributed to the differential plant responses. For example, the relevant field season of 2014 experienced an “El Niño” event, with more frequent rains and colder mean temperatures during the winter months (corresponding to our field season) (CONAGUA 2014).
The observed differences in time to reproduction and seed size can have important ecological and evolutionary consequences for plant populations and plant–insect interactions (Elzinga et al. 2007; Brody et al. 2007). Plants that flower relatively early or late typically receive less damage from flower and seed herbivores than plants that flower at the peak flowering period (Johnson et al. 2015). This could be very important for lima bean, as its seeds are frequently consumed by larvae of bruchid beetles (Alvarez et al. 2006; Aebi et al. 2008, Shlichta et al. 2014). These beetles start appearing in the field as pods mature in early January, and their densities build up as the season progresses reaching a peak towards the end of January (Hernandez-Cumplido et al. 2016a, b; Bustos et al. unpublished data). Any shift in the timing of seed production may have important consequences for the exposure to seed predators.
We found that seeds from control plants were in general heavier than seeds produced by plants exposed to the two herbivory treatments (with and without parasitoids) (Fig. 4a). This appeared to be mainly due to the fact that, within each treatment, seeds produced early in the season (first 4 weeks) were heavier than seeds produced later in the season (last 4 weeks) (Online Resource 2). We could speculate that by producing more seeds earlier in the season, plants exposed to herbivores + parasitoids produced on average heavier seeds than plants with caterpillars alone. Larger seeds may have a selective advantage during adverse conditions (Leishman et al. 2000), can better tolerate pre-dispersal seed predation (Mack 1998), and can improve seedling vigor and competitive ability (Moles and Westoby 2004). Nevertheless, larger seed size may not always be advantageous. In lab and field studies, we have consistently found that seed beetles lay more eggs on larger seeds, in which they perform better (Campan and Benrey 2006; Zaugg et al. 2013; Hernandez-Cumplido et al. 2016b).
Overall impact of parasitoids on plant fitness
One of the unexpected but most interesting results from this study was that, in the absence of parasitoids, herbivores trigger an overcompensation response (i.e. more trifolia were produced by herbivore-damaged than by control plants). This runs against the general assumption that the action parasitoids may benefit plants. Indeed, only a handful of studies suggest that parasitoids may positively affect plant fitness (Gomez and Zamora 1994; Van Loon et al. 2000; Hoballah and Turlings 2001; Smallegange et al. 2008; Gols et al. 2015), and only three of these studies were conducted under (semi-)field conditions. Gomez and Zamora (1994) found that by excluding parasitoids from fruits of Hormathophylla spinosa, Brassicaceae, the incidence of damage by seed weevils was increased and seed production significantly decreased. Hoballah and Turlings (2001) found that maize plants produced more seeds when attacked by parasitized caterpillars than plants attacked by unparasitized caterpillars. Finally, in a recent study, Gols et al. (2015) showed in an outdoor garden experiment that the fitness of Sinapsis arvensis (Brassicaceae) was significantly increased when Pieris brassicae larvae were parasitized by two parasitoid species. Our study adds to this scarce field evidence on the potential beneficial effects of parasitoids for plant fitness, but also shows that parasitoids may indirectly affect plant compensatory growth in responses to herbivory.
Conclusions and future directions
The beneficial effects of parasitoids on plant performance in natural and agricultural systems have been widely accepted, but the mechanisms underlying these effects, particularly under natural conditions, remain largely underexplored. Our results provide further insight into how the presence of parasitoids can alter the outcome of plant–herbivore interactions. We conclusively showed that the combined effects of the plant’s ability to tolerate and compensate for herbivore damage, and the parasitoid-mediated reduction in leaf damage, mitigated the negative effects of herbivory, which ultimately resulted in more and heavier seeds produced earlier in the season. It should be noted that in this study we only looked at one herbivore species and one parasitoid species during one particular time of the season. To fully understand the impact of parasitoids on herbivore-mediated plant responses, it would be necessary to manipulate herbivore pressure and parasitoid presence throughout the entire growing season.
References
Abdala-Roberts L, Hernández-Cumplido J, Chel-Guerrero L et al (2016) Effects of plant intraspecific diversity across three trophic levels: underlying mechanisms and plant traits. Am J Bot 103:1810–1818. https://doi.org/10.3732/ajb.1600234
Ables JR, Bradleigh Vinson S (1981) Regulation of host larval development by the egg-larval endoparasitoid Chelonus insularis [Hym.: Braconidae]. Entomophaga 26:453–458. https://doi.org/10.1007/BF02374720
Aebi A, Shani T, Hansson C et al (2008) The potential of native parasitoids for the control of Mexican bean beetles: a genetic and ecological approach. Biol Control 47:289–297. https://doi.org/10.1016/j.biocontrol.2008.07.019
Agrawal AA (2000) Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends Plant Sci 5:309–313. https://doi.org/10.1007/s00468-007-0181-8
Alvarez N, Mercier L, Hossaert-Mckey M, Contreras-Garduno J, Kunstler G, Aebi A, Benrey B (2006) Ecological distribution and niche segregation of sibling species: the case of bean beetles, Acanthoscelides obtectus Say and A. obvelatus Bridwell. Ecol Entomol 31:582–590. https://doi.org/10.1111/j.1365-2311.2006.00817.x
Askew R, Shaw M (1986) Parasitoid communities: their size, structure and development. In: Greathead D (ed) Wagge J. Insect parasitoids, Academic p, pp 225–264
Ballhorn DJ, Kautz S, Heil M, Hegeman AD (2009) Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defence in nature. PLoS ONE 4:1–7. https://doi.org/10.1371/journal.pone.0005450
Beckage NE, Riddiford LM (1982) Effects of parasitism by Apanteles congregatus on the endocrine physiology of the tobacco hornworm Manduca sexta. Gen Comp Endocrinol 47:308–322. https://doi.org/10.1016/0016-6480(82)90238-6
Blue E, Kay J, Younginger BS, Ballhorn DJ (2015) Differential effects of type and quantity of leaf damage on growth, reproduction and defence of lima bean (Phaseolus lunatus L.). Plant Biol 17:712–719. https://doi.org/10.1111/plb.12285
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20:441–448. https://doi.org/10.1016/j.tree.2005.05.001
Boege K, Dirzo R, Siemens D, Brown P (2007) Ontogenetic switches from plant resistance to tolerance: minimizing costs with age? Ecol Lett 10:177–187. https://doi.org/10.1111/j.1461-0248.2006.01012.x
Brody AK, Price MV, Waser NM (2007) Life-history consequences of vegetative damage in scarlet gilia, a monocarpic plant. Oikos 116:975–985. https://doi.org/10.1111/j.2007.0030-1299.15705.x
Campan EDM, Benrey B (2006) Effects of seed type and bruchid genotype on the performance and oviposition behavior of Zabrotes subfasciatus (Coleoptera: Bruchidae). Insect Sci 13:309–318. https://doi.org/10.1111/j.1744-7917.2006.00099.x
Capinera JL (2001) Handbook of vegetable pests, 1st edn. Academic Press, Cambridge. https://doi.org/10.1007/s13398-014-0173-7.2
Coleman RA, Barker AM, Fenner M (1999) Parasitism of the herbivore Pieris brassicae L. (Lep., Pieridae) by Cotesia glomerata L. (Hym., Braconidae) does not benefit the host plant by reduction of herbivory. J Appl Entomol 123:171–177. https://doi.org/10.1046/j.1439-0418.1999.00334.x
CONAGUA Comisión Nacional del agua (2014) Servicio meteorológico nacional. Reporte del Clima en México. http://smn1.conagua.gob.mx/climatologia/analisis/reporte/Anual2013.pdf. http://smn1.conagua.gob.mx/climatologia/analisis/reporte/RC-Septiembre14.pdf
Coudron TA, Kelly TJ, Puttler B (1990) Developmental responses of trichoplusia-Ni (Lepidoptera, Noctuidae) to parasitism by the Ectoparasite Euplectrus plathypenae (Hymenoptera, Eulophidae). Arch Insect Biochem Physiol 13:83–94
Crawley M (1989) Insect herbivores and plant population dynamics. Annu Rev Entomol. https://doi.org/10.1146/annurev.en.34.010189.002531
Dietrich R, Ploss K, Heil M (2005) Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environ 28:211–222. https://doi.org/10.1111/j.1365-3040.2004.01265.x
Edenius L, Danell K, Bergström R, Bergstrom R (1993) Impact of herbivory and competition on compensatory growth in woody winter plants: on winter browsing by moose on Scots pine. 66:286–292
Elzinga J, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G (2007) Time after time: flowering phenology and biotic interactions. Trends Ecol Evol 22:432–439. https://doi.org/10.1016/j.tree.2007.05.006
Fatouros NE, Van Loon JJA, Hordijk KA, Smid HM, Dicke M (2005) Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J Chem Ecol 31:2033–2047. https://doi.org/10.1007/s10886-005-6076-5
Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Funct Ecol 25:399–407. https://doi.org/10.1111/j.1365-2435.2010.01805.x
Freytag GF, Debouck DG (2002) Taxonomy, distribution and ecology of the genus Phaseolus (Leguminosae-Papilionoideae) in North America. BRIT Press, Mexico and Central America
Godfray H (1994) Parasitoids: behavioral and evolutionary ecology
Gols R, Wagenaar R, Poelman EH, Kruidhof HM, Van Loon JJA, Harvey JA (2015) Fitness consequences of indirect plant defence in the annual weed, Sinapis arvensis. Funct Ecol 29:1019–1025. https://doi.org/10.1111/1365-2435.12415
Gomez JM, Zamora R (1994) Top-down effects in a tritrophic system: parasitoids enhance plant fitness. Ecology 75:1023–1030. https://doi.org/10.2307/1939426
Harvey JA (2005) Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomol Exp Appl 117:1–13. https://doi.org/10.1111/j.1570-7458.2005.00348.x
Harvey JA, Harvey IF, Thompson DJ (1994) Flexible larval growth allows use of a range of host sizes by a parasitoid wasp. Ecology 75:1420–1428. https://doi.org/10.2307/1937465
Heil M (2004) Induction of two indirect defences benefits lima bean (Phaseolus lunatus, Fabaceae) in nature. J Ecol 92:527–536. https://doi.org/10.1111/j.0022-0477.2004.00890.x
Heil M (2010) Plastic defence expression in plants. Evol Ecol 24:555–569. https://doi.org/10.1007/s10682-009-9348-7
Hernandez-Cumplido J, Forter B, Moreira X, Heil M, Benrey B (2016a) Induced floral and extrafloral nectar production affect ant-pollinator interactions and plant fitness. Biotropica 48:342–348. https://doi.org/10.1111/btp.12283
Hernandez-Cumplido J, Glauser G, Benrey B (2016b) Cascading effects of early-season herbivory on late-season herbivores and their parasitoids. Ecology 97:1283–1297. https://doi.org/10.1890/15-1293.1/suppinfo
Hernández-Cumplido J, Benrey B, Heil M (2010) Attraction of flower visitors to plants that express indirect defence can minimize ecological costs of ant—pollinator conflicts. J Trop Ecol 26:555–557. https://doi.org/10.1017/S0266467410000234
Hoballah MEF, Turlings TCJ (2001) Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol Ecol Res 3:553–565
Huhta AP, Rautio P, Hellstrom K, Saari M, Tuomi J (2009) Tolerance of a perennial herb, Pimpinella saxifraga, to simulated flower herbivory and grazing: immediate repair of injury or postponed reproduction? Plant Ecol 201:599–609. https://doi.org/10.1007/sl1258-008-9535-6
Johnson MTJ, Campbell SA, Barrett SCH (2015) Evolutionary interactions between plant reproduction and defense against herbivores. Annu Rev Ecol Evol Syst 46:191–213. https://doi.org/10.1146/annurev-ecolsys-112414-054215
Jourdie V, Alvarez N, Molina-Ochoa J, Williams T, Bervinson D, Benrey B, Turlings TCJ, Franck P (2010) Population genetic structure of two primary parasitoids of Spodoptera frugiperda (Lepidoptera), Chelonus insularis and Campoletis sonorensis (Hymenoptera): to what extent is the host plant important? Mol Ecol 19:2168–2179. https://doi.org/10.1111/j.1365-294X.2010.04625.x
Juenger T, Lennartsson T, Tuomi J (2012) The evolution of tolerance to damage in Gentianella campestris: natural selection and quantitative genetics of tolerance. Evol Ecol 14:393–419. https://doi.org/10.1023/A:1010908800609
Kaplan I, Carrillo J, Garvey M, Ode PJ (2016) Indirect plant-parasitoid interactions mediated by changes in herbivore physiology. Curr Opin Insect Sci 14:112–119. https://doi.org/10.1016/j.cois.2016.03.004
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Karban R, Myers JH (1989) Induced plant responses to herbivory. Ann Rev Ecol Syst 20:331–348
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328. https://doi.org/10.1146/annurev.arplant.53.100301.135207
Koptur S, Smith CL, Lawton JH (1996) Effects of artificial defoliation on reproductive allocation in the common vetch, Vicia sativa (Fabaceae: Papilionoideae). Am J Bot 83:886–889. https://doi.org/10.2307/2446265
Korpita T, Gómez S, Orians CM (2014) Cues from a specialist herbivore increase tolerance to defoliation in tomato. Funct Ecol 28:395–401. https://doi.org/10.1111/1365-2435.12184
Kotanen PM, Rosenthal JP (2000) Tolerating herbivory: does the plant care if the herbivore has a backbone? Evol Ecol 14:537–549. https://doi.org/10.1023/A:1010862201331
Leishman MR, Wright IJ, Moles AT, Westoby M (2000) The evolutionary ecology of seed size. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities, 2nd edn. CAB int., Wallingford, UK, pp 31–57
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. Cary, NC
Lucas-Barbosa D, Dicke M, Kranenburg T, Aartsma Y, Van Beek TA, Huigens ME, Van Loon JJA (2016) Endure and call for help: strategies of black mustard plants to deal with a specialized caterpillar. Funct Ecol 31:325–333. https://doi.org/10.1111/1365-2435.12756
Mack AL (1998) An advantage of large seed size: tolerating rather than succumbing to seed predators. Biotropica 30:604–608. https://doi.org/10.1111/j.1744-7429.1998.tb00100.x
Mackauer M, Sequeira R (1993) Patterns of development in insect parasites. Parasites and pathogens of insects. Parasites, vol 1. Academic Press, San Diego, pp 1–23
Manzaneda AJ, Prasad KVSK, Mitchell-Olds T (2010) Variation and fitness costs for tolerance to different types of herbivore damage in Boechera stricta genotypes with contrasting glucosinolate structures. New Phytol 188:464–477. https://doi.org/10.1111/j.1469-8137.2010.03385.x
Martínková J, Klimešová J, Mihulka S (2008) Compensation of seed production after severe injury in the short-lived herb Barbarea vulgaris. Basic Appl Ecol 9:44–54. https://doi.org/10.1016/j.baae.2006.12.001
Mauricio R, Bowers MD, Bazzaz FA (1993) Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae). Ecology 74:2066–2071
Mauricio R, Rausher MD, Burdick DS (1997) Variation in the defense strategies of plants: are resistance and tolerance mutually exclusive? Ecology 78:1301–1311. https://doi.org/10.2307/2266125
Moles AT, Westoby M (2004) Seedling survival and seed size: a synthesis of the literature. J Ecol 92:372–383. https://doi.org/10.1111/j.0022-0477.2004.00884.x
Moreira X, Abdala-Roberts L, Hernandez-Cumplido J, Cuny MAC, Glauser G, Benrey B (2015) Specificity of induced defenses, growth, and reproduction in lima bean (Phaseolus lunatus) in response to multispecies herbivory. Am J Bot 102:1300–1308. https://doi.org/10.3732/ajb.1500255
Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38:541–566. https://doi.org/10.1146/annurev.ecolsys.38.091206.095822
Ode PJ, Harvey JA, Reichelt M et al (2016) Differential induction of plant chemical defenses by parasitized and unparasitized herbivores: consequences for reciprocal, multitrophic interactions. Oikos 125:1398–1407. https://doi.org/10.1111/oik.03076
Poelman EH, Zheng S-J, Zhang Z, Heemskerk NM, Cortesero A, Dicke M (2011) Parasitoid-specific induction of plant responses to parasitized herbivores affects colonization by subsequent herbivores. Proc Natl Acad Sci 108:19647–19652. https://doi.org/10.1073/pnas.1110748108
Poelman EH, Bruinsma M, Zhu F, Weldegergis BT, Boursault AE, Jongema YDE, Van Loon JJA, Vet LEM, Harvey JA, Dicke M (2012) Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol. https://doi.org/10.1371/journal.pbio.1001435
Pogue GM (2002) A world revision of the genus Spodoptera Guenée (Lepidoptera: Noctuidae). Mem Am Entomol Soc 43:1–202
Puettmann KJ, Saunders MR (2001) Patterns of growth compensation in eastern white pine (Pinus strobus L.): the influence of herbivory intensity and competitive environments. Oecologia 129:376–384. https://doi.org/10.1007/s004420100741
Rosenheim JA, Wilhoit LR, Goodell PB, Grafton-Cardwell EE, Leigh TF (1997) Plant compensation, natural biological control, and herbivory by Aphis gossypii on pre-reproductive cotton: the anatomy of a non-pest. Entomol Exp Appl 85:45–63. https://doi.org/10.1023/A:1003026507687
Shlichta JG, Glauser G, Benrey B (2014) Variation in cyanogenic glycosides across populations of wild lima beans (Phaseolus lunatus) has no apparent effect on bruchid beetle performance. J Chem Ecol 40:468–475. https://doi.org/10.1007/s10886-014-0434-0
Smallegange RC, Van Loon JJA, Blatt SE, Harvey JA, Dicke M (2008) Parasitoid load affects plant fitness in a tritrophic system. Entomol Exp Appl 128:172–183. https://doi.org/10.1111/j.1570-7458.2008.00693.x
Smilowitz Z, Iwantsch GF (1973) Relationships between the parasitoid Hyposoter exiguae and the cabbage looper, Trichoplusia ni: effects of host age on developmental rate of the parasitoid. Environ Entomol 2:759–763. https://doi.org/10.1093/ee/2.5.759
Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31:565–595. https://doi.org/10.1146/annurev.ecolsys.31.1.565
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185. https://doi.org/10.1016/S0169-5347(98)01576-6
Thorpe WH (1933) Notes on the natural control of Coleophora laricella, the larch case-bearer. Bull Entomol Res 24:271–291. https://doi.org/10.1017/S0007485300031448
Tiffin P (2000) Mechanisms of tolerance to herbivore damage: what do we know? Evol Ecol 14:523–536. https://doi.org/10.1023/A:1010881317261
Tito R, Castellani TT, Fáveri SB, Lopes BC, Vasconcelos HL (2016) From over to undercompensation: variable responses to herbivory during ontogeny of a neotropical monocarpic plant. Biotropica 48:608–617. https://doi.org/10.1111/btp.12340
Utsumi S, Ando Y, Roininen H, Takahashi J, Ohgushi T (2013) Herbivore community promotes trait evolution in a leaf beetle via induced plant response. Ecol Lett 16:362–370. https://doi.org/10.1111/ele.12051
Van der Meijden E, Klinkhamer PGL (2000) Conflicting interests of plants and the natural enemies of herbivores. Oikos 89:202–208
Van Loon JJA, De Boer JG, Dicke M (2000) Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol Exp Appl 97:219–227. https://doi.org/10.1023/A:1004032225239
Wise MJ, Abrahamson WG (2005) Beyond the compensatory continuum: environmental resource levels and plant tolerance of herbivory. Oikos 109:417–428. https://doi.org/10.1111/j.0030-1299.2005.13878.x
Wise MJ, Abrahamson WG (2007) Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. Am Nat 169:443–454. https://doi.org/10.1086/512044
Xi X, Eisenhauer N, Sun S (2015) Parasitoid wasps indirectly suppress seed production by stimulating consumption rates of their seed-feeding hosts. J Anim Ecol 84:1103–1111. https://doi.org/10.1111/1365-2656.12361
Xiao Q, Ye W, Zhu Z, Chen Y, Zheng H (2005) A simple non-destructive method to measure leaf area using digital camera and Photoshop software. Chinese J Ecol 6:711–714
Zaugg I, Benrey B, Bacher S (2013) Bottom-up and top-down effects influence bruchid beetle individual performance but not population densities in the field. PLoS ONE 8:e55317. https://doi.org/10.1371/journal.pone.0055317
Acknowledgements
We thank Xoaquin Moreira for advice on statistical analysis, Christer Hansson for the determination of Euplectrus platyhypenae, Thomas Degen for the drawings presented in the graphs and Alfredo López-Rojas, Quint Rusman, Stéphanie Morelon, Yasmin Emery and William K. Petry for their help in the field. We thank Ted Turlings for helpful advice and discussions during this study and the Universidad del Mar of Puerto Escondido (Oaxaca, Mexico) for logistic support and infrastructure. We are grateful to Caroline Mueller, Jeff Harvey and an anonymous reviewer for their many constructive and insightful comments that helped improve this manuscript. The authors declare no conflict of interest. This research was financially supported by the Swiss National Science Foundation (Project No. 3100AO-10923) awarded to BB.
Author information
Authors and Affiliations
Contributions
BB originally formulated the idea, MACC, JG, JHC and BB designed the experiments, MACC, JG and JHC conducted fieldwork, MACC analyzed the data, MACC and BB wrote the manuscript.
Corresponding author
Additional information
Communicated by Caroline Müller.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cuny, M.A.C., Gendry, J., Hernández-Cumplido, J. et al. Changes in plant growth and seed production in wild lima bean in response to herbivory are attenuated by parasitoids. Oecologia 187, 447–457 (2018). https://doi.org/10.1007/s00442-018-4119-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4119-1