Abstract
Perennial, polycarpic herbs can respond to herbivory either by (1) regrowth in the same season in order to compensate for lost reproductive structures or by (2) postponing reproduction until the following growing season. We tested these response patterns with the perennial umbellifer Pimpinella saxifraga by simulating flower herbivory and shoot grazing both in the field and in a common garden experiment. In the field, both simulated flower herbivory and grazing effectively suppressed current reproduction, whereas no statistically significant effects of previous-year treatments on growth or reproduction were found in the following year. In the common garden, in the first year the species fully compensated for simulated flower herbivory in vegetative parameters but seed set was reduced by 26%. After 2 years of flower removal, the plants overcompensated in shoot and root biomass by 47 and 46%, respectively, and compensated fully in reproductive performance. Simulated grazing resulted in 21% lower shoot biomass in the first season, but the root biomass was not affected. In the second season the root biomass increased by 43% as compared to the control plants. However, regrowth following simulated grazing resulted in a significant delay in flowering with the consequence that the seed yield of fertile plants was reduced by 55% as compared to the control plants. These results suggest that in resource-rich garden conditions P. saxifraga may immediately repair injuries caused by flower herbivory, but repairs more extensive shoot injury less successfully. Delayed phenology decreases the benefits of immediate repair. In resource-poor conditions, the benefits of regrowth can be negligible. Accordingly, in our field population, the plants postponed their reproduction until the following year in response to simulated grazing and frequently in response to flower removal. When the plants gain very little from regrowth, the costs of reproduction would select for postponed reproduction in response to injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When strict biennials and other monocarpic species are grazed during the flowering stage, they have only one choice. They must immediately repair the injury to compensate for the lost biomass and damaged reproductive structures. If conditions are favourable, the immediate repair may result in equal and even overcompensation compared to the reproductive success of ungrazed plants (Paige and Whitham 1987; Whitham et al. 1991; Lennartsson et al. 1998; Huhta et al. 2000a). However, compensatory regrowth is often associated with delayed flowering and seed maturation (Benner 1988; Bergelson and Crawley 1992) which may, in unfavourable conditions, result in a complete reproductive failure or at best only a partial compensation for the lost reproductive potential (Lennartsson et al. 1998). Consequently, monocarpic plants are in a “bet-hedging” situation, not only in relation to herbivory (Vail 1992; Nilsson et al. 1996), but also in relation to resource and weather conditions during regrowth and seed maturation (Lennartsson et al. 1998; Huhta et al. 2000b; Levine and Paige 2004).
In response to grazing (or mowing), polycarpic species have more options as compared to monocarpic species (Fig. 1). A perennial plant may postpone its reproductive attempt into the following season rather than to retry flowering and seed setting within the same season. Besides grazing and mowing, insect herbivory may cause considerable losses for flowering plants (Louda 1984; Hendrix and Trapp 1989; Cox and McEvoy 1983; Karban and Strauss 1993). One could expect that it would be easier to immediately repair flower herbivory than extensive injury by grazing and browsing. On the other hand, the plant may postpone reproductive investment also in this case. In fact, clipping of flower stalks and removal of developing flowers are treatments sometimes used to manipulate the current reproductive investment in order to test how the reduced reproductive effort affects future reproductive success in perennial plants (e.g. Tolvanen and Laine 1997; Hemborg 1998; Houle 2001; Obeso 2002; Knight 2003). If the plants do postpone their reproductive investment in response to flower removal, their improved future reproductive success would indicate the costs of reproduction.
Strategy tree of mono- and polycarpic species in relation to grazing and immediate repair versus postponing reproduction until the next growing season. Undercompensation = the current reproductive success of grazed plants is lower compared to ungrazed plants, equal compensation = grazed and ungrazed plants have an equal seed production, overcompensation = the current reproductive success of grazed plants is higher than that of ungrazed plants
An important implication of these manipulative studies is that herbivory during the flowering stage of a perennial plant has in fact three consequences: (A) the immediate cost of herbivory referring to the immediately lost reproductive capacity of injured plants in relation to intact plants, (B) the delayed cost of herbivory in terms of reduced future reproductive capacity of previously injured plants and (C) altered reproductive investment of injured plants influencing the potential costs of reproduction. According to Venecz and Aarssen (1998), if current reproduction is prevented by herbivory or clipping, resources that would have been otherwise invested in reproduction may be stored below-ground and invested in reproduction in the following year. In such a case, the greater fruit and seed yield of injured plants in the subsequent year compared to uninjured plants would indicate the costs of reproduction (i.e. C > B ≥ 0). On the other hand, when the injured plants immediately regrow and flower in order to mitigate the costs of herbivory damage on current reproduction (A), this investment may reduce their future fecundity as a consequence of the costs of compensatory reproduction. This implies that it would not be useful to postpone reproduction in conditions where the injured plant gains more in reducing the costs of injury than it loses in terms of the costs of compensatory reproduction. If there are no costs associated with reproduction, it would be always useful to restart reproduction in order to reduce the adverse effects of herbivory. However, if the reproductive costs are high and the compensatory reproduction is likely to fail, it would be useful to postpone reproduction until the following growing season.
We studied the responses of the perennial umbellifer, Burnet saxifrage (Pimpinella saxifraga), after simulated flower herbivory and ungulate grazing or browsing in two consecutive years in garden conditions (1998–1999), and in a field population (1999–2000). P. saxifraga occurs mainly in human-influenced habitats, such as dry to mesic meadows and pastures, fields, road verges and ridges (Wells et al. 1976; Hämet-Ahti 1980; Grime et al. 1988). It is a mid-seral species and occurs in moderately stressful but undisturbed habitats (Grime et al. 1988). Although it contains several chemical defence compounds (Cornu et al. 2001), it is a food source for Swallowtail butterfly larvae (Papilio machaon) (Marttila et al. 1990) and also ungulate grazing may cause serious injuries to P. saxifraga. Because it does not occur very abundantly in intensively grazed grasslands (Jantunen and Saarinen 2003), we hypothesized that the species may be well able to compensate for the loss of reproductive organs caused by insect herbivores but less so for more comprehensive biomass losses caused by mowing or ungulate grazing. We monitored the effects of two levels of damage both in vegetative and reproductive performance parameters in order to test for the costs of injury and the costs of reproduction.
Material and methods
Study species
Pimpinella saxifraga (L.) is a perennial plant that has large and deep penetrating rootstock. Most of the above-ground parts die at the end of the growing season. Rosette leaves overwinter and support new growth in the following spring. In field conditions the plants grow about 50 cm tall (Kalela and Väänänen 1960). White to pale reddish flowers are commonly hermaphrodite and the inflorescence is typically umbelliferous with main umbels and umbellules (Fig. 2a). Each hermaphroditic flower can produce a fruit consisting of two-one-seeded mericarps.
Schematic presentation a of an umbelliferous plant and flower structure along with the treatment effects (b–d) on reproductive characteristics (umbel structure) of Pimpinella saxifraga in the garden during the years 1998 and 1999 (mean ± SE). The bars denoted by a dot differ statistically from the unclipped control plants of the corresponding treatment group (filled = 1998, open = 1999, P < 0.05)
The worldwide distribution of P. saxifraga includes the whole of Europe, Asia ranging to the Baikal area and north-eastern parts of North America (Hämet-Ahti 1980). According to Kalela and Väänänen (1960), P. saxifraga is native in Finland as a seashore species. In contrast, according to Hämet-Ahti (1980), it occurs only in human-influenced habitats as an archaeophyte. In Finland P. saxifraga is common extending to northern parts of Central Finland (Kalela and Väänänen 1960). In northern Finland, it occurs in only a few locations, such as Kiiminki (the origin of one of the populations studied here), the Kuusamo district and Kemi on the coast of the Gulf of Bothnia.
Experimental design
The garden experiment was carried out during 1997–1999 in the Botanical Gardens of the University of Oulu, Finland (65° N, 25.5° E). The target plants were grown from seeds collected from two separate populations located in Joensuu (62.5° N, 30° E) and in Kiiminki (65°10′ N, 25°50′ E). The seedlings were planted (1997) at regular distances (50 cm) into planters in five adjacent rows. At the time of sprouting (1998) the plants (N = 23–33 per treatment) were randomly assigned into three different treatment groups: (1) control, (2) removal of all developing flower buds (referred to hereafter as simulated flower herbivory) and (3) 75% clipping of shoot biomass measured as height of an each individual, which represents realistic amount of biomass loss during heavy grazing pressure by domestic animals (Huhta, pers. obs.), and is hereafter referred to as simulated grazing. In the first growing period (1998), after measuring the initial height and number of stems, the treatments were carried out just before flowering (at the bud stage), on 29 June 1998. The phenological state of each plant was surveyed on 21 July, 20 August and 5 October using four classes: (I) the plant with flowers in the bud stage, (II) flowering, (III) unripe (green) fruits and (IV) ripe (brown) fruits. At the end of the season (19–20 October) the plants from rows two and four (N = 27, 26 and 25 per treatment) were harvested in order to evaluate the impacts of the treatments after the first season, while the plants in the three remaining rows (N = 23, 33 and 33 per treatment) were allowed to grow for another year. During the second season (1999), the treatments (1, 2 and 3 above) were repeated at a phenologically corresponding time on 24–25 June 1999. The phenology of plants was monitored on 15 July and 18 August. In addition to the phenological stages of flowering plants I–IV, sterile and dead plants were recorded. The surviving plants were harvested for analysis on 28–29 October 1999.
The harvested plants (the above-ground structures were cut away first, whereafter the underground structures were dug out from the soil) were dried at room temperature for several weeks and the following parameters were recorded: stem height, stem number, shoot and root weight, root:shoot ratio, the number of umbels, and seed weight. Further, five to six plants from each treatment were randomly chosen and six of their primary umbels were chosen for closer examination. This included counting the number of umbellules per umbel and the number of seed bearing branches per umbellule (umbellule branches, see Fig. 2a). All this together allowed us to calculate an estimate for total seed production per plant: seed number = number of umbels × number of umbellules per umbel × number of umbellule branches × 2. The multiplier 2 is used because of the two-one-seeded mericarp is carried by each umbellule branch.
In 1999, sterile plants were included in the vegetative parameters and dead and sterile plants were included when calculating reproductive success in order to find out the true effects of clippings in time, i.e. if the treatments were to be realized in exhaustion of resources in the following year leading into resource deficiency causing “resting period” or even death. In vegetative parameters we did this by recording sterile plants as zeros in case that particular parameter was not available (e.g. number of stems). In the seed parameters only fertile plants were included as these data were not measured from every plant (e.g. umbellule branches). In case of reproductive success we estimated the final performance by multiplying the number of seeds per fertile plant by survival probability (% of sterile and fertile plants in 1999) and by flowering probability (% of fertile plants in 1999) as well as by seed weight.
The treatments (control, flower removal and 75% clipping) were repeated in the Kiiminki field population on 6 July 1999. The soil in Kiiminki habitat is nutrient poor compared to the garden soil, nitrogen concentration being only about 1/4 of that in the garden (0.41% vs. 1.83%) and while the organic layer in Kiiminki is on average less than 5 cm, the soil in garden is mainly organic material (OM% 67.3). Similar sized plants growing on two small hillocks, an area of 0.1 ha, were randomly allotted to treatments. The plant performance was followed until the 2000 season during which no clippings of the target plants were performed in order to see if the effects of the treatments in 1999 were still affecting plant performance. Their phenology was estimated on 20 July, seeds were collected on 21 September and plants were harvested on 6 October 2000. This experiment also included the removal of vegetation around the immediate surroundings of the target plants by mowing the neighbouring plants (referred to below as no competition). The original number of replicates per specific treatment combination was 15.
Data analyses
Because we tested a single hypothesis with multiple variables we performed a protected ANOVA on the data. This approach combines MANOVA and ANOVA (i.e. univariate analyses are performed if the multivariate analysis yields a significant result) and is less conservative than, for example, Bonferroni correction used to correct the P-values of multiple tests of a single hypothesis (Scheiner 1993). Because in the garden experiment the MANOVA yielded significant results (Table 1), we also conducted ANOVA for the data. In the garden experiment in both MANOVA (Pillai’s trace) and the subsequent ANOVA, we applied a two-way factorial design with treatment (control, simulated flower herbivory and simulated grazing) and the number of treatments (clippings during 1 or 2 years) as grouping factors. Further, because we collected seeds from two populations (Joensuu and Kiiminki) and since seeds from the Joensuu population seemed to produce plants that were both longer (Joensuu 50.4 ± 1.2 cm and Kiiminki 47.2 ± 1.1 cm; F 1,191 = 3.74, P = 0.054) and tended to have more stems (9.5 ± 0.4 and 8.5 ± 0.4, F 1,191 = 3.17, P = 0.077) (measured before the treatments were carried out in 1998), we used the origin as a blocking factor (here considered as a fixed factor) in further analyses. Our intention is not, however, to study closer the possible differences between the two populations, and hence, we did not include origin in the interactions terms in the models (cf. Newman et al. 1997). MANOVA was performed separately for those variables measured in all studied plants (Table 1a: height, stem number, umbel number, above-ground weight and root weight) and for those parameters measured only in a subsample of studied plants (Table 1b: umbellule branch number, umbel branch number, number of seeds per umbel, seeds per fertile plant and seed weight). We present here the MANOVA results, but instead of all the individual ANOVA results we present planned contrasts (control versus other treatments performed separately for plants treated during 1 or 2 years) performed under the above ANOVA model.
For the data from the field, we performed the MANOVA using two-way factorial design with cutting treatment (control, simulated flower herbivory and simulated grazing) and competition (neighbouring plants mown or left intact) as grouping factors. The test was performed separately for those variables measured in all studied plants (Table 3a: height, stem number and above-ground weight) and those measured only in plants that produced flowers (Table 3b: number of umbels, number of umbellules per umbel, number of umbellule branches and number of seeds per umbel as well as number of seeds per plant). In the field data no significant cutting effect was found (Table 3), and hence we did not continue to ANOVA and planned cutting treatment comparisons. Seed production and initial height, number of leaves and stem diameter of the plants that re-flowered in 1999 were compared to those that postponed flowering to 2000 by means of one-way ANOVA.
The results of garden- and field experiments are presented in Table 2 where we tabulated vegetative and reproductive traits case-wise and calculated ratios expressing relative performance of plants under different treatments in the following formula: 100 × [(treatment − control)/control]. Thus, positive values indicate overcompensation and negative values, undercompensation (sensu Belsky 1986), respectively.
The impact of injury level and origin on plant phenology during the two seasons in the garden experiment was tested using a four-way contingency table, where the grouping factors were phenological stage (I–IV, see above) of the plant, treatment, population and time of observation, whereas the dependent variable was the number of the plants in each class. The effects of grouping factors (and their interactions) were examined using a loglinear model. Because the phenology in 1998 was monitored three times and in 1999 only twice, the examination described above was performed separately for each year. We performed the analysis using SPSS software’s “Loglinear model selection” option suitable for hierarchical loglinear models to multidimensional cross tabulations using an iterative proportional-fitting algorithm. Iterations start from a saturated model of which factors (and their interactions) are gradually dropped out until the best-fit model has been reached. The best-fit model only has factors (or factor combinations) that explain the observed frequencies. Since we are testing (with log-likelihood coefficient G 2) the compatibility between observed and expected frequencies, a good model takes a low G 2-value (and high P-value). As the data included zero frequencies, a constant (0.5) was added to each frequency before the analysis (cf. Caswell 1989). Statistical differences between the number of dead, sterile and fertile plants in different treatments in 1999 was tested with the G 2-test.
Results
Growth and reproductive success in the garden
In the garden experiment (1998–1999), the treatments significantly affected plant growth and shoot structure (Table 1a) and reproductive parameters (Table 1b). In the first year (1998), there were only minor treatment effects on stem height and number (Table 2). Neither flower removal nor simulated grazing had any marked effects on above- or below-ground biomass (Table 2). In the second year, flower removal increased above- and below-ground biomass by 47 and 46%, respectively, and simulated grazing by 16% (NS) and 43% compared to control plants.
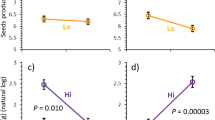
Reproductive parameters showed a different pattern as most of the treatment effects were negative (Table 2). The adverse effects of simulated grazing were slightly more pronounced than the effects of flower removal, throughout the hierarchical structure of the inflorescences (Fig. 2a–d). The pattern was the same in the seed number per umbel (Fig. 3a), the seed production of fertile plants (Fig. 3b), and seed weight (Table 2). When the proportion of dead and sterile plants was taken into account for evaluating reproductive success (total seed production per plant), this did not qualitatively change the result (Fig. 3c), because all plants survived to flowering in 1998 and sterility and mortality did not significantly differ between the treatment groups in 1999 (Fig. 3d; G 2 = 2.4, df = 4, P = 0.662).
Treatment effects on reproductive characteristics and fitness of Pimpinella saxifraga in the garden during the years 1998 and 1999 (mean ± SE): a mean seed number per umbel, b mean seed number of fertile plants, c reproductive success taking account probability of survival and flowering and mean seed number of fertile plants, d proportion of dead, sterile, and fertile plants in 1999. In a and b the bars denoted by a dot differ statistically from the unclipped control plants of the corresponding treatment group (filled = 1998, open = 1999, P < 0.05). Number of replicates for each treatment group (control, floral removal and simulated grazing) are: 1998: 11, 11 and 10 and for 1999: 10, 11 and 12, respectively. For arrays used in calculation of reproductive success and survival and flowering, see Material and methods
Phenological effects in the garden
The injured plants flowered and set seed considerably later than the uninjured controls and this delay increased with injury intensity. In the first year, the peak of flowering was around 21 July among control plants, while the injured plants were still in the bud-stage (stage I). On 20 August, 37% of control plants had passed flowering but the fruits were still immature (stage III) while injured plants had just started to flower (stage II). On 5 October, 90, 50, and 30% of the control, flower removed, and plants subjected to simulated grazing, respectively, had fully matured seeds (stage IV). In the second year, the same pattern was repeated. The injured plants had their flowering peak approximately 4 weeks later than the controls. On 20 August, control and flower-removed plants had produced immature fruits (stage III), but a half of the plants subjected to simulated grazing were still flowering (stage II). The observed frequencies could be best explained by the log linear model of the three factor interaction “phenological stage × injury level × observation time” in the both years (1998: G 2 = 11.17, df = 45, P = 1.00; 1999: G 2 = 6.19, df = 30, P = 1.00). Hence, the effect of clipping on the phenological stage was dependent on the time of observation, but the origin of populations did not significantly explain plant phenology (i.e. this factor could be dropped from the model).
Compensation ability in the field
In the field experiment (1999–2000), P. saxifraga could hardly compensate at all by repairing injuries in the first summer. Although some injured plants re-flowered in most treatment groups (40% in flower removal + no competition, 47% in flower removal + competition, 0% in simulated grazing + no competition and 7% in simulated grazing + competition), only two flower-removed plants managed to produce seeds. When the plants were left untouched during summer 2000, no statistically significant effects of previous flower or shoot damage were detected in vegetative (Table 3a) or reproductive variables (Table 3b). In the presence of competition, there was a slight decreasing trend in the number of stems, above-ground biomass and number of umbels in previously injured plants (Table 2).
Competition as such reduced plant growth (Tables 2 and 3a), but had no effect on reproductive performance (Table 3b). There was no indication of the cost of reproduction among plants recovering from previous flower removal since the seed production in 2000 of the plants that re-flowered in 1999 was the same (1378 ± 293, mean ± SE) as those that postponed flowering until the following season (1373 ± 261, one-way ANOVA: F 1,28 < 0.001, P = 0.991). At the time of injury in 1999, these two groups of plants did not differ from each other in relation to initial height (flowered 51.1 ± 1.8 cm vs. postponed 54.1 ± 2.0 cm; F 1,28 = 1.217, P = 0.279), number of leaves (flowered 10.2 ± 1.2 vs. postponed 10.2 ± 1.9; F 1,28 < 0.001, P = 0.998) or stem diameter (flowered 3.2 ± 0.1 mm vs. postponed 3.2 ± 0.2 mm; F 1,28 = 0.069, P = 0.794).
Discussion
Immediate repair or postponed reproduction?
In perennial plants, the fitness consequences of herbivory are rather complicated since the costs of injury on current and/or future reproductive success are confounded by the costs of reproduction. If the plant escapes grazing altogether (Fig. 1), the optimal reproductive tactics include its investment in current reproduction and its expected future reproductive success. In conditions where reproduction is costly, the plant has to trade current reproduction for expected future reproduction (e.g. Obeso 2002). A possible strategy is that the plant invests resources in current reproduction and, as a consequence, suffers costs in the survival and future fertility. Alternatively, the plant may totally refrain from current reproduction and thus it will avoid the potential costs of reproduction. The injured plants may also postpone their reproduction until the following growing season, and therefore they will suffer from the costs of herbivory but not from the costs of reproduction. Thus the overall effect of herbivore injury on reproductive success in the second season could be positive if, as suggested by Venecz and Aarssen (1998), by postponing reproduction, the injured plant can avoid the costs of reproduction and can accumulate more resource reserves for the following growing season. This notion fits rather well with the responses of P. saxifraga in our field experiment. The control plants escaped the costs of grazing, but suffered from the potential costs of reproduction. The plants which were subjected to simulated grazing postponed their reproduction until the following growing season (2000). They thus suffered the cost of grazing in terms of reproductive failure in 1999. However, 1 year later, the previously grazed and control plants performed equally which does not support the presumption that there is a cost of reproduction. In the case of flower removal, the situation was similar except that 40–47% of the injured plants re-flowered in the same year that they were damaged (1999), but could not produce seeds. The failure in setting seeds may result from missing peak of pollinator availability (e.g. Juenger and Bergelson 1997) or insufficient response time before the end of the growing season (Trumble et al. 1993; Venecz and Aarssen 1998). Anyhow, it is likely that the reproductive failure is related to a shift in the phenology of the injured plants that started to re-flower after injury, as we observed in the garden conditions.
The fact that compensatory reproduction is likely to fail in the field suggests that the costs of reproduction need not be high in order to select for postponed reproduction in P. saxifraga. We found no evidence supporting the cost of compensatory reproduction, since the plants which re-flowered in the first season (1999) in the field produced seeds in the following summer as equally well as the plants that postponed flowering until the following summer. There was no indication that, e.g. differences in plant size could have confounded this comparison.
Tolerance to repeated grazing
The field experiment indicates that both simulated flower herbivory and grazing have direct costs on the reproductive success of P. saxifraga in the same growing season. There are no delayed costs of grazing in the following growing season, or they are outweighed by the costs of reproduction when comparing ungrazed flowering plants and grazed plants which fail to flower due to grazing. From these results, one could expect that repeated grazing in subsequent years will more or less completely suppress flowering of P. saxifraga. However, the results of our garden experiment were not in line with this expectation. In the garden, the plants tolerated both flower removal and simulated grazing well. The treatments did not suppress the current reproduction as dramatically as in the field. In the garden, vegetative traits showed mainly positive response to the injury, whereas seed production declined and this decline was more pronounced in plants subjected to repeated grazing. As a result of the greater current reproductive investment one could expect that control plants would have accordingly suffered a greater cost of previous reproduction in the following season (Venecz and Aarssen 1998). Indeed, in the second season, the control plants had 47 and 16% lower above-ground biomass and 47 and 43% lower below-ground biomass compared to flower removal and simulated grazing. They also produced 24% fewer umbels than plants under flower removal, but 7% more umbels than plants subjected to simulated grazing. The costs of previous reproduction may also be realized as lower survival rates of the control plants compared to the flower removal treatment, or to a greater probability of remaining sterile in the second season. The figures supported this idea (mortality: 26% vs. 16%; sterility: 13% vs. 8% in control and flower removal, respectively), but the differences were not statistically significant.
Resource allocation and availability
Both flower removal and simulated grazing promote the increase of below-ground biomass. This in turn made extensive compensation of above-ground structures possible. This observation strongly supports the notion that the ability to shunt carbon reserves from root to shoot is one of the most central mechanisms of herbivore tolerance in perennial plants (e.g. McNaughton 1983; Rosenthal and Kotanen 1994; McNaughton et al. 1998; Strauss and Agrawal 1999; Wise et al. 2008). In our case, it is possible that the reserves are related to the costs of reproduction which are, at least partially, avoided in injured plants compared to the uninjured flowering plants. Alternatively, the resources are gained as a result of improved shoot growth (i.e. increased photosynthetic biomass) in the year of injury. The increased vegetative growth enhances the ability to assimilate carbon and acquire mineral nutrients (through increased sink demand) compared to uninjured controls (Sadras 1996). The acquired resources are stored in the root and in the following season are invested in above-ground growth and reproduction (Venecz and Aarssen 1998). In the garden experiment, flower removal and simulated grazing had no effect on shoot or root growth in the first year, but in the second year flower removal improved both shoot and root biomass and simulated grazing root biomass compared to the control plants.
It is interesting to note that the plants grown in the garden attempted to repair the injuries rather than postpone their reproduction until the following season. This is consistent with the fact that tolerance via compensatory growth seems to be more beneficial in the resource-rich garden conditions than in the field (cf. Maschinski and Whitham 1989; Huhta et al. 2000a). If the costs of reproduction were greater among the uninjured plants in the garden than in the field conditions, this would accordingly favour compensatory growth in the garden compared to the field conditions. We can neither confirm nor reject this possibility in the case of P. saxifraga. In our experiment, the compensation responses are linked to soil fertility. In the garden, during the second year the plants compensated seemingly well despite that they were injured for the second time in their lifetime. Also control plants maintained their high productivity, although their combined sterility and mortality was over 10% units higher compared to both clipping treatments. The control plants in the garden (in 1999) were about 14 times heavier (46.2 g vs. 3.34 g) and produced nearly 50 times more seeds (69,808 vs. 1460) than plants in the field in a non-competitive environment. Comparison of the results from the field experiment to the results from the garden experiment suggests that the compensation capacity is resource limited: after herbivory damage in a resource-poor environment reproduction is postponed until the following growing season, but not necessarily in resource-rich environments. In the field, there was also a slight decreasing trend in the number of stems, above-ground biomass and number of umbels in previously injured plants in the presence of competition. There was not, however, any statistically significant injury × competition interaction on plant performance.
In all, our results suggest that polycarpic plants may more readily allocate resources to immediate compensation in the resource-rich environment (garden) and postpone reproduction until the next growing season in the resource limited field conditions. Consequently, injured polycarpic perennials may either postpone their reproduction until the following summer or try to compensate immediately after the injury during the first summer (Fig. 1). Our results showed that the reproductive tactics that the plants use depends on resource availability. In the field, grazed plants postponed their reproduction until the following growing season and in the second season the previously injured plants performed equally well as the control plants. The plants that re-flowered during the first season could not produce seeds which suggest a time-limitation for seed maturation. In the resource-rich garden conditions, on the other hand, plants compensated fully for simulated flower herbivory in vegetative parameters but seed set was reduced. Hence, it seems that after herbivory damage in a resource-poor environment reproduction is postponed until the following growing season, but in resource-rich environments plants seem to be ready to take the chance to compensate already in the first season.
References
Belsky AJ (1986) Does herbivory benefits plants? A review of evidence. Am Nat 127:870–892. doi:10.1086/284531
Benner BL (1988) Effects of apex removal and nutrient supplementation on branching and seed production in Thlaspi arvense (Brassicaceae). Am J Bot 75:645–651. doi:10.2307/2444198
Bergelson J, Crawley MJ (1992) Herbivory and Ipomopsis aggregata the disadvantages of being eaten. Am Nat 139:870–882. doi:10.1086/285362
Caswell H (1989) Matrix population models. Sinauer, Sunderland
Cornu A, Carnat A-P, Martin B, Coulon J-B, Lamaison J-L, Berdaque J-L (2001) Solid-phase microextraction on volatile components from natural grassland plants. J Agric Food Chem 49:203–209. doi:10.1021/jf0008341
Cox CS, McEvoy PB (1983) Effect of summer moisture stress on the capacity of tansy ragwort (Senecio jacobaea) to compensate for defoliation by cinnabar moth (Tyria jacobaeae). J Appl Ecol 20:225–234. doi:10.2307/2403388
Grime JP, Hodgson JG, Hunt R (1988) Comparative plant ecology. A functional approach to common British species. Unwin Hyman, London
Hämet-Ahti L (1980) Pukinjuuri—Pimpinella saxifraga L. In: Jalas J (ed) Suuri kasvikirja III. Otava, Keuruu, pp 216–217
Hemborg AM (1998) Cost of reproduction in subarctic Ranunculus acris: a five-year field experiment. Oikos 83:273–282. doi:10.2307/3546838
Hendrix SD, Trapp EJ (1989) Floral herbivory in Pastinaca sativa: do compensatory responses offset reductions in fitness. Evolution 43:891–895
Houle G (2001) Reproductive costs are associated with both male and female functions in Alnus viridis ssp. crispa. Ecoscience 8:220–229
Huhta A-P, Hellström K, Rautio P, Tuomi J (2000a) A test of the compensatory continuum: fertilization increases and below-ground competition decreases tolerance of tall wormseed mustard (Erysimum strictum). Evol Ecol 14:353–372. doi:10.1023/A:1010808925284
Huhta A-P, Lennartsson T, Tuomi J, Rautio P, Laine K (2000b) Tolerance of Gentianella campestris in relation to damage intensity: an interplay between apical dominance and herbivory. Evol Ecol 14:373–392. doi:10.1023/A:1011028722860
Jantunen J, Saarinen K (2003) A comparison of vegetation in grazed, formerly grazed and ungrazed valuable semi-natural grasslands in SE Finland. Memoranda Societatis Fauna Flora Fenn 78:55–61
Juenger T, Bergelson J (1997) Pollen and resource limitation of compensation to herbivory in scarlet gilia, Ipomopsis aggregata. Ecology 78:1684–1695
Kalela A, Väänänen H (1960) Pimpinella saxifraga L. Tavallinen pukinjuuri. In: Kalela A, Väänänen H (eds) Pohjolan luonnonkasvit 3. WSOY, Porvoo, pp 1328–1330
Karban R, Strauss SH (1993) Effects of herbivores on growth and reproduction of their perennial host, Erigeron glaucus. Ecology 74:39–46. doi:10.2307/1939499
Knight TM (2003) Effects of herbivory and its timing across populations of Trillium grandiflorum (Liliaceae). Am J Bot 90:1207–1214. doi:10.3732/ajb.90.8.1207
Lennartsson T, Nilsson P, Tuomi J (1998) Induction of overcompensation in the field gentian, Gentianella campestris. Ecology 79:1061–1072
Levine MT, Paige KN (2004) Direct and indirect effects of drought on compensation following herbivory in scarlet gilia. Ecology 85:3185–3191. doi:10.1890/03-0748
Louda SM (1984) Herbivore effect on stature, fruiting, and leaf dynamics of a native crucifer. Ecology 65:1379–1386
Marttila O, Haahtela T, Aarnio H, Ojalainen P (1990) Suomen päiväperhoset, (Finnish Butterflies). Kirjayhtymä, Helsinki
Maschinski J, Whitham GT (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability and timing. Am Nat 134:1–19. doi:10.1086/284962
McNaughton SJ (1983) Compensatory plant growth as a response to herbivory. Oikos 40:329–336. doi:10.2307/3544305
McNaughton SJ, Banyikwa FF, McNaughton MM (1998) Root biomass and productivity in a grazing ecosystem: the Serengeti. Ecology 79:587–592
Newman JA, Bergelson J, Grafen A (1997) Blocking factors and hypothesis testing in ecology: is your statistics text wrong? Ecology 78:1312–1320
Nilsson P, Tuomi J, Åström P (1996) Bud dormancy as a bet-hedging strategy. Am Nat 147:269–281. doi:10.1086/285849
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155:321–348. doi:10.1046/j.1469-8137.2002.00477.x
Paige KN, Whitham TG (1987) Flexible life history traits: shifts by Scarlet gilia in response to pollinator abundance. Ecology 68:1691–1695. doi:10.2307/1939861
Rosenthal JP, Kotanen PM (1994) Terrestrial plant tolerance to herbivory. Trends Ecol Evol 9:145–148. doi:10.1016/0169-5347(94)90180-5
Sadras VO (1996) Cotton compensatory growth after loss of reproductive organs as affected by availability of resources and duration of recovery period. Oecologia 106:432–439. doi:10.1007/BF00329698
Scheiner SM (1993) MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 94–112
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185. doi:10.1016/S0169-5347(98)01576-6
Tolvanen A, Laine K (1997) Effects of reproduction and artificial herbivory on vegetative growth and resource levels in deciduous and evergreen dwarf shrubs. Can J Bot 75:656–666
Trumble JT, Kolodny-Hirsch DM, Ting IP (1993) Plant compensation for arthropod herbivory. Annu Rev Entomol 38:93–119. doi:10.1146/annurev.en.38.010193.000521
Vail SP (1992) Selection for overcompensatory plant responses to herbivory: a mechanism for the evolution of plant-herbivore mutualism. Am Nat 139:1–8. doi:10.1086/285309
Venecz JI, Aarssen LW (1998) Effects of shoot apex removal in Lythrum salicaria (Lythraceae): assessing the costs of reproduction and apical dominance. Ann Bot Fenn 35:101–111
Wells TCE, Sheail J, Ball DL, Ward LK (1976) Ecological studies on the Porton ranges: relationships between vegetation, soils and land-use history. J Ecol 64:589–626. doi:10.2307/2258775
Whitham TG, Maschinski J, Larson KC, Paige KN (1991) Plant responses to herbivory: the continuum form negative to positive and underlying physiological mechanisms. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley, New York, pp 227–256
Wise MJ, Cummins JJ, De Young C (2008) Compensation for floral herbivory in Solanum carolinense: identifying mechanisms of tolerance. Evol Ecol 22:19–37. doi:10.1007/s10682-007-9156-x
Acknowledgements
The work was financially supported by the Academy of Finland (projects #40951 and #80486), the Emil Aaltonen Foundation and Kone Foundation. We thank the technical staff of Oulanka Research Station and Department of Biology at the University of Oulu for their valuable help in the field and laboratory. Aaron Bergdahl kindly checked the language. Finally, sincerest thanks to S.-M. Horttanainen for her field assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huhta, AP., Rautio, P., Hellström, K. et al. Tolerance of a perennial herb, Pimpinella saxifraga, to simulated flower herbivory and grazing: immediate repair of injury or postponed reproduction?. Plant Ecol 201, 599–609 (2009). https://doi.org/10.1007/s11258-008-9535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9535-6