Abstract
Water conservation strategies are well documented in species living in water-limited environments, but physiological adaptations to water availability in temperate climate environments are still relatively overlooked. Yet, temperate species are facing more frequent and intense droughts as a result of climate change. Here, we examined variation in field hydration state (plasma osmolality) and standardized evaporative water loss rate (SEWL) of adult male and pregnant female common lizards (Zootoca vivipara) from 13 natural populations with contrasting air temperature, air humidity, and access to water. We found different patterns of geographic variation between sexes. Overall, males were more dehydrated (i.e. higher osmolality) than pregnant females, which likely comes from differences in field behaviour and water intake since the rate of SEWL was similar between sexes. Plasma osmolality and SEWL rate were positively correlated with environmental temperature in males, while plasma osmolality in pregnant females did not correlate with environmental conditions, reproductive stage or reproductive effort. The SEWL rate was significantly lower in populations without access to free standing water, suggesting that lizards can adapt or adjust physiology to cope with habitat dryness. Environmental humidity did not explain variation in water balance. We suggest that geographic variation in water balance physiology and behaviour should be taken account to better understand species range limits and sensitivity to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is a vital resource for animals that influences many aspects of species’ functional traits including physiological performance, such as locomotion and immunity, and life history traits such as growth, reproduction and survival (Whitehead et al. 1996; Lorenzon et al. 1999; Taylor et al. 2006; Marquis et al. 2008; Tingley et al. 2012; Moeller et al. 2013; Zylstra et al. 2013). The clarification of functional responses related to water balance (i.e. the balance between water intake and loss) is, therefore, critical in understanding and predicting general ecological patterns such as distribution (Dunkin et al. 2013; Peterman and Semlitsch 2014), population dynamics (Foley et al. 2008; Marquis et al. 2008; McKechnie and Wolf 2010), or habitat use (Davis and DeNardo 2009; Dunkin et al. 2013; Rozen-Rechels et al. 2015). Species’ water balance may be challenged as soon as water availability is restricted and this is especially true in terrestrial ectotherms due to their high physiological sensitivity to climatic conditions (Deutsch et al. 2008; Kearney et al. 2009).
Several studies have focused on flexible water conservation strategies including changes in behavioural activity (Lorenzon et al. 1999; Davis and DeNardo 2009; Hetem et al. 2010), shifts in thermoregulatory strategies (Lorenzon et al. 1999; Angilletta et al. 2010; Köhler et al. 2011), metabolic depression (Kennett and Christian 1994; Tieleman et al. 2002; Muir et al. 2007), or a combination of these. These adjustments aim at lowering the rate of water loss and limiting dehydration when individuals are exposed to or colonize water-restricted environments (Moen et al. 2005). Among the functional traits linked with water balance, standardized evaporative water loss (i.e. evaporative water loss at rest and in standardized conditions or SEWL) is a common physiological measure to determine the water balance regulation in intra- and inter-specific comparisons. SEWL is critically influenced by surface area and skin permeability, which determine cutaneous loss of water, and by metabolism and breathing activity, which determine ventilatory water loss (Mautz 1982; Woods and Smith 2010). According to previous comparative analyses, there is a general relationship between the SEWL and habitat aridity, where species and/or populations living in water-restricted habitats or climates are characterised by lower SEWL (Tieleman et al. 2003; Williams et al. 2004; Moen et al. 2005; Van Sant et al. 2012; Guillon et al. 2014; Cox and Cox 2015; Belasen et al. 2016). This relationship likely reflects functional genetic adaptations and/or physiological acclimation to buffer the effects of more frequent heat stress, lower air humidity and more restricted water availability on water balance and energy expenditure (Webster et al. 1985; Lillywhite 2006; Dupoué et al. 2015b). Yet, comparative studies of SEWL across geographic gradients of temperature, humidity and free standing water availability are rare, and whether these factors influence intra-specific variation in water balance remain unclear. In addition, comparative studies of SEWL have rarely examined concurrent variation in hydration state. If physiological components of the water balance such as SEWL are adapted or adjusted to environmental conditions, we expect that geographic variation in SEWL will buffer environmental variation in hydration state such that animals maintain to some extant more similar hydration states across environments.
Within the same population, variation in physiological state (e.g. breeding, moulting, or digesting) also impacts the rate of water loss and the hydration state (e.g. Dupoué et al. 2015a). During the reproductive season, this variation is often closely related to sex and may generate sexual differences in SEWL and hydration state. In particular, adult females experience multiple changes specifically associated with pregnancy or gravidity and involving their behaviour (e.g. increased thermoregulatory precision; Lorioux et al. 2013; Shine 2006), physiology (e.g. higher metabolic rate; Dupoué and Lourdais 2014; Schultz et al. 2008), and morphology (e.g. greater physical burden; Miles et al. 2000; Le Galliard et al. 2003). These changes can induce higher rates of SEWL in adult females through increased rates of ventilation and transpiration (Webster et al. 1985; Woods and Smith 2010; Dupoué et al. 2015b). Furthermore, offspring production requires a considerable amount of water investment to support vitellogenesis and/or embryonic development (Du 2004; Lourdais et al. 2015). Either of these two investments may lead to a higher reliance of adult females on free standing water and water allocation trade-offs between mothers and their offspring (Dupoué et al. 2015a). As a result, individuals may adjust their drinking behaviour to decrease the level of risk induced by dehydration (Lourdais et al. 2015). It is, however, still unclear if environmental conditions such as air temperature, air humidity or the access to free standing water could add with sexual differences and reproductive state requirements in driving the regulation of water balance.
In this study, we examined geographic and sex-specific sources of variation in water balance physiology in a widespread, viviparous lizard (Z. vivipara) living in cool, wet temperate environments. We sampled adult males and females from 13 natural populations distributed across the Massif Central mountain range in France. Sampling was done during the same reproductive season at the end of the mating period when females are undergoing pregnancy. At the adult stage in this species, all females engage into reproduction, and thereby we did not investigate the specific cost of pregnancy. Instead, we checked how water balance regulation may naturally differ between pregnant females and males, and we further examined the influence of reproductive stage and reproductive effort. In these populations, rainfall intensity during the activity season has immediate positive effects on offspring survival and delayed effects on female reproductive performance (Marquis et al. 2008). Past experiments on water restriction revealed that intense water restriction during pregnancy can result in a dramatic impairment of reproductive success (Dauphin-Villemant and Xavier 1986). In contrast, limited restriction of water availability leads to reduced activity and growth in yearlings (Lorenzon et al. 1999) and has complex effects on reproduction in pregnant females (Lorenzon et al. 2001).
We focused on two functional traits related to water balance, namely plasma osmolality (an indicator of hydration state in species lacking salt-glands; Peterson 2002) and SEWL. We tested the influence of access to free water (availability of water in the environment), ambient temperature, water vapour pressure in the air, and individual reproductive investment on those physiological traits. It is noteworthy that natural populations also differ in other parameters including altitude and vegetation cover (Lorenzon et al. 1999; Rutschmann et al. 2016), or slope orientation and local wind speed conditions (pers. obs.), which may influence water balance. However, we focused on the environmental covariates that have previously been shown to influence the regulation of water balance in laboratory experiments. We hypothesized that lizards’ hydration state and SEWL rate should differ according to environmental conditions and physiological state (pregnancy). Specifically, we tested three predictions. First, we expected pregnant females to be more dehydrated than males due to a higher rate of water loss during gestation (Webber et al. 2015; Dupoué et al. 2015b), and the investment of water into offspring production (Dupoué et al. 2015a). Because of this, females should also be more sensitive to environmental conditions than males. Second, we predicted that lizards from sites with lower access to free standing water should have lower SEWL rates than lizard populations with greater water access to maintain water homeostasis (i.e. similar plasma osmolality). Third, because the rate of water loss directly positively correlates with temperature and negatively with humidity (e.g. Dupoué et al. 2015a), we predicted these effects to be mitigated so that lizards should have a similar hydration state; that is, the SEWL rates would be lower in warmer climates and lower in drier climate and osmolality would not correlate either with temperature or humidity.
Materials and methods
Study species, population descriptions, and captive husbandry
The common lizard, Z. vivipara, is a small (adult snout–vent length ~ 50–75 mm), widespread species in the family Lacertidae that inhabits peat bogs and heathlands across northern Eurasia. While the species has populations that are oviparous and other populations that are viviparous, we limited our study to 13 viviparous populations of the Massif Central mountain range in south-central France. These populations are located at the southern range limits for the viviparous form of the species (Pilorge et al. 1983). Populations were distributed along an elevational gradient and have different water access and local climate conditions (Table S1, see below). In these populations, males emerge in mid-April while females emerge in early May. Males copulate with females shortly after their emergence with fertilization occurring in mid-late May. Pregnancy lasts 2–3 months, with parturition occurring between mid-July and early August. Litter size varies from 1 to 12, and neonates do not receive any post-natal parental care. After parturition, females are lean and restore energy reserves before entering into hibernation in late September.
At each locality, we recorded the presence or absence of water sources available to the lizards (e.g. ponds, streams, peat bogs), as well as temperature and humidity using two temperature data loggers (Thermochron iButtons, Maxim Integrated Products, Sunnyvale, CA, USA, ± 0.0625 °C) and one temperature–humidity data logger (Hygrochron iButtons, Maxim Integrated Products, Sunnyvale, CA, USA, ± 0.0625 °C and 0.04% relative humidity—RH). Loggers were placed where we found most of lizards within vegetation at ground level completely shaded to avoid the effect of radiation. Because evaporative water loss depends on water vapour density gradients (Mautz 1982), we used water vapour density (in g m−3) as an index of “air humidity” with the approximation of a stable barometric pressure of 1013.25 mbar (see details in Tieleman et al. 2002). Air temperature and humidity were recorded every hour, and we standardized the sampling period from 29th June to 17th July to compare populations. These three weeks sampling period was the best compromise we could achieve to characterize accurately the differences in microclimatic conditions during the active season among populations. Compared to long-term meteorological data collected with nearby permanent stations that are difficult to extrapolate at high spatial resolutions (Rutschmann et al. 2016), our data more accurately reflect population characteristics and microclimatic conditions experiences by lizards. Over this sampling period, we extracted the daily mean, minimum, and maximum temperatures (T mean, T min, and T max, respectively) and humidities (H mean, H min, and H max) to assess the climate of each population (Table S1).
We caught a total of 246 females (mean ± SE, body mass (BM) = 4.84 ± 0.07 g, snout–vent length (SVL) = 61.44 ± 0.24 mm) and 135 males (BM = 3.47 ± 0.06 g, SVL = 54.30 ± 0.31 mm) between the 19th and 26th of June 2015. On the day of capture, lizards from 8 populations (males) and 12 populations (females) were transferred to a field laboratory and housed in individual terraria (18 × 12 × 12 cm) with sterilized soil, a shelter, and opportunities for thermoregulation to record standardized water loss rates (Table S2, see below). During captivity, we provided a 20–30 °C thermal gradient for 6 h per day (09:00–12:00 and 14:00–17:00) using a 25 W incandescent light bulb placed over one end of each terrarium. We also provided water 3 times per day and fed lizards with 2 crickets (Acheta domesticus) every 2 days. We recorded litter mass (i.e. the total mass of neonates) after parturition to examine the influence of reproductive investment on female water balance. Within 3 days after parturition, we released each female with her litter at her exact capture location. At the end of July, upon completion of experiments, we released males at their exact capture locations.
Plasma osmolality
All lizards from all populations were bled in the field immediately after capture (within 5 min) using a standard protocol (Meylan et al. 2003). Blood samples (40–60 µl whole blood) were collected from the post-orbital sinus using 2–3 20 µl microcapillary tubes. In the laboratory, blood samples were centrifuged for 5 min (3000 rpm), plasma was separated from blood cells and kept frozen in airtight tubes until used for subsequent analyses. Plasma osmolality was then determined using a vapour pressure osmometer (model 5500, Wescor, Logan, UT, USA) and the protocol described in Wright et al. (2013). Before analyses, plasma was diluted (1:1) in reptile Ringer’s solution (300 mOsm kg−1) prepared following methods from Secor et al. (1994) so that plasma osmolality could be determined from 10 µl duplicates (intra-individual variation: 3.9%). High osmolality values indicate high dehydration.
Water loss estimations
On the day of capture, all lizards from a sub-set of 8 populations (males) and 12 populations (females, see Table S2) were returned to the laboratory, weighed (BM1, ± 1 mg) and then maintained under constant temperature (23.5 ± 0.1 °C) and humidity (14.6 ± 0.1 g m−3) without any access to water or food. After 24 h, all individuals were weighed again (BM2, ± 1 mg), and we estimated the rate of water loss (in mg h−1) using the loss of mass (BM2–BM1) over this period. We used body mass loss as a proxy of total evaporative water loss (i.e. the sum of ventilatory and cutaneous evaporative water losses) because, in squamate reptiles, variation in body mass is highly correlated with variation in water loss (DeNardo et al. 2004; Moen et al. 2005; Dupoué et al. 2015b). However, it is possible that the animals could have lost some mass due to defecation and urination, and we did not measure faeces and urine mass for technical reasons related to husbandry priorities. The long period between the two body mass measurements decreased the potential biases related to small faeces production, since over 24 h the loss of body water are more likely to contribute to body mass loss. Regardless, based on the previously reported mass of faeces in this species (mean males: 37.9 mg, females: 52.2 mg; González-Suárez et al. 2011), any defecation would have represented a significant proportion of mass loss (males: 22.5%, females: 33.9%) and would have been detected. Thus, upon reviewing the dataset, we excluded two females from analyses because they showed extremely high mass losses (655 and 1059 mg) that are likely attributable to faeces or egg loss.
Statistical analyses
All analyses were performed with R software (R Development Core Team, version 3.2.0, http://cran.r-project.org/). Initially, we used linear models to test the effects of SVL, population, and sex and their interactions on plasma osmolality and the rate of SEWL. Next, we investigated the effect of water access and climatic conditions on plasma osmolality and the rate of SEWL. We performed these latter analyses separately for each sex because females may vary in rates of water loss due to the effects associated with pregnancy and not to general sex-specific factors. We used mixed-effects linear models (package nlme, Pinheiro et al. 2016) in which population identity was included as a random factor to account for repeated measurements within the same population. Water access was treated as a categorical factor while temperature metrics (i.e. T mean, T min and T max) and humidity metrics (i.e. H mean, H min and H max) were treated as linear and quadratic covariates to test for non-linear relationships. We centred covariates by subtracting the mean from each observation. Furthermore, we also estimated embryonic development (ED) to account for the pregnancy stage: ED was estimated as the number of days between capture date and parturition date. We tested the influence of two estimates of reproductive effort: the absolute reproductive effort (ARE; estimated as the mass of all neonates) and the relative reproductive effort (RRE, derived from the linear relationship between litter mass and female size, F 1,169 = 89.5, p < 0.001, r 2 = 0.35). We only present results based on ARE since they were similar to those obtained from RRE analyses.
Whenever we found significant variation in water balance indicators between populations, we further checked the potential correlation with population characteristics. To do so, we used a model selection approach using the Akaike information criterion corrected for small sample size (AICc, package AICcmodavg, Mazerolle 2016). We compared the contribution of each environmental and reproductive variable to the model, as well as additive models of each environmental and reproductive variable, to a model including only the random effect of the population (i.e. null model). The best model was chosen as the one with the lowest AICc. Models that have a difference of AICc lower than 2 have comparable support of the data. In our analyses, one or more models had a ΔAICc that was less than 2 when compared to the best model. In the latter cases, we focused on the model with the lowest number of parameters (k) and tested the significance of covariates with likelihood ratio tests (LRT). We did not record humidity measures in three populations due to logger failure; therefore, we first restricted the analyses to a dataset without those populations (Table S3). However, since model selection did not retain the influence of humidity (Table S3), we tested the influence of water access, temperature, and reproductive investment on the full dataset. Results were similar for the full and the restricted data set. Finally, we used linear models to test the correlation between plasma osmolality and the rate of water loss within and between populations (Speakman et al. 2003). Results are presented as mean ± SE unless otherwise stated.
Results
Variation in water balance among populations and between the sexes
Plasma osmolality and the rate of SEWL were not significantly influenced by lizard SVL (F 1,363 = 2.17, p = 0.142 and F 1,332 = 0.52, p = 0.473, respectively) but differed significantly among populations (F 12,366 = 2.47, p = 0.004, and F 11,333 = 6.88, p < 0.001, respectively). Moreover, osmolality was different between sexes (males: 311.1 ± 3.1 mOsm kg−1, females: 301.5 ± 2.1 mOsm kg−1, F 1,366 = 6.61, p = 0.011), while the rate of SEWL was similar between sexes (males: 6.73 ± 0.38 mg h−1, females: 6.48 ± 0.20 mg h−1, F 1,333 = 0.37, p = 0.544). Both osmolality and the rate of SEWL were impacted by the interaction between population and sex (F 12,354 = 2.88, p < 0.001, and F 7,272 = 2.56, p = 0.014, respectively), which indicates sex-specific geographic patterns of water balance physiology.
Influence of environmental conditions and individual state
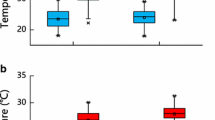
In males, the two best models retained mean temperature as the primary environmental factor influencing plasma osmolality (Table 1). Models for water loss in males included significant effects of water access (no access versus access: β = 3.66 ± 1.42, t 6,71 = 2.57, p = 0.042, Fig. 1a, c) and minimum temperature. That is, males from populations without water access had a rate of SEWL rates that were only about half that of males from population with access to water (no access: 4.85 ± 1.13 mg h−1, access: 8.50 ± 0.86 mg h−1, Fig. 1c) while remaining in similar hydration states (no access: 302.7 ± 7.7 mOsm kg−1, access: 315.1 ± 5.1 mOsm kg−1; β = 12.37 ± 9.22, t 11,104 = 1.34, p = 0.207, Fig. 1a). In addition, plasma osmolality was positively correlated with mean temperature (β = 10.67 ± 4.85, t 11,104 = 2.20, p = 0.050, Fig. 2a), whereas the rate of SEWL tended to be higher for populations with higher minimum temperature (β = 1.21 ± 0.50, t 6,71 = 2.42, p = 0.052, Fig. 2b).
Effects of the access to water in natural populations of common lizards on the indicators of water balance a, b plasma osmolality, and c, d the rate of standardized evaporative water loss (SEWL) in males (left panel) and females (right panels). Points represent mean ± SE and significant effects of water access are symbolised: *p < 0.05, n.s. non-significant
Positive relationships between indicators of water balance and thermal conditions in males. Trend lines are included for significant (solid line) or marginal (dashed line) correlations between a plasma osmolality and mean temperature (p = 0.050, r 2 = 0.10) and b the rate of standardized evaporative water loss (SEWL) and minimal temperature (p = 0.052, r 2 = 0.25)
In females, there was more uncertainty among statistical models. The best model (Table 1) did not retain any influence of environmental conditions or reproduction for plasma osmolality, but included an effect of water access on the rate of SEWL (no access versus access: β = 1.39 ± 0.62, t 10,228 = 2.24, p = 0.048, Fig. 1b, d). None of the remaining top-ranking models included significant covariates. Females from populations without access to water lost almost 25% less water than did females from populations with access to water (no access: 5.49 ± 0.50 mg h−1, access: 6.88 ± 0.37 mg h−1, Fig. 1d) yet remained in similar hydration states (no access: 304.2 ± 5.7 mOsm kg−1, access: 301.3 ± 4.0 mOsm kg−1, β = − 2.87 ± 6.92, t 11,213 = − 0.41, p = 0.686, Fig. 1b). Model comparisons also retained a slight non-linear relationship between the rate of SEWL and the minimal temperature (water loss ~ T min + T 2min : β (T min) = 0.23 ± 0.23, t 9,228 = 1.03, p = 0.328, β (T 2min ) = 0.21 ± 0.11, t 9,228 = 1.85, p = 0.098). We did not find any influence of reproductive advancement (ED) or reproductive investment (ARE, and RRE) on either plasma osmolality (Fig. 3a, b, c) or the rate of SEWL (Fig. 3d, e, f) (Table 1).
No relationship between the indicators of water balance and reproductive performance in pregnant females, regarding the influence of embryonic development (ED), absolute reproductive effort (ARE, estimated from the mass of the litter), and relative reproductive effort (RRE, derived from the linear relationship between litter mass and female snout–vent length), on a, b, c plasma osmolality and on d, e, f the rate of standardized evaporative water loss (SEWL)
Relationship between osmolality and SEWL
In both sexes, osmolality was not correlated with the rate of SEWL either among or within populations (Table S4).
Discussion
In this study, we investigated variation in water balance (i.e. hydration state and water loss) in wild populations of a widespread lizard species (Z. vivipara) that differ in their access to water and in local climate conditions. Males were more dehydrated than females, whereas the rate of SEWL was similar between sexes. In addition, the rate of SEWL was higher in individuals from populations with access to water, which is consistent with our second prediction. Finally and contrary to our last prediction, plasma osmolality and the rate of SEWL were positively correlated with environmental temperature in males, yet there was no correlation with environmental humidity.
Sex differences in dehydration rate and/or the rate of SEWL have been previously documented in other species including humans (Stachenfeld et al. 2001; Cryan and Wolf 2003; Weldon et al. 2013). In this study, we expected pregnant females to be more dehydrated than males due to physiological changes associated with pregnancy (Dupoué et al. 2015a). In particular, pregnant or gravid females have higher metabolic rates and higher transpiration rates caused by body distension (Schultz et al. 2008; Dupoué and Lourdais 2014; Webber et al. 2015; Dupoué et al. 2015b), which should increase the rate of SEWL (Mautz 1982; Woods and Smith 2010). Furthermore, developing embryos also need water for somatic growth (Du 2004; Lourdais et al. 2015), and water allocation to embryonic development can impair female hydration state (Dupoué et al. 2015a). Yet, we found no difference in mean SEWL between sexes and males were slightly more dehydrated on average than females. This indicates that factors other than breeding state per se may be responsible for the observed sexual differences in water balance. Indeed, despite higher water demands caused by pregnancy in females compared to males, behavioural factors may contribute to buffer sexual differences in field evaporative water loss or water balance. For instance, pregnant females of Z. vivipara select lower temperature and stay relatively inactive in the field (Van Damme et al. 1986; Le Galliard et al. 2003). These differences in thermoregulation and activity might reduce water loss and thus dehydration in females. Besides, the regulation of hydration state can also be adjusted by water intake. Pregnancy may be associated to an increase drinking behaviour caused by a decrease in the osmotic threshold of thirst (Cheung and Lafayette 2013; Lourdais et al. 2015). Together, these behavioural adjustments of thermoregulation and water intake might be particularly relevant to better understand the functional regulation of the water balance.
When investigating the effects of water access, we found that individuals from habitats with permanent access to water had, in general, higher SEWL rates compared to lizards from populations without access to water. Interestingly, hydration state was not different between those populations suggesting that a lower rate of water loss might compensate for lower water availability to maintain hydration state, and therefore, physiological homeostasis. That is, individuals from water-restricted populations may remain normosmotic by having a lower rate of water loss, either via acclimation or genetic adaptations to the drier environment, for example through reduced ventilatory rate or reduced peripheral perfusion (Tieleman et al. 2003; Williams et al. 2004; Moen et al. 2005; Van Sant et al. 2012; Guillon et al. 2014; Cox and Cox 2015; Belasen et al. 2016). Yet, contrary to our last set of predictions, we found that water balance indicators did not correlate with air humidity. This suggests that the access to free-standing water is a better descriptor of the rate of water loss than environmental humidity, which was relatively high in sampled areas of dense vegetation used by lizards in all populations.
In addition, the SEWL rate was slightly and positively correlated with environmental temperature in males, whereas it tended to increase non-linearly with temperature in females. Our best statistical models for females included correlations with minimum daily temperature instead of mean or maximum values. Although the AICc suggested some uncertainty among the best statistical models, and therefore, point to the need of further studies with a larger sample size, this result may reflect the ecological relevance of minimal temperatures for ectotherms. During summer season, lizards are exposed to minimal temperatures measured on the ground since they stay inactive in shelter very close from the surface such as inside grass tufts, shallow crevices in soil and rocks, and dead trunk cavities (pers. obs.). Therefore, lizards must endure minimal conditions for a relatively long period inside their night shelters, which are likely closed from surface (e.g. T min > 4 h, ~ 03:00–08:00 h). Instead, maximal conditions experienced during activity, daytime last for a shorter duration (e.g. T max < 1 h, ~ 15:00 h) and can be avoided through microhabitat selection (Davis and DeNardo 2009; Guillon et al. 2014). Rate of water loss increases with temperature due to lower skin resistance and higher metabolic rate of reptiles at higher body temperatures (Webster et al. 1985; Lillywhite 2006; Dupoué et al. 2015b). To buffer this biophysical relationship, we would have expected a negative correlation between the rate of SEWL and environmental temperature, so that osmolality would have not correlated with temperature. Instead, we observed a positive correlation between water loss and air temperature and between osmolality and temperature in males.
We hypothesize the geographic variation in water access and temperature was due to permanent and consistent differences among populations related to altitude, slope orientation and habitat type (pers. obs.). Thus, we propose that geographic differences in the rate of SEWL may reflect local acclimation and/or adaptations to prevailing environmental conditions. For example, natural selection related to the water balance could favour plastic and/or genetic changes in the properties of the skin barrier, likely resulting from changes in lipid composition, organization and/or mobilization among populations (Kattan and Lillywhite 1989; Lillywhite 2006). In uricotelic species (e.g., squamate reptiles and birds), transcutaneous water loss is the main avenue for water loss (Kattan and Lillywhite 1989; Lillywhite 2006; Williams et al. 2012). The keratin–lipids complex (sandwich-like layers) localized in the stratum corneum (i.e. the outer layer of the epidermis) constitute the main barrier limiting transcutaneous water loss (Bouwstra et al. 2003; Lillywhite 2006; Champagne et al. 2012). The permeability of this water barrier can be adjusted by modifying its thickness (quantity of lipids), the proportion of the different lipids with specific polarity (e.g. cholesterol, fatty acids, phospholipids and ceramides), and/or their geometry (Lillywhite 2006; Williams et al. 2012). Further investigations are needed to quantify the contribution of geographic differences in skin water permeability and distinguish whether these differences are caused by plastic responses or genetic adaptations to short- or long-term exposure to climatic conditions.
Although comparative studies cannot determine the causes of relationships, ecological comparisons across geographical distributions such as this one provide useful opportunities to understand and predict how species may respond to climatic conditions (Somero 2011; Rezende and Diniz-Filho 2012). Water is an essential yet relatively overlooked resource in ecological studies. As demonstrated here, water balance may be affected by local climate conditions and water availability, and therefore, geographic variation in the water balance strategies should be integrated into global change studies (Todgham and Stillman 2013). For instance, further studies should specifically examine the relative contributions of physiological adaptation, acclimation, and behavioural mitigation in adjusting the rate of water loss to minimize dehydration. Such information is essential to improve the accuracy of models that predict species’ responses to climate change and to promote effective conservation measures (Wikelski and Cooke 2006; Cooke et al. 2013).
References
Angilletta MJ, Cooper BS, Schuler MS, Boyles JG (2010) The evolution of thermal physiology in endotherms. Front Biosci 26:861–881. doi:10.1093/intimm/dxu021
Belasen A, Brock K, Li B et al (2016) Fine with heat, problems with water: microclimate alters water loss in a thermally adapted insular lizard. Oikos. doi:10.1111/oik.03712
Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M (2003) Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res 42:1–36. doi:10.1016/S0163-7827(02)00028-0
Champagne AM, Munoz-Garcia A, Shtayyeh T et al (2012) Lipid composition of the stratum corneum and cutaneous water loss in birds along an aridity gradient. J Exp Biol 215:4299–4307. doi:10.1242/jeb.077016
Cheung KL, Lafayette RA (2013) Renal physiology of pregnancy. Adv Chronic Kidney Dis 20:209–214. doi:10.1053/j.ackd.2013.01.012
Cooke SJ, Sack L, Franklin CE et al (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1:1–23. doi:10.1093/conphys/cot001
Cox CL, Cox RM (2015) Evolutionary shifts in habitat aridity predict evaporative water loss across squamate reptiles. Evolution (NY) 69:2507–2516. doi:10.1111/evo.12742
Cryan PM, Wolf BO (2003) Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. J Exp Biol 206:3381–3390. doi:10.1242/jeb.00574
Dauphin-Villemant C, Xavier F (1986) Adrenal activity in the females Lacerta vivipara Jacquin: possible involvement in the success of gestation. In: Assemacher I, Boissin J (eds) Endocrine regulation as adaptive mechanism to environment. CNRS, Paris, pp 241–250
Davis JR, DeNardo DF (2009) Water supplementation affects the behavioral and physiological ecology of gila monsters (Heloderma suspectum) in the sonoran desert. Physiol Biochem Zool 82:739–748. doi:10.1086/605933
DeNardo DF, Zubal TE, Hoffman TCM (2004) Cloacal evaporative cooling: a previously undescribed means of increasing evaporative water loss at higher temperatures in a desert ectotherm, the Gila monster Heloderma suspectum. J Exp Biol 207:945–953. doi:10.1242/jeb.00861
Deutsch CA, Tewksbury JJ, Huey RB et al (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci U S A 105:6668–6672
Du W (2004) Water exchange of flexible-shelled eggs and its effect on hatchling traits in the Chinese skink, Eumeces chinensis. J Comp Physiol B Biochem Syst Environ Physiol 174:489–493. doi:10.1007/s00360-004-0435-3
Dunkin RC, Wilson D, Way N et al (2013) Climate influences thermal balance and water use in African and Asian elephants: physiology can predict drivers of elephant distribution. J Exp Biol 216:2939–2952. doi:10.1242/jeb.080218
Dupoué A, Lourdais O (2014) Relative reproductive effort drives metabolic changes and maternal emaciation during pregnancy in a viviparous snake. J Zool 293:49–56. doi:10.1111/jzo.12116
Dupoué A, Brischoux F, Angelier F et al (2015a) Intergenerational trade-off for water may induce a mother–offspring conflict in favour of embryos in a viviparous snake. Funct Ecol 29:414–422. doi:10.1111/1365-2435.12349
Dupoué A, Stahlschmidt ZR, Michaud B, Lourdais O (2015b) Physiological state influences evaporative water loss and microclimate preference in the snake Vipera aspis. Physiol Behav 144:82–89. doi:10.1016/j.physbeh.2015.02.042
Foley C, Pettorelli N, Foley L (2008) Severe drought and calf survival in elephants. Biol Lett 4:541–544. doi:10.1098/rsbl.2008.0370
González-Suárez M, Mugabo M, Decencière B et al (2011) Disentangling the effects of predator body size and prey density on prey consumption in a lizard. Funct Ecol 25:158–165. doi:10.1111/j.1365-2435.2010.01776.x
Guillon M, Guiller G, DeNardo DF, Lourdais O (2014) Microclimate preferences correlate with contrasted evaporative water loss in parapatric vipers at their contact zone. Can J Zool 92:81–86
Hetem RS, Strauss WM, Fick LG et al (2010) Variation in the daily rhythm of body temperature of free-living Arabian oryx (Oryx leucoryx): does water limitation drive heterothermy? J Comp Physiol B Biochem Syst Environ Physiol 180:1111–1119. doi:10.1007/s00360-010-0480-z
Kattan GH, Lillywhite HB (1989) Humidity acclimation and skin permeability in the lizard Anolis carolinensis. Physiol Zool 62:593–606
Kearney M, Shine R, Porter WP (2009) The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc Natl Acad Sci U S A 106:3835–3840. doi:10.1073/pnas.0808913106
Kennett R, Christian KA (1994) Metabolic depression in estivating long-neck turtles (Chelodina rugosa). Physiol Zool 67:1087–1102
Köhler A, Sadowska J, Olszewska J et al (2011) Staying warm or moist? Operative temperature and thermal preferences of common frogs (Rana temporaria) and effects on locomotion. Herpetol J 21:17–26
Le Galliard J-F, Le Bris M, Clobert J (2003) Timing of locomotor impairment and shift in thermal preferences during gravidity in a viviparous lizard. Funct Ecol 17:877–885
Lillywhite HB (2006) Water relations of tetrapod integument. J Exp Biol 209:202–226. doi:10.1242/jeb.02007
Lorenzon P, Clobert J, Oppliger A, John-Alder H (1999) Effect of water constraint on growth rate, activity and body temperature of yearling common lizard (Lacerta vivipara). Oecologia 118:423–430
Lorenzon P, Clobert J, Massot M (2001) The contribution of phenotypic plasticity to adaptation in Lacerta vivipara. Evolution (NY) 55:392–404. doi:10.1554/0014-3820(2001)055[0392:TCOPPT]2.0.CO;2
Lorioux S, Lisse H, Lourdais O (2013) Dedicated mothers: predation risk and physical burden do not alter thermoregulatory behaviour of pregnant vipers. Anim Behav 86:401–408. doi:10.1016/j.anbehav.2013.05.031
Lourdais O, Lorioux S, Dupoué A et al (2015) Embryonic water uptake during pregnancy is stage- and fecundity-dependent in the snake Vipera aspis. Comp Biochem Physiol Part A Mol Integr Physiol 189:102–106. doi:10.1016/j.cbpa.2015.07.019
Marquis O, Massot M, Le Galliard JF (2008) Intergenerational effects of climate generate cohort variation in lizard reproductive performance. Ecology 89:2575–2583. doi:10.1890/07-1211.1
Mautz WJ (1982) Patterns of evaporative water loss. In: Gans C, Pough FH (eds) Biology of the Reptilia. Academic Press, London, pp 443–481
Mazerolle MJ (2016) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c)
McKechnie AE, Wolf BO (2010) Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett 6:253–256. doi:10.1098/rsbl.2009.0702
Meylan S, Dufty AM, Clobert J (2003) The effect of transdermal corticosterone application on plasma corticosterone levels in pregnant Lacerta vivipara. Comp Biochem Physiol Part A Mol Integr Physiol 134:497–503
Miles DB, Sinervo B, Frankino WA (2000) Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution (NY) 54:1386–1395. doi:10.1554/0014-3820(2000)054[1386:Rblpat]2.0.Co;2
Moeller KT, Butler MW, DeNardo DF (2013) The effect of hydration state and energy balance on innate immunity of a desert reptile. Front Zool 10:23–33. doi:10.1186/1742-9994-10-23
Moen DS, Winne CT, Reed RN (2005) Habitat-mediated shifts and plasticity in the evaporative water loss rates of two congeneric pit vipers (Squamata, Viperidae, Agkistrodon). Evol Ecol Res 7:759–766
Muir TJ, Costanzo JP, Lee RE (2007) Osmotic and metabolic responses to dehydration and urea-loading in a dormant, terrestrially hibernating frog. J Comp Physiol B 177:917–926. doi:10.1007/s00360-007-0190-3
Peterman WE, Semlitsch RD (2014) Spatial variation in water loss predicts terrestrial salamander distribution and population dynamics. Oecologia 176:357–369
Peterson CC (2002) Temporal, population, and sexual variation in hematocrit of free-living desert tortoises: correlational tests of causal hypotheses. Can J Zool 80:461–470. doi:10.1139/Z02-021
Pilorge T, Xavier F, Barbault R (1983) Variations in litter size and reproductive effort within and between some populations of Lacerta vivipara. Ecography (Cop) 6:381–382
Pinheiro J, Bates D, DebRoy S et al (2016) nlme: Linear and nonlinear mixed effects models
Rezende EL, Diniz-Filho JAF (2012) Phylogenetic analyses: comparing species to infer adaptations and physiological mechanisms. Compr Physiol 2:639–674. doi:10.1002/cphy.c100079
Rozen-Rechels D, van Beest FM, Richard E et al (2015) Density-dependent, central-place foraging in a grazing herbivore: competition and tradeoffs in time allocation near water. Oikos 124:1142–1150. doi:10.1111/oik.02207
Rutschmann A, Miles DB, Le Galliard JF et al (2016) Climate and habitat interact to shape the thermal reaction norms of breeding phenology across lizard populations. J Anim Ecol 85:457–466. doi:10.1111/1365-2656.12473
Schultz TJ, Webb JK, Christian KA (2008) The physiological cost of pregnancy in a tropical viviparous snake. Copeia 2008:637–642. doi:10.1643/CP-06-182
Secor SM, Stein ED, Diamond J (1994) Rapid upregulation of snake intestine in response to feeding: a new model of intestinal adaptation. Am J Physiol 266:G695–G705
Shine R (2006) Is increased maternal basking an adaptation or a pre-adaptation to viviparity in lizards? J Exp Zool Part A Comp Exp Biol 305A:524–535
Somero GN (2011) Comparative physiology: a “crystal ball” for predicting consequences of global change. Am J Physiol Regul Integr Comp Physiol 301:R1–R14. doi:10.1152/ajpregu.00719.2010
Speakman JR, Ergon T, Cavanagh R et al (2003) Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. Proc Natl Acad Sci USA 100:14057–14062
Stachenfeld NS, Splenser AE, Calzone WL et al (2001) Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol 91:1893–1901
Taylor BE, Scott DE, Gibbons JW (2006) Catastrophic reproductive failure, terrestrial survival, and persistence of the marbled salamander. Conserv Biol 20:792–801. doi:10.1111/j.1523-1739.2005.00321.x
Tieleman BI, Williams JB, Buschur ME (2002) Physiological adjustments to arid and mesic environments in larks (Alaudidae). Physiol Biochem Zool 75:305–313. doi:10.1086/341998
Tieleman BI, Williams JB, Bloomer P (2003) Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc R Soc B Biol Sci 270:207–214. doi:10.1098/rspb.2002.2205
Tingley R, Greenlees MJ, Shine R (2012) Hydric balance and locomotor performance of an anuran (Rhinella marina) invading the Australian arid zone. Oikos 121:1959–1965. doi:10.1111/j.1600-0706.2012.20422.x
Todgham AE, Stillman JH (2013) Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr Comp Biol 53:539–544. doi:10.1093/icb/ict086
Van Damme R, Bauwens D, Verheyen RF (1986) Selected body temperatures in the lizard Lacerta vivipara: variation within and between populations. J Therm Biol 11:219–222
Van Sant MJ, Oufiero CE, Muñoz-Garcia A et al (2012) A phylogenetic approach to total evaporative water loss in mammals. Physiol Biochem Zool 85:526–532. doi:10.1086/667579
Webber MM, Gibbs AG, Rodríguez-Robles JA (2015) Hot and not-so-hot females: reproductive state and thermal preferences of female Arizona Bark Scorpions (Centruroides sculpturatus). J Evol Biol 28:368–375. doi:10.1111/jeb.12569
Webster MD, Campbell GS, King JR (1985) Cutaneous resistance to water-vapor diffusion in pigeons and the role of the plumage. Physiol Zool 58:58–70
Weldon CW, Daniels SR, Clusella-Trullas S, Chown SL (2013) Metabolic and water loss rates of two cryptic species in the African velvet worm genus Opisthopatus (Onychophora). J Comp Physiol B Biochem Syst Environ Physiol 183:323–332. doi:10.1007/s00360-012-0715-2
Whitehead FJ, Couper RTL, Moore L et al (1996) Dehydration deaths in infants and young children. Am J Forensic Med Pathol 17:73–78
Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21:38–46. doi:10.1016/j.tree.2005.10.018
Williams JB, Muñoz-Garcia A, Ostrowski S, Tieleman BI (2004) A phylogenetic analysis of basal metabolism, total evaporative water loss, and life-history among foxes from desert and mesic regions. J Comp Physiol B Biochem Syst Environ Physiol 174:29–39. doi:10.1007/s00360-003-0386-0
Williams JB, Muñoz-garcia A, Champagne A (2012) Climate change and cutaneous water loss of birds. J Exp Biol 215:1053–1060. doi:10.1242/jeb.054395
Woods HA, Smith JN (2010) Universal model for water costs of gas exchange by animals and plants. Proc Natl Acad Sci U S A 107:8469–8474. doi:10.1073/pnas.0905185107
Wright CD, Jackson ML, DeNardo DF (2013) Meal consumption is ineffective at maintaining or correcting water balance in a desert lizard, Heloderma suspectum. J Exp Biol 216:1439–1447. doi:10.1242/jeb.080895
Zylstra ER, Steidl RJ, Jones CA, Averill-Murray RC (2013) Spatial and temporal variation in survival of a rare reptile: a 22-year study of Sonoran desert tortoises. Oecologia 173:107–116. doi:10.1007/s00442-012-2464-z
Acknowledgements
We thank Pauline Blaimont, Pauline Dufour, Laurène Duhalde, Amélie Faure, Julia Rense, and Qiang Wu for their help with fieldwork. We also thank Clotilde Biard for lending us some of the loggers. We are grateful to the ‘Office Nationale des Forêts’, the ‘Parc National des Cévennes’, and the regions Auvergne, Rhône Alpes and Languedoc Roussillon for allowing us to sample lizards. This study was funded by the Centre National de la Recherche Scientifique (CNRS) the Agence Nationale de la Recherche (ANR-13-JSV7-0011-01 to SM) and the National Science Foundation (NSF-EF1241848 to DBM).
Author information
Authors and Affiliations
Contributions
AD, AR, JFLG, DBM, JC, and SM conceived the ideas and designed methodology; AD, AR, JFLG, DBM, JC, and SM captured lizards; AD and AR collected water loss data; AD, GAB, and DD collected osmolality data; AD analysed the data; AD and AR led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Additional information
Communicated by Hannu J. Ylonen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dupoué, A., Rutschmann, A., Le Galliard, J.F. et al. Water availability and environmental temperature correlate with geographic variation in water balance in common lizards. Oecologia 185, 561–571 (2017). https://doi.org/10.1007/s00442-017-3973-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3973-6