Abstract
Extreme high temperatures are occurring more frequently with ongoing anthropogenic climate warming, but the experimental tests of the effects of high temperatures on terrestrial vertebrates in natural conditions are rare. In this study, we investigated the effects of extreme high temperatures on female reproduction and offspring traits of multi-ocellated racerunners (Eremias multiocellata) kept in field enclosures in the desert steppe of Inner Mongolia. Our studies indicate that high temperatures significantly affect the gestation period and reproductive output of females and the offspring sex ratio, but have little impact on offspring body size and mass. More interestingly, we found that the effect of extreme high temperatures on female reproductive output was not consistent between two consecutive years that differed in precipitation. Low precipitation may aggravate the impact of climate warming on lizards and negatively affect the survival of lizards in the desert steppe. Our results provide evidence that temperature interacts with precipitation to determine the life history of lizards, and they suggest that a drier and hotter environment, such as the future climate in arid mid-latitude areas, will likely impose severe pressure on lizard populations, which are an important component of the food web in desert areas around the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ongoing anthropogenic climate warming is undisputable. The global mean surface temperature increased by about 0.85 °C during the last century, and further warming is predicted to occur this century (Pachauri et al. 2014). Climate warming is accompanied by more frequent extreme temperatures (Easterling et al. 2000; Meehl and Tebaldi 2004; Perkins et al. 2012; Seneviratne et al. 2014), and has pervasive effects on the physiology, life history traits, phenology, and distribution of species, and therefore on biodiversity, community structure, and ecosystem function (Dillon et al. 2010; Memmott et al. 2007; Ohlberger 2013; Parmesan 2006; Root et al. 2003). Despite wide recognition and increasing scientific evidence for the profound impact of climate warming on biological systems, gaps still exist in our knowledge of how climate warming affects animal life.
Previous studies have mainly focused on the effects of increasing mean ambient temperature on the survival and reproduction of animals, with much less focus on extreme temperatures (Bradshaw and Holzapfel 2006; Easterling et al. 2000). The current and forecasted climate warming, however, encompasses other aspects of climate change, such as an increasing frequency of extreme temperatures, alteration of the diurnal temperature range (Adler and Drake 2008; Choi et al. 2009; Sun et al. 2007; Vose et al. 2005), and altered precipitation patterns (IPCC 2013). Recent studies have suggested that the changes in the magnitude and frequency of extreme temperatures modify many physiological and life history traits, such as thermal tolerance, growth rate, and reproduction, and thus are ecologically as important for the performance and local persistence of certain species as increasing mean temperatures (Angilletta 2009; Bauerfeind and Fischer 2014; Hoffmann et al. 2002; Jentsch et al. 2007; Smith 2011). The impact of extreme temperatures on organisms thus needs to be studied to comprehensively understand the impact of climate warming.
Experiments that identify how temperature affects the physiology and life history traits of animals have largely been carried out in the laboratory rather than in the field (Angilletta 2009; Du and Shine 2015; Johnston and Bennett 1996). This is especially true for vertebrates, presumably due to the logistic difficulty of providing realistic environmental conditions for relatively large animals. The simplified thermal environment of the laboratory prevents the results of these studies from being extrapolated to field populations that experience fluctuating climatic conditions (Triggs and Knell 2012). Experimental field studies on the effects of climate factors (e.g., temperature, precipitation, and oxygen) on organisms are, therefore, needed to understand the mechanisms underpinning the possible impact of climate warming on biological systems (Parmesan 2006; Wernberg et al. 2012; Wolkovich et al. 2012).
Experimental manipulation of multiple factors may provide insight into the synergistic effects of climate warming on biological systems (Rosa and Seibel 2008; Zittier et al. 2013). Climatic factors other than temperature change are associated with climatic warming (Adler et al. 2008; Thompson et al. 2015). For example, changes in precipitation in the context of climate warming are complex, both temporally and spatially (Meehl et al. 2007; Qian et al. 2007; Sun et al. 2007). The interaction between precipitation and temperature change is likely to be significant (Bonebrake and Mastrandrea 2010), but the impact of this interaction on organisms is largely unknown.
The response of reproductive traits to climate warming is fundamental to the biological effects of global warming, because reproductive success determines the recruitment of offspring and therefore population dynamics and viability. Lizards provide an excellent model to study the influence of climate warming on reproduction, because their behavior and physiology are highly dependent on environmental temperatures. Climate change may not only affect female reproductive output, including offspring number and size, but also influence embryonic development and the phenotypes of offspring (e.g., Chamaille-Jammes et al. 2006; Dubey and Shine 2011; Lu et al. 2013; Ma et al. 2014; Marquis et al. 2008). More critically, climate change has caused population decreases and species extinction in lizards, and more local and global extinctions of this kind are expected in the future (Sinervo et al. 2010).
In this study, we experimentally increased temperatures in field enclosures and investigated the effect on female reproduction and offspring traits in a viviparous lizard, the multi-ocellated racerunner (Eremias multiocellata), in a desert steppe region of Inner Mongolia. This desert species provides a model system to study the effects of extreme high temperatures on reproductive traits for the following reasons. First, staying cool is critical for ectotherms in desert areas, because they are vulnerable to high ambient temperatures in areas with sparse vegetation cover and where the times and places suitable for activity are constrained (Kearney et al. 2009). Second, ectotherms in arid mid-latitude zones are most at risk from future climate warming owing to the low thermal safety margin (Clusella-Trullas et al. 2011). Third, this arid region has become hotter and drier since 1950 (Fig. S1), and this may have a severe impact on local lizard populations. We conducted our experiments during two consecutive years, and hypothesized that the high temperatures produced by the experimental conditions would have the overall negative effects on female and offspring traits. More specifically, we expected that (1) the racerunners from the high-temperature treatment group would have a shorter gestation period and breed earlier than those from the control group, because high temperatures may result in faster embryonic development in viviparous lizards (Ji et al. 2006; Tang et al. 2012); (2) the females from the high-temperature treatment group would produce fewer or smaller neonates than the control group owing to the energy constraints imposed by high temperatures (Du et al. 2000; Qu et al. 2011); and (3) high temperatures would skew the offspring sex ratio of the racerunners, because temperature affects sex determination in this species (Tang et al. 2012).
Materials and methods
Study site and experimental animals
The field experiments were conducted at Ordos Key Research Station for Field Observation of Ecological Environments on Sandy Grasslands, China. The field station is located in the Shierliancheng countryside, Jungar Banner, Inner Mongolia, China (40°12′17″N, 111°07′43″E; elevation 1036 m). In this cold semiarid desert steppe region, the air temperature and heat-wave frequency during the three summer months (June, July, and August) increased from 1951 to 2014, whereas the Palmer Drought Severity Index (PDSI) decreased during the same period, suggesting that this area is becoming both warmer and drier (Fig. S1). A heat wave is defined as a spell of three or more days on each of which the maximum shade temperature reaches or exceeds 32.2 °C (Glickman 2000). The PDSI is based on precipitation, temperature, and the available water content (AWC) of the soil (Fieldhouse and Palmer 1965) and was calculated using the scPDSI software ver. 2.0 (available at http://greenleaf.unl.edu). This index provides a realistic metric of relative periods of drought and excessive soil moisture (Wells et al. 2004; Table S1). Climate data, including air temperature, precipitation, and AWC, were collected from China’s meteorological data-sharing service system (http://cdc.cma.gov.cn/). This region is predominantly sandy grassland with low-to-moderate levels of sparse vegetation dominated by Artemisia ordosica.
The multi-ocellated racerunner (E. multiocellata), a small viviparous lizard inhabiting desert and semiarid areas, is one of the most common lizards at our study site (Zeng et al. 2014; Zhao et al. 1999). Previous studies of this species have indicated that female reproductive traits and offspring phenotypes not only differ among populations, but are also affected by maternal thermal environments (Li et al. 2011; Tang et al. 2012). Females produce more male offspring when kept under high constant temperatures above 32 °C, and more female offspring at 25 °C, suggesting that this lizard may be a temperature-dependent sex determination (TSD) species with a pivotal temperature of approximately 29 °C (Tang et al. 2012; Zhang et al. 2010).
Experimental design
We buried steel sheeting in the sand (0.2 m depth) to build 20 round enclosures (1.8 m diameter, 0.4 m high) situated at random locations in the natural habitat of the racerunner (Fig. S2). The enclosures were randomly divided into two groups. The high-temperature conditions were created by covering the enclosures with transparent plastic film, whereas the control enclosures were only covered with bird nets to eliminate avian predation. The transparent plastic film covering the high-temperature enclosures was pierced with round holes (3 cm in diameter) every 30 cm to facilitate the exchange of air and moisture between the enclosures and the environment.
Experiments were conducted in 2013 and 2014. Because the lizards are not only active on the ground surface but also burrow to a depth of 10–20 cm underground during the reproductive season (personal observation), we recorded the temperatures with i-Buttons (Dallas Semiconductor, Dallas, Texas, USA) both at the soil surface and at a 10-cm depth underground in three enclosures in each treatment to compare the thermal regimes between the high-temperature treatment and the control during the experiment. The highest soil-surface temperature each day in the high-temperature enclosures was about 60 °C, which is similar to the extreme temperatures previously recorded at the study site (Fig. S3). We also collected climate data, including air temperature, precipitation, wind speed, and relative humidity at our study site in June 2013 and 2014 from China’s meteorological data-sharing service system (http://cdc.cma.gov.cn/).
Gravid female racerunners were collected by hand in the late May 2013 (n = 54) and 2014 (n = 52). We measured snout–vent length (SVL, to 0.01 mm) and body mass (BM, to 0.001 g), and then randomly assigned two or three females to each enclosure on 1 June. Food (mealworms and crickets dusted with additional vitamins and minerals) was provided daily ad libitum. The experiments lasted for 30 days (the entire month of June), covering the main period of gestation in this species (Zhao et al. 1999). On 30 June, we collected the females (n = 28 in both 2013 and 2014) from the enclosures with multiple recapture sessions (≥5) and brought them back to our laboratory in the Ordos field station. There was no sign of lizards escaping from enclosure, so we assume that those females were not recovered and died during the treatments.
Reproductive traits and offspring phenotypes
Females collected from the field enclosures were maintained in small cages (310 × 210 × 180 mm) with a substrate of sand and two small pieces of brick as shelters. The cages were exposed to the natural light regime of the field station, and a 60-W incandescent light bulb was suspended 5 cm above each cage to allow thermoregulation by the females from 8:00 to 12:00. Food (mealworms and crickets dusted with additional vitamins and minerals) was provided daily ad libitum. Each cage contained two females and was checked once per day for neonates, and four times per day following the first parturition. We collected and measured the SVL (1 mm) and BM (0.01 g) of neonates within a few hours after birth; we calculated the birth date as x days, where x is the number of days between the beginning of the treatment and parturition, the litter size as the number of neonates, and the litter mass as the total mass of neonates produced by a female. The SVL and BM of the postpartum females were measured again, and the females were then released at the sites, where they were collected. The neonates were kept in the cages and were sexed by observing the preanal scales. Males have large, square, regularly distributed preanal scales, while female preanal scales are small, round, and scattered (Fig. S4). We confirmed the viability of sexing using this between-sex morphological difference by dissecting some hatchlings and identifying their gonads.
All experiments in this study were performed under approval (IOZ14001) from the Animal Ethics Committee at the Institute of Zoology, Chinese Academy of Sciences.
Statistical analysis
All analyses were performed with the SPSS statistics software (version 22.0). We tested the normality of distributions and the homogeneity of all variances in the data with the Kolmogorov–Smirnov test and Bartlett’s test prior to the analysis. For the observations in the field enclosures, the repeated-measure ANOVAs were used to detect differences in mean daily temperatures at the soil surface and at a 10-cm depth between treatments. A 2 × 2 frequency table was used to determine differences in the percentages of surviving and breeding females between the high-temperature treatment and control groups.
Factorial ANOVAs were used to test the differences in the initial SVL and BM of females receiving thermal treatment, with year and surviving status as the fixed factors. Female body condition was quantified with residual scores from the linear regression of log e -transformed BM to log e -transformed SVL (Schulte-Hostedde et al. 2005). Because postpartum body condition was strongly correlated with the initial body condition (R 2 = 0.707, P < 0.001), we used two-way ANCOVAs to determine the between-treatment and between-year differences in postpartum body condition, with the initial body condition as the covariate.
Mixed-model ANOVAs were used to determine the effects of high temperature and year on female reproduction and on most neonate traits. Mixed-model ANCOVAs were used to determine the effects of high temperature and year on neonate SVL and BM, with maternal initial SVL and BM as the covariate, since neonate SVL values were significantly correlated with maternal SVL (R 2 = 0.473, P = 0.001) and BM (R 2 = 0.307, P = 0.044) values. Prior to ANCOVAs, our analyses indicated that the data met the assumption of homogeneity of regression slopes among treatments. Enclosure number was introduced as a random factor to avoid pseudoreplication of individuals within an enclosure. Litter means of the neonate SVL and BM were used for the analysis to avoid the pseudoreplication of individuals within a litter. Mixed-model ANOVAs were further used to test for differences in female reproductive traits and neonate traits between the high-temperature treatment and control groups in each year.
Results
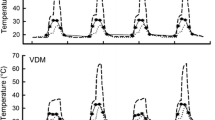
The soil-surface temperatures (F 1,8 = 650.79, P < 0.001) and 10-cm depth temperatures (F 1,8 = 268.99, P < 0.001) inside the enclosures were higher in the high-temperature treatment group than in the control group (Fig. 1). The soil-surface temperatures were slightly higher in 2014 than in 2013, but this difference was not statistically different (F 1,8 = 2.38, P = 0.16; Fig. 1a). However, the temperatures 10 cm underground were significantly lower in 2013 than in 2014 (F 1,8 = 12.40, P < 0.01; Fig. 1b), probably because the precipitation in 2013 (96.1 mm) was about twice that in 2014 (49.9 mm). Accordingly, the water vapor pressure was somewhat higher in 2013 than in 2014 (Table S2). Wind speed and relative humidity, by contrast, were similar in 2 years (Table S2).
The percentage of surviving females did not differ between the high-temperature treatment group and the control group in either 2013 (χ 2 = 1.76, P = 0.18) or 2014 (χ 2 = 0.43, P = 0.51) (Fig. 2a). The percentage of breeding females was a little lower in the high-temperature treatment group than in the control group in 2014 (χ 2 = 4.556, P < 0.05), but not in 2013 (χ 2 = 1.51, P = 0.22) (Fig. 2b). In addition, there were no between-year differences in the percentages of survival (χ 2 = 1.24, P = 0.27) or breeding (χ 2 = 1.17, P = 0.28) for females in the control group. For the high-temperature treatment group, however, the percentage of breeding females was significantly lower in 2014 than in 2013 (χ 2 = 5.38, P < 0.05), although the percentage of surviving females did not differ between 2 years (χ 2 = 0.29, P = 0.59) (Fig. 2).
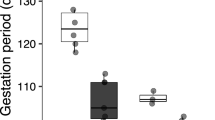
The initial SVL and BM values of the females did not differ either between thermal treatments or between years, but differed significantly between surviving and non-surviving females (Table 1; Fig. 3). Further analysis in each year indicated that surviving females were larger and heavier than non-surviving females in 2014 (SVL: F 1,48 = 42.00, P < 0.0001; BM: F 1,48 = 34.63, P < 0.0001), but not in 2013 (SVL: F 1,50 = 0.32, P = 0.57; BM: F 1,50 = 0.155, P = 0.70) (Fig. 4). Moreover, breeding females had worse postpartum body conditions in 2014 than in 2013 (F 1,24 = 13.42, P = 0.001), although thermal treatment did not affect the postpartum body condition of females (F 1,24 = 0.001, P = 0. 97) (Fig. 5).
The high-temperature treatment significantly affected female birth date, litter size, litter mass, and neonate sex ratio, but not neonate SVL or BM (Table 2). Neither birth date nor any of the measured traits of neonates differed between 2 years (Table 2). Further analysis in each year indicates that the high-temperature treatment did not affect any reproductive traits with the exception of 2013 when it stimulated earlier parturition (Table 3). In 2014, however, gravid females from the high-temperature treatment group gave birth earlier and produced litters with more male neonates than in the control group, although offspring body size and mass did not differ between the two groups (Table 3).
Discussion
Our study found that extreme high temperatures significantly affected the gestation period and reproductive output of racerunner females as well as the offspring sex ratio, but had little impact on offspring size and mass. More interestingly, the effect of extreme high temperatures on female reproductive output was not consistent between the experiments in the two consecutive years (years that differed in precipitation). Compared with the control group, females from the high-temperature treatment group produced fewer offspring and more males in the dry year, but not in the wet year. These results are generally consistent with our predictions of the effects of warming on female reproduction and hatchling traits. Below, we explain how these predictions were confirmed using the evidence from our experiments.
Female racerunners that experienced higher temperatures had a shorter gestation period, confirming our first prediction. A similar pattern of thermal dependence for gestation has also been reported in other viviparous lizards (Tang et al. 2012; Wang et al. 2014), as well as for oviparous species (Lu et al. 2013; Ma et al. 2014). The physiological basis underlying this pattern is that higher body temperatures may accelerate the development of embryos inside the female’s body cavity (Deeming 2004; Du and Shine 2015). This may underpin the observed phenomenon that climate warming has changed the phenology of marine, freshwater, and terrestrial animals, with earlier breeding associated with the rise in temperatures (Parmesan 2006, 2007; Root et al. 2003). In addition, offspring produced earlier may benefit from the additional time they receive for gaining energy and seeking shelter for winter survival, which can increase fitness in ectothermic animals (Olsson and Shine 1997; Rodriguez-Diaz et al. 2010; Warner and Shine 2007). Nonetheless, these benefits of earlier breeding may not always convey a selective advantage under global warming. For example, earlier hatching may sometimes lead to trophic mismatch; a lack of synchrony between animal hatching and food availability could decrease growth and survival rates (McKinnon et al. 2012).
Females from the high-temperature treatment group had smaller litter sizes and, therefore, fewer offspring than those from the control group, which is consistent with our second prediction. This may indicate that females from the high-temperature treatment group faced a higher mortality of embryos, given that high extreme temperatures are harmful for embryonic development and even lead to the death of embryos in lizards as well as other reptiles (Du and Ji 2003, 2006; Birchard and Deeming 2004; Bell et al. 2013). Previous studies on life history responses to experimental warming have yielded contradictory results. Some investigations have found that reproductive life history may change in response to temperature variation (Costantini et al. 2010; Lu et al. 2014; Ma et al. 2014), but others have found that it does not (Ji et al. 2006; Lu et al. 2013; Telemeco et al. 2010). This may reflect species-specific responses to experimental warming, or different experimental treatments associated with the magnitude of changes in the thermal environment mimicked by these studies. The fitness cost of energy constraints is faced in the field by populations of lizards and other animals in the context of global warming. For example, Sceloporus lizards in Mexico are vulnerable to local extinction, probably because they are forced to restrict their activity by more than 4 h per day due to climate warming and, therefore, experience a restricted net energy gain (Sinervo et al. 2010).
As we expected, extremely high temperatures skewed the offspring sex ratio, with more males observed in the high-temperature treatment group. This is consistent with results from constant-temperature experiments on other populations of this species (Tang et al. 2012; Zhang et al. 2010). These studies demonstrated that female racerunners produce male-skewed litters at high temperatures, but female-skewed litters at low temperatures, with a pivotal temperature of approximately 29 °C (Tang et al. 2012; Zhang et al. 2010). The offspring sex of many oviparous reptiles, including tuatara, all crocodiles, most turtles, and some lizards, is determined by the temperatures experienced by the growing embryo (Valenzuela and Lance 2004). These species of reptiles with TSD are particularly vulnerable to climate warming, because climate warming may skew the sex ratio of the offspring, and hence the population sex ratio (Janzen 1994). Recently, TSD has been found in several species of viviparous reptiles, including the racerunner (Robert and Thompson 2001; Tang et al. 2012; Zhang et al. 2010). Although maternal thermoregulation in these viviparous species may buffer the temperature variation experienced by the growing embryos to some degree (Huey and Tewksbury 2009; Kearney et al. 2009), the offspring sex ratio may be skewed by climate change in natural populations (Wapstra et al. 2009) as well as by extreme temperatures, as in this study. Our results further highlight the potentially severe impacts of climate warming on TSD reptiles, a critical issue that has attracted intense concern in ecological studies, and in animal conservation in the last few decades, because skewed sex ratios may impact the reproduction of the next generation and hence the population dynamics (Ihlow et al. 2012; Janzen 1994; Mitchell et al. 2008; Valenzuela and Lance 2004).
More interestingly, the effects of extreme high temperatures on female reproduction and offspring sex were significant in 2014, but not in 2013. A smaller proportion of larger females gave birth in 2014, and they produced a smaller litter with more male neonates (Figs. 2, 4). The between-year differences in mean and maximum temperatures were not statistically significant, and may not be the main reason for the different effects. More likely, the between-year difference in climate warming effects was due to precipitation, which was higher in 2013 than in 2014. First, although soil-surface temperatures did not differ between the two years, underground soil temperatures were lower in 2013 than in 2014 due to the between-year difference in precipitation (Fig. 1). In 2013, lizards might have been able to explore underground thermal opportunities to achieve suitable body temperatures, which can alleviate the impact of high-temperature treatment, as shown in the laboratory warming experiment (Fig. S2). Such thermal opportunities largely decreased in 2014, however, due to the increase in underground soil temperature. For example, during the active period of the lizards—from 8:00 to 18:00 each day—in 2013, 43 % of the 10-cm depth soil temperatures were over 29 °C, which is the pivotal temperature for TSD in this species (Tang et al. 2012; Zhang et al. 2010). In 2014, by contrast, this increased to 54 %. Second, field investigations have demonstrated that the precipitation may affect the reproductive output and, therefore, the population size of animals, including reptiles (Dobkin et al. 1987; Lorenzon et al. 2001; Madsen and Shine 2000). Third, water constraints during pregnancy can directly decrease offspring size and survival (Marquis et al. 2008), or indirectly affect offspring number by influencing food availability for reptiles (James and Whitford 1994). In addition, a macro-physiological analysis predicts that temperature may interact with precipitation to determine the response of squamate reptiles to climate change (Clusella-Trullas et al. 2011). Experimental tests of the interaction between temperature and precipitation on life history are rare, however. Our experimental results provide evidence that temperature interacts with precipitation to determine the life history of lizards. The effect of extreme high temperatures on female reproduction and offspring traits was more pronounced in the dry year than in the wet year, which suggests that low precipitation may aggravate the impact of climate warming on lizards.
Our results have important implications for animal conservation in arid mid-latitude areas undergoing climate warming. Our study region has experienced increasing temperatures, extreme high temperatures, and decreasing rainfall over the last 65 years (Fig. S1), and this trend is expected to continue in the future at other mid-latitude regions similar to our study site (Adler et al. 2008; Meehl et al. 2007). The negative impact of extreme high temperatures on lizard reproduction would reduce recruitment and, therefore, the sustainability of lizard populations in the desert steppe of our study site. More severely, the drier environment in this area would render the survival of the lizards even more difficult. Given that lizards are the principal vertebrates of the arid region and a critical component of local food webs (Pianka 1986), the decrease or disappearance of lizard populations due to climate warming would lower the regional biodiversity. Ectothermic animals, such as lizards, should, therefore, receive more attention in future studies if we are to understand how climate warming affects animals and influences the conservation of biodiversity under global warming in arid mid-latitude areas.
References
Adler PB, Drake JM (2008) Environmental variation, stochastic extinction, and competitive coexistence. Am Nat 172:E186–E195. doi:10.1086/591678
Adler RF, Gu GJ, Wang JJ, Huffman GJ, Curtis S, Bolvin D (2008) Relationships between global precipitation and surface temperature on interannual and longer timescales (1979–2006). J Geophys Res-Atmos 113:D22104. doi:10.1029/2008jd010536
Angilletta MJ (2009)Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford University Press
Bauerfeind SS, Fischer K (2014) Simulating climate change: temperature extremes but not means diminish performance in a widespread butterfly. Popul Ecol 56:239–250. doi:10.1007/s10144-013-0409-y
Bell K, Blomberg S, Schwarzkopf L (2013) Detrimental influence on performance of high temperature incubation in a tropical reptile: is cooler better in the tropics? Oecologia 171:83–91. doi:10.1007/s00442-012-2409-6
Birchard GF, Deeming DC (2004) Effects of incubation temperature. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham
Bonebrake TC, Mastrandrea MD (2010) Tolerance adaptation and precipitation changes complicate latitudinal patterns of climate change impacts. P Natl Acad Sci USA 107:12581–12586. doi:10.1073/pnas.0911841107
Bradshaw WE, Holzapfel CM (2006) Climate change: evolutionary response to rapid climate change. Science 312:1477–1478. doi:10.1126/science.1127000
Chamaille-Jammes S, Massot M, Aragon P, Clobert J (2006) Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Glob Chang Biol 12:392–402. doi:10.1111/j.1365-2486.2005.01088.x
Choi G et al (2009) Changes in means and extreme events of temperature and precipitation in the Asia-Pacific Network region, 1955–2007. Int J Climatol 29:1906–1925. doi:10.1002/joc.1979
Clusella-Trullas S, Blackburn TM, Chown SL (2011) Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am Nat 177:738–751. doi:10.1086/660021
Costantini D, Carello L, Dell’Omo G (2010) Patterns of covariation among weather conditions, winter North Atlantic Oscillation index and reproductive traits in Mediterranean kestrels. J Zool 280:177–184. doi:10.1111/j.1469-7998.2009.00649.x
Deeming DC (2004) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham
Dillon ME, Wang G, Huey RB (2010) Global metabolic impacts of recent climate warming. Nature 467:704–706. doi:10.1038/nature09407
Dobkin DS, Olivieri I, Ehrlich PR (1987) Rainfall and the interaction of microclimate with larval resources in the population-dynamics of checkerspot butterflies (Euphydryas editha) inhabiting serpentine grassland. Oecologia 71:161–166. doi:10.1007/Bf00377280
Du WG, Ji X (2003) The effects of incubation thermal environments on size, locomotor performance and early growth of hatchling soft-shelled turtles, Pelodiscus sinensis. J Therm Biol 28:279–286. doi:10.1016/S0306-4565(03)00003-2
Du WG, Ji X (2006) Effects of constant and fluctuating temperatures on egg survival and hatchling traits in the northern grass lizard (Takydromus septentrionalis, Lacertidae). J Exp Zool 305A:47–54. doi:10.1002/jez.a.243
Du W-G, Shine R (2015) The behavioural and physiological strategies of bird and reptile embryos in response to unpredictable variation in nest temperature. Biol Rev 90:19–30. doi:10.1111/brv.12089
Du W-G, Yan S-J, Ji X (2000) Selected body temperature, thermal tolerance and thermal dependence of food assimilation and locomotor performance in adult blue-tailed skinks, Eumeces elegans. J Therm Biol 25:197–202. doi:10.1016/S0306-4565(99)00022-4
Dubey S, Shine R (2011) Predicting the effects of climate change on reproductive fitness of an endangered montane lizard, Eulamprus leuraensis (Scincidae). Clim Change 107:531–547. doi:10.1007/s10584-010-9963-x
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modeling, and impacts. Science 289:2068–2074. doi:10.1126/science.289.5487.2068
Fieldhouse DJ, Palmer WC (1965) The climate of the northeast: meteorological and agricultural drought. University of Delaware, Agricultural Experiment Station, Newark
Glickman TS (2000) Glossary of Meteorology, 2nd edn. American Meteorological Society, Boston, Mass
Hoffmann AA, Anderson A, Hallas R (2002) Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett 5:614–618. doi:10.1046/j.1461-0248.2002.00367.x
Huey RB, Tewksbury JJ (2009) Can behavior douse the fire of climate warming? P Natl Acad Sci USA 106:3647–3648. doi:10.1073/pnas.0900934106
Ihlow F et al (2012) On the brink of extinction? How climate change may affect global chelonian species richness and distribution. Glob Chang Biol 18:1520–1530. doi:10.1111/j.1365-2486.2011.02623.x
IPCC (2013) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
James CD, Whitford WG (1994) An experimental study of phenotypic plasticity in the clutch size of a lizard. Oikos 70:49–56. doi:10.2307/3545698
Janzen FJ (1994) Climate-change and temperature-dependent sex determination in reptiles. P Natl Acad Sci USA 91:7487–7490. doi:10.1073/pnas.91.16.7487
Jentsch A, Kreyling J, Beierkuhnlein C (2007) A new generation of climate-change experiments: events, not trends. Front Ecol Environ 5:365–374. doi:10.1890/1540-9295(2007)5[365:angoce]2.0
Ji X, Lin L-H, Luo L-G, Lu H-L, Gao J-F, Han J (2006) Gestation temperature affects sexual phenotype, morphology, locomotor performance and growth of neonatal brown forest skink, Sphenomorphus indicus. Biol J Linn Soc 88:453–463. doi:10.1111/j.1095-8312.2006.00633.x
Johnston IA, Bennett AF (1996) Animals and temperature: phenotypic and evolutionary adaptation. Cambridge University Press, Cambridge
Kearney M, Shine R, Porter WP (2009) The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. P Natl Acad Sci USA 106:3835–3840. doi:10.1073/pnas.0808913106
Li H, Qu Y-F, Ding G-H, Ji X (2011) Life-history variation with respect to experienced thermal environments in the lizard, Eremias multiocellata (Lacertidae). Zool Sci 28:332–338. doi:10.2108/zsj.28.332
Lorenzon P, Clobert J, Massot M (2001) The contribution of phenotypic plasticity to adaptation in Lacerta vivipara. Evolution 55:392–404
Lu H-L, Wang Y, Tang W-Q, Du W-G (2013) Experimental evaluation of reproductive response to climate warming in an oviparous skink. Integr Zool 8:175–183. doi:10.1111/1749-4877.12025
Lu Z-C, Wang Y-M, Zhu S-G, Yu H, Guo J-Y, Wan F-H (2014) Trade-offs between survival, longevity, and reproduction, and variation of survival tolerance in Mediterranean Bemisia tabaci after temperature stress. J Insect Sci 14:1–11
Ma L, Sun B-J, Li S-R, Sha W, Du W-G (2014) Maternal thermal environment induces plastic responses in the reproductive life history of oviparous lizards. Physiol Biochem Zool 87:677–683. doi:10.1086/678050
Madsen T, Shine R (2000) Rain, fish and snakes: climatically driven population dynamics of Arafura filesnakes in tropical Australia. Oecologia 124:208–215. doi:10.1007/s004420050008
Marquis O, Massot M, Le Galliard JF (2008) Intergenerational effects of climate generate cohort variation in lizard reproductive performance. Ecology 89:2575–2583. doi:10.1890/07-1211.1
McKinnon L, Picotin M, Bolduc E, Juillet C, Bêty J (2012) Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Can J Zool 90:961–971. doi:10.1139/z2012-064
Meehl GA, Tebaldi C (2004) More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305:994–997. doi:10.1126/science.1098704
Meehl GA, Tebaldi C, Teng H, Peterson TC (2007) Current and future US weather extremes and El Nino. Geophys Res Lett 34:6. doi:10.1029/2007gl031027
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant–pollinator interactions. Ecol Lett 10:710–717. doi:10.1111/j.1461-0248.2007.01061.x
Mitchell NJ, Kearney MR, Nelson NJ, Porter WP (2008) Predicting the fate of a living fossil: how will global warming affect sex determination and hatching phenology in tuatara? P Roy Soc B-Biol Sci 275:2185–2193. doi:10.1098/rspb.2008.0438
Ohlberger J (2013) Climate warming and ectotherm body size—from individual physiology to community ecology. Funct Ecol 27:991–1001. doi:10.1111/1365-2435.12098
Olsson M, Shine R (1997) The seasonal timing of oviposition in sand lizards (Lacerta agilis): why early clutches are better. J Evol Biol 10:369–381. doi:10.1007/s000360050030
Pachauri RK, Meyer LA, IPCC (2014) Climate change 2014: synthesis report. IPCC, Geneva, Switzerland
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol S 37:637–669. doi:10.1146/annurev.ecolsys.37.091305.110100
Parmesan C (2007) Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob Chang Biol 13:1860–1872. doi:10.1111/j.1365-2486.2007.01404.x
Perkins SE, Alexander LV, Nairn JR (2012) Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys Res Lett 39:L20714. doi:10.1029/2012gl053361
Pianka ER (1986) Ecology and natural history of desert lizards. Princeton University Press, Princeton
Qian T, Dai A, Trenberth KE (2007) Hydroclimatic trends in the Mississippi River basin from 1948 to 2004. J Climate 20:4599–4614. doi:10.1175/Jcli4262.1
Qu Y-F, Li H, Gao J-F, Xu X-F, Ji X (2011) Thermal preference, thermal tolerance and the thermal dependence of digestive performance in two Phrynocephalus lizards (Agamidae), with a review of species studied. Curr Zool 57:684–700
Robert KA, Thompson MB (2001) Sex determination—Viviparous lizard selects sex of embryos. Nature 412:698–699. doi:10.1038/35089135
Rodriguez-Diaz T, Gonzalez F, Ji X, Brana F (2010) Effects of incubation temperature on hatchling phenotypes in an oviparous lizard with prolonged egg retention: are the two main hypotheses on the evolution of viviparity compatible? Zoology 113:33–38. doi:10.1016/j.zool.2009.05.001
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. doi:10.1038/nature01333
Rosa R, Seibel BA (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. P Natl Acad Sci USA 105:20776–20780. doi:10.1073/pnas.0806886105
Schulte-Hostedde A, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86:155–163
Seneviratne SI, Donat MG, Mueller B, Alexander LV (2014) No pause in the increase of hot temperature extremes. Nat Clim Chang 4:320
Sinervo B et al (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899. doi:10.1126/science.1184695
Smith MD (2011) The ecological role of climate extremes: current understanding and future prospects. J Ecol 99:651–655. doi:10.1111/j.1365-2745.2011.01833.x
Sun Y, Solomon S, Dai A, Portmann RW (2007) How often will it rain? J Clim 20:4801–4818. doi:10.1175/Jcli4263.1
Tang X-L et al (2012) Effects of gestation temperature on offspring sex and maternal reproduction in a viviparous lizard (Eremias multiocellata) living at high altitude. J Therm Biol 37:438–444. doi:10.1016/j.jtherbio.2012.03.002
Telemeco RS, Radder RS, Baird TA, Shine R (2010) Thermal effects on reptile reproduction: adaptation and phenotypic plasticity in a montane lizard. Biol J Linn Soc 100:642–655. doi:10.1111/j.1095-8312.2010.01439.x
Thompson DM, Cole JE, Shen GT, Tudhope AW, Meehl GA (2015) Early twentieth-century warming linked to tropical Pacific wind strength. Nat Geosci 8:117–121. doi:10.1038/Ngeo2321
Triggs A, Knell RJ (2012) Interactions between environmental variables determine immunity in the Indian meal moth Plodia interpunctella. J Anim Ecol 81:386–394. doi:10.1111/j.1365-2656.2011.01920.x
Valenzuela N, Lance V (2004) Temperature dependent sex determination in vertebrates. Smithsonian Books, Washington DC
Vose RS, Easterling DR, Gleason B (2005) Maximum and minimum temperature trends for the globe: an update through 2004. Geophys Res Lett 32:L23822. doi:10.1029/2005gl024379
Wang Z, Lu H-L, Ma L, Ji X (2014) Viviparity in high-altitude Phrynocephalus lizards is adaptive because embryos cannot fully develop without maternal thermoregulation. Oecologia 174:639–649. doi:10.1007/s00442-013-2811-8
Wapstra E et al (2009) Climate effects on offspring sex ratio in a viviparous lizard. J Anim Ecol 78:84–90. doi:10.1111/j.1365-2656.2008.01470.x
Warner DA, Shine R (2007) Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154:65–73. doi:10.1007/s00442-007-0809-9
Wells N, Goddard S, Hayes MJ (2004) A self-calibrating palmer drought severity index. J Clim 17:2335–2351. doi:10.1175/1520-0442(2004)017<2335:Aspdsi>2.0.Co;2
Wernberg T, Smale DA, Thomsen MS (2012) A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob Chang Biol 18:1491–1498. doi:10.1111/j.1365-2486.2012.02656.x
Wolkovich EM et al (2012) Warming experiments underpredict plant phenological responses to climate change. Nature 485:494–497. doi:10.1038/nature11014
Zeng Z-G et al (2014) Effects of habitat alteration on lizard community and food web structure in a desert steppe ecosystem. Biol Conserv 179:86–92. doi:10.1016/j.biocon.2014.09.011
Zhang D-J, Tang X-L, Yue F, Chen Z, Li R-D, Chen Q (2010) Effect of gestation temperature on sexual and morphological phenotypes of offspring in a viviparous lizard, Eremias multiocellata. J Therm Biol 35:129–133. doi:10.1016/j.jtherbio.2010.01.003
Zhao E-M, Zhao K-T, Zhou K-Y (1999) Fauna Sinica Reptilia Vol. 2 Squamata. Chinese Science Press, Beijing
Zittier ZMC, Hirse T, Portner HO (2013) The synergistic effects of increasing temperature and CO2 levels on activity capacity and acid-base balance in the spider crab, Hyas araneus. Mar Biol 160:2049–2062. doi:10.1007/s00227-012-2073-8
Acknowledgments
We thank Shao-Yong Chen and Zhi-Liang Jie for their assistance in the field and laboratory. This work was supported by grants from the One Hundred Talents Program of the Chinese Academy of Sciences and the National Natural Sciences Foundation of China (31372203).
Author contribution statement
W. G. D. designed the study, Y. W., Z. G. Z., S. R. L. and J. H. B. conducted experiments, W. G. D. and Y. W. analysed data, and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hannu J. Ylonen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zeng, ZG., Li, SR. et al. Low precipitation aggravates the impact of extreme high temperatures on lizard reproduction. Oecologia 182, 961–971 (2016). https://doi.org/10.1007/s00442-016-3727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3727-x