Abstract
Seed dispersal may involve different vectors of dispersal in two or more sequential phases (i.e., diplochory). However, contributions of each phase to the overall seed dispersal effectiveness (SDE) are poorly understood and hard to evaluate due to post-dispersal processes that affect seed and seedling survival. We investigated the simultaneous bird (phase 1, in plant canopy) and ant (phase 2, on the floor) contributions to SDE with the ornithochoric shrub Erythroxylum ambiguum in a Brazilian Atlantic forest. Twelve species of birds fed on fruit and dispersed approximately 26 % of the seed crop. The remaining seed crop, 90 % of which contained viable seeds, fell to the ground beneath the parental plant. Ants either cleaned seeds in fruits or carried fallen fruit and seeds from bird feces to their nests. Although E. ambiguum has no adaptation for ant dispersal, ants were as quantitatively important as birds. Birds and ants equally increased germination rates compared to controls. However, birds deposited seeds farther from the parent, where seedling survival was higher (78 %) than it was beneath the parent (44 %), whereas ants carried seeds to their nests, where seedling survival was higher (83 %) than in controls away from their nests (63 %). Diplochory allowed a 42 % increase in SDE compared to dispersal in phase 1 alone. High lipid content in the fruit pulp of E. ambiguum may facilitate the inclusion of ants in a second step of dispersal after diaspores reach the floor. Ants can also buffer the dispersal of diplochorous plants against decreases in phase 1 dispersers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed dispersal is important for its influence on seed survival and seedling establishment and therefore for plant population and community dynamics (Howe and Smallwood 1982; Wang and Smith 2002; Nathan 2006; Clark et al. 2007; González-Castro et al. 2015). Seed dispersal can reduce the mortality of seeds and seedlings due to density-dependent effects such as sibling competition and herbivore damage that are often greater near the parent plant (Janzen 1970; Connell 1971). Seed dispersers may also consistently take seeds to specific sites (directed dispersal), where the chances of recruitment are much greater than in random sites (Howe and Smallwood 1982; Wenny 2001). Dispersal can also favor the colonization of new sites (Howe and Smallwood 1982; Calviño-Cancela and Martín-Herrero 2009), thereby playing a role in metapopulation dynamics and gene flow (e.g., Nathan 2006).
Animals are often important seed dispersal agents, and plants attract them by providing an edible reward (e.g., fleshy fruit pulp) with seeds. Dispersers have shaped fruit and seed traits in unrelated plant clades (Lengyel et al. 2009; Lomáscolo et al. 2010). Nevertheless, different dispersers (a variety of bird, mammal, reptile, or invertebrate species) may participate in the dispersal of any given plant species and thus together contribute to seed dispersal (Jordano 2000; Gove et al. 2007; González-Castro et al. 2015). In such diffuse mutualisms, a plant benefits from the service of multiple seed disperser species that may vary in their dispersal effectiveness (Gove et al. 2007; Calviño-Cancela and Martín-Herrero 2009; Leal et al. 2014; González-Castro et al. 2015).
Seed dispersal effectiveness (SDE) can be estimated by combining measures of quantitative and qualitative processes that influence plant recruitment likelihood, such as the number of seeds dispersed and the quality of seed dispersal (Schupp 1993; Schupp et al. 2010). Ideally, measuring SDE should include both the quantity and quality components of dispersal that influence seed germination, survival and growth (Schupp 1993; Schupp et al. 2010). Quantifying seed dispersal and its delayed consequences for post-dispersal seed predation, germination and seedling survival in the same system is quite difficult but essential to expand our knowledge on the complex dispersal systems of tropical plants (Culot et al. 2015).
Until recently, studies of the seed dispersal loop usually encompassed just one stage of the seed dispersal sequence (phase 1 or primary seed dispersal). Phase 1 is the part in which seeds are removed from the plant canopy (e.g., by an avian disperser) and dropped at some distance (Wang and Smith 2002). Following primary dispersal, post-dispersal factors (e.g., secondary seed dispersal and predation) influence seed fate and plant recruitment (Forget et al. 2004). Rodents (Vander Wall et al. 2005), beetles (Andersen and Feer 2005; Pérez-Ramos et al. 2013), ants (Passos and Oliveira 2002) and water (Hampe 2004) may rearrange seed shadows generated in phase 1 by secondarily dispersing seeds (phase 2), thereby changing recruitment probabilities. Such multi-phased seed dispersal systems involving subsequent steps of dispersal in which different vectors participate are known as diplochory (Vander Wall and Longland 2004). Careful measures of seed fate after each step of dispersal are required to estimate independent contributions to SDE by phase 1 or phase 2 dispersers (Culot et al. 2015). Empirical data for this type of approach are rare, and those few data suggest context dependency (e.g., Böhning-Gaese et al. 1999; Culot et al. 2015). Dispersers that are relatively unimportant numerically may become important qualitatively and thus determine plant recruitment (Pérez-Ramos et al. 2013).
Myrmecochory (seed dispersal by ants) is common among herbs of temperate forests of North America, Europe and Japan and in shrubs growing in nutrient-poor soils and fire-prone habitats in South Africa and Australia (reviewed in Rico-Gray and Oliveira 2007; Lengyel et al. 2009). Myrmecochorous plants produce seeds attached to a lipid-rich appendage called an elaiosome that elicits seed removal by ants. The ants consume the elaiosome and discard the unharmed seed in the nest midden, where the seed can germinate (Giladi 2006). Although ants usually remove seeds short distances, they often provide seed escape from predators or fire and directed dispersal to nutrient-rich soils of ant nests (Giladi 2006). Indeed, the repeated evolution of myrmecochory in unrelated plant lineages suggests that seed dispersal mutualisms between plants and ants were a powerful evolutionary source of plant diversification (Lengyel et al. 2009). Nevertheless, the near absence of myrmecochorous plants in Neotropical rainforests (Lengyel et al. 2009) contrasts with the abundance of litter-foraging ants (Hölldobler and Wilson 1990), the organisms most likely to encounter fallen plant diaspores (i.e., fruits and seeds). Most Neotropical shrubs and trees are clearly associated with vertebrate seed dispersers (Howe and Smallwood 1982; Jordano 2000). However, remains of fruit pulp left on seeds previously handled by vertebrates may attract foraging ants, so the possibility exists for post-dispersal interactions between ants and fallen diaspores (Passos and Oliveira 2002). Although the occurrence and potential benefits of ant-seed interactions to non-myrmecochorous plants have been increasingly recognized (e.g., Pizo and Oliveira 2001; Passos and Oliveira 2002, 2004; Vander Wall and Longland 2004; Christianini and Oliveira 2009, 2010) it is not yet clear which are the costs of the inclusion of ants in a phase 2 of dispersal, as well as the consequences for overall SDE. For instance, ants may be quantitatively important seed dispersers but seedling recruitment may still be largely driven by vertebrates acting in phase 1, possibly due to benefits related to dispersal distance (Böhning-Gaese et al. 1999). On the other hand, ants may rescue seeds dropped by birds beneath parent plant canopies and provide seeds with additional chances of dispersal and recruitment (Christianini and Oliveira 2009). Without careful evaluations of phases 1 and 2 of dispersal and their delayed consequences for seed predation, germination and seedling survival we have to rely only on partial and possible misleading conclusions about the role of each stage of dispersal in plant regeneration.
Here we investigate bird and ant contributions to SDE in the bird-dispersed shrub Erythroxylum ambiguum Peyr., during both phase 1 and phase 2 of dispersal. We specifically attempt to answer: (1) what is the quantitative and qualitative contribution of birds and ants to seed dispersal in our multi-phased system? And (2) how do phase 1 and phase 2 contribute to total SDE? To our knowledge this is the first study that partitions in detail the relative contribution of phase 1 and phase 2 of dispersal provided by birds and ants, respectively, to a non-myrmecochorous plant.
Materials and methods
Study site
We examined bird and ant contributions to overall SDE in Carlos Botelho State Park (CBSP) in southeastern Brazil (24º08′S, 47º55′W). CBSP is part of the largest remaining parcel of the Atlantic forest (Fundação Florestal 2008; Lima et al. 2011) and comprises 37,644 ha, most containing old-growth evergreen subtropical rainforest (Lima et al. 2011). CBSP has at least 1143 vascular plant species (Lima et al. 2011), 342 bird and 35 mammal species (Fundação Florestal 2008). The study plot (ca. 30 ha) was at ca. 700 m a.s.l. Mean annual rainfall varies from 1700 mm to 2400 mm and temperatures from 17 to 22 °C (Fundação Florestal 2008).
Plant species
Erythroxylum ambiguum Peyr. (Erythroxylaceae) is a shrub that fruits from September to March (P. H. S. A. Camargo, unpublished data). The fruit is a spherical red drupe (1 cm) with a single seed surrounded by a fleshy red pulp rich in lipids (88 % dry weight), 4 % carbohydrates, <1 % protein, 5 % fiber and 2 % ash (P. H. S. A. Camargo and A. V. Christianini, unpublished data), a set of traits indicative of bird-dispersal syndrome (Jordano 2000). E. ambiguum fruits have alkaloids that are toxic to mammals (Colodel et al. 2004). The plants only reproduce by seeds that can remain viable up to 40 days after dispersal (P. H. S. A. Camargo, personal observation).
Fruit crop size and seed fate
To estimate fruit production, in August 2012 we haphazardly selected 11 fruiting plants (3.2 ± 1.1 m high; mean ± SD), each of which was a minimum of 10 m from any other conspecific plant. We counted the entire fruit crop in each plant canopy. We placed fruit/seed traps beneath plants to estimate fruit fall. Traps were 1500-cm2 plastic trays lined with 0.2-mm nylon mesh, supported 20 cm above the ground by wooden stakes. The stakes were smeared with Tanglefoot to prevent ant access, and trays were covered with a wire screen to prevent vertebrates from removing seeds and fruits. Two to five traps were used per plant, sampling 42 ± 23 % of the canopy area. Trap contents were collected weekly. Diaspores were classified as: (1) ripe fruit (bright red and intact), (2) unripe fruit (green or yellowish pulp), (3) preyed on before dispersal (broken seeds or seeds with emergence holes of insects), (4) seeds with part of fruit pulp still attached to them but with signs of being manipulated by birds (bill marks), and (5) seeds in feces.
We estimated the number of undispersed seeds by the ratio between the number of diaspores in the trays and the proportion of the canopy area sampled (Christianini and Oliveira 2010). Although we considered the above-mentioned categories 4 and 5 as encompassing diaspores from the plant growing above the traps, they may include an unknown fraction of diaspores brought by birds from other plants and dropped on the spot. Therefore, it is possible that the estimate of undispersed seeds is slightly overestimated. We obtained for each plant the amount of diaspores removed by primary seed dispersers by calculating the difference between the crop size and the estimate of undispersed diaspores.
Plant-frugivore interactions in the plant: dispersal phase 1
To gather data on frugivores at the plants, we monitored 19 shrubs during two fruiting seasons: 2012/2013 and 2013/2014. We observed frugivores with the aid of binoculars from 0600–1100 to 1530–1830 hours, totaling 148.5 h of direct observations. We noted the time of the visit, the visiting species and the number of diaspores consumed or dropped. Seeds swallowed by the visitors were considered dispersed. For birds that swallowed fruits, we noted the distance from the focal tree to the first place where they subsequently perched or to the spot we lost them from sight (post-feeding flight distance) as a proxy for minimum seed dispersal distance (Jordano and Schupp 2000). We recognize that this protocol is limited in that gut retention time is longer than the elapsed time between perches, but this enabled us to produce minimum estimates of seed dispersal distances in phase 1 that could be compared with phase 2. We complemented data on bird frugivory (but not potential distances of dispersal) from video recordings of visitors to the shrubs obtained with seven camera traps (Bushnell Natureview Cam HD—119438; Bushnell, Overland Park, KS; S. M. Rodrigues and A. J. Piratelli, unpublished data). Each camera set on separate plants recorded 30-s videos after being triggered by a movement sensor. The cameras operated 24 h/day from December 2013 to February 2014 for a total of 4015 h (S. M. Rodrigues and A. J. Piratelli, unpublished data).
On-ground, ant-diaspore interactions: dispersal phase 2
Ant-diaspore interactions were measured in 31 transects, each 90 m long and with nine on-ground sampling stations separated by 10 m from one another, for a total of 279 stations. This distance was enough to ensure independent diaspore discovery by different ant colonies (see distances of diaspore removal in results). Each station had five individually marked (Testors enamel paint; Testors, Rockford) fruits placed on a 4 × 4-cm white filter paper (Passos and Oliveira 2002). The stations were checked at 15-min intervals over 2 h between 0800 and 1800 hours. When an ant was seen at and touching a diaspore, we noted the species and behavior. Ants removing diaspores were followed to their colony or were lost in the leaf litter, at which point the displacement distance was measured. Ant voucher specimens were deposited in the Entomological Collection Padre Jesus Santiago Moure (DZUP) of the Universidade Federal do Paraná, Brazil.
To determine the fate of fallen diaspores (e.g., secondary dispersal and predation), we measured removal rates with exclosure experiments in two paired treatments. The first treatment was a vertebrate-exclusion treatment, in which diaspores were covered by a 1.5-cm wire mesh cage (15 × 15 × 10 cm) on the top and sides and staked to the ground. Adjacent to the first treatment was the control treatment, accessible to all organisms (free access). Each treatment received a pair of diaspores, one of which was an entire ripe fruit and the other a seed whose pulp we manually removed. Five paired treatments were placed equidistant under each of 26 fruiting E. ambiguum, for a total of 130 replicates. Diaspores were marked with a small dot of enamel paint (Testors) to distinguish them from naturally fallen diaspores. We counted the number of diaspores removed after 24 h. To test whether removal rates differed for diaspores beneath or away from parents (simulating diaspores that had dispersed), a second set of five paired treatments (as above) was established at least 20 m away from any conspecific fruiting plant under other shrub species.
We compared removal rates in treatments using factorial ANOVA in the R program version 3.0.0 (R Development Core Team 2013). Residuals from the analysis met the normality and homocedasticity assumptions of ANOVA. Each reproductive E. ambiguum and its non-conspecific pair were considered as a block, and the exclusion treatment (exclosure, free access), location treatment (beneath, away from fruiting conspecifics) and diaspore type (whole fruit, clean seed) were the independent variables. The pooled number of diaspores removed in each treatment per plant was the dependent variable.
We tested in January 2014 whether ants reshape the bird-generated seed shadow by following the fate of seeds in bird feces. Four captive Turdus albicollis and five Turdus leucomelas that were maintained with a regular diet of fruits, seeds, and insect larvae were also fed on the fruits of E. ambiguum. On average, birds defecated seeds 11.3 ± 5.9 min (n = 27) after consumption. Fresh fecal samples (<24 h after defecation) with a single seed embedded (n = 21) were placed on the forest floor early in the morning and covered with a wire cage. We recorded the ant species and their behavior toward the seeds in feces every 15 min for 2 h. After 24 h, we recorded the number of seeds remaining.
Effects of birds and ants on seed germination
We examined how bird gut passage or manipulation by ants influenced germination using germination trials. Seeds were tested in controls, both with 32 diaspores—(1) whole fruits, and (2) cleaned seeds in which we manually removed pulp; and two treatments—(1) seeds that were defecated by birds, and (2) handled by ants (32 seeds in each treatment). The captive T. albicollis and T. leucomelas provided the defecated seeds. The ant refuse pile of a Pachycondyla striata colony raised in the laboratory (see Pizo and Oliveira 2001) provided the ant-manipulated seeds. All seeds were rinsed in a 0.5 % sodium hypochlorite solution and sown in vermiculite, moistened regularly with sterilized water and kept at room temperature and natural light. Treatments were checked daily for 45 days for germination, identified as emergence of the radicle.
We compared the percentage of germination between treatments with G-tests. Germination speed (i.e., the cumulative proportion of germinated seeds) was compared among treatments by Kolmogorov–Smirnov tests, with adjustments in critical level using Dunn–Sidak’s method due to multiple comparisons (Gotelli and Ellison 2011).
Seedling distribution and survival
We followed ant workers to their nests after attracting them to tuna baits on the ground. We sampled 23 ant nests: Pachycondyla striata (n = 21), Ectatomma edentatum (n = 1), and Atta sexdens (n = 1). We compared seedling distribution and survival near ant nests and far from ant nests (random direction, 1–5 m from a nest) in 0.5 × 0.5-m plots. We also sampled three 100 × 2-m quadrats haphazardly established in the study plot, tagged all seedlings and noted the seedling distance from an ant nest, and if farther than 1 m, we considered them as distant. The distance of seedlings to the nearest adult shrub was also measured.
We compared the number of seedlings near and far from ant nests by the Wilcoxon paired sample test. Seedlings were visited monthly for 1 year to note survival. We compared survival near and far from ant nests by Peto and Peto’s generalized Wilcoxon test (Pyke and Thompson 1986). We also compared the proportion of seedlings surviving beneath or away from conspecific E. ambiguum using Peto and Peto’s logrank test (Pyke and Thompson 1986). The relationship between 1-year seedling survival and its distance to the nearest conspecific was modeled using logistic regression.
Contribution of dispersal phase 1 and phase 2 to SDE
We calculated the proportion of fruits removed from plant canopy by primary dispersers as described above (“Fruit crop size and seed fate”). The contribution of ants to dispersal was calculated as the sum of: (1) the product of the proportion of fruits removed by ants in removal experiments by the proportion of crop size that falls as viable diaspores under the parent plant, and (2) the product of the proportion of seeds removed from the bird feces in removal experiments by ants times the proportion of diaspores dispersed by birds.
We constructed a path diagram (see Culot et al. 2015) that incorporated the transition probabilities from seeds to 1-year-old seedlings, with respect to the combined effects of dispersal (birds, phase 1; ants, phase 2), seed deposition location, probability of seed predation by granivores, effect of seed treatment on germination and 1-year seedling survival (Fig. 4). We estimated the number of seeds dispersed in phase 1 or phase 2 and at the deposition location (beneath or away from the parental plant) as described above (fruit crop size and seed fate). We calculated separate probabilities of seed loss to granivores by taking the mean difference between control and cage-paired treatments in removal experiments performed beneath and away from parental plants. We assumed that all diaspores removed on the ground by vertebrates were preyed on because seeds as small as those of E. ambiguum are likely to be eaten rather than dispersed by rodents (Vieira et al. 2003). We also considered all diaspores removed by the leaf-cutter ant A. sexdens as preyed on because of the low likelihood of survival of seeds removed by this ant species (see Christianini and Oliveira 2009). The mean seed loss to rodents found in diaspore-removal experiments away from the parental plant was used as a measure of seed predation of those seeds embedded in bird droppings. Estimates for the appropriate part are indicated above and in Table 1 and Fig. 4.
We compared the proportion of fruits removed by birds (phase 1) and the relative contribution of ants (phase 2) at the same plant by the Mann–Whitney U-test. The potential contribution of phase 1 to SDE was calculated as the number of 1-year seedlings that would have been produced without phase 2 dispersers (i.e., probability of occurrence of phase 2 = 0), following Culot et al. (2015). The contribution of phase 2 was obtained by the difference between the number of seedlings produced in the field (i.e., after phase 1 and phase 2) and the contribution of phase 1 alone (Culot et al. 2015).
Our approach has two shortcomings. First, we broadly evaluated the role of two types of vectors (birds and ants) but disregarded possible differences among species within each type. For instance, we assume that the effect of seed passage through bird gut is similar among species, which may not be true (see Traveset et al. 2001). However, all bird species observed visiting E. ambiguum are typical frugivores that behave as seed dispersers. Second, removal experiments were run for 24 h, a time span adequate for capturing most diaspore removal by ants (Christianini and Oliveira 2010) but which likely underestimates long-term rodent seed predation. Thus, our results should be interpreted with caution.
Results
Fruit crop and seed fate
Erythroxylum ambiguum produced 381 ± 329 fruits (mean ± SD; n = 11) in a fruiting season. Despite a low visitation rate (see below), birds removed 25.9 ± 12.3 % of the total fruit crop (Table 1). Most of the fruit crop (57.4 ± 16.1 %) fell under the parent plant as ripe intact fruits. They were followed by ripe fruits dropped by birds evidenced by bill marks in the fleshy pulp (Table 1). On average, 5.0 ± 11.2 % of the fruit crop was wasted as unripe fruit, and 2.2 ± 2.7 % had seeds preyed upon. Approximately 67 % of the fruits reached the ground ripe and undamaged (sum of fallen ripe diaspores and dropped by birds) and could germinate or be rescued from beneath the parental plant by secondary dispersal agents (Table 1).
Plant-frugivore interactions in the crown: dispersal phase 1
Only birds (12 species) fed on E. ambiguum fruits, comprising a low visitation rate of 0.06 visits/10 h along both seasons (data for all species pooled). Birds of variable body sizes ate the fruits, from Ilicura militaris (13 g), Turdus albicollis (67 g), Celeus flavescens (166 g) to Ramphastos dicolorus (500 g) (Fig. 1). Each visitor remained in the fruiting plant an average of 36 ± 42 s (n = 25). A bird fed on 3.6 ± 2.3 fruits per visit (range 2–10; n = 25). Although some fruit was dropped beneath the parental plant by birds that only consumed the fruit’s fleshy portion, 90 % of the fruits were swallowed whole and the seeds thereafter were moved away. Estimates of seed displacement from the fruiting plants by birds were 11.4 ± 6.8 m (n = 18) based on the first landing perch.
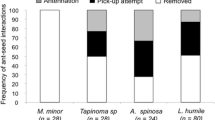
Interactions of frugivorous birds (a) and ants (b) with diaspores of Erythroxylum ambiguum in the plant crown and on the forest floor, respectively, in an Atlantic rainforest in southeastern Brazil. Birds act as primary dispersers by removing seeds from the plant canopy, and ants interact with fallen fruits/seeds dropped to the forest floor by birds or after fruit fall
Ant-diaspore interaction on the ground: dispersal phase 2
We recorded a total of 20 ant species attending E. ambiguum fruits on the forest floor (Fig. 1). Ant attendance followed three patterns: displacement of the diaspore to the nest by a single large ant or with the aid of recruited nestmates; recruitment of nestmates and local consumption of the pulp without removal of the diaspore by small ants; and inspection and handling of the diaspore without displacing it, irrespective of ant body size. Only ants performing diaspore removal (the poneromorphs Pachycondyla striata, Ectatomma edentatum, Odontomachus meinerti, Gnamptogenys striatula and the leaf-cutter ant Atta sexdens) may rescue seeds fallen beneath parent plants. These ants accounted for a total of 22.7 % of the records and displaced diaspores to 0.91 ± 0.93 m (n = 24). No ant nest was located beneath a parental plant. A. sexdens may behave as a seed predator, but they were responsible for only 8.3 % of diaspore removal by ants. Ants removed 62 % of seeds embedded in bird feces within 24 h. Thus, ants were able to quickly reshape a substantial amount of the seed shadow generated during phase 1.
Exclosure experiments revealed that diaspores outside the cages (control treatments) had higher removal rates than diaspores in exclosures, indicating that vertebrates (possibly rodents) played a role in the removal of fallen diaspores (Table 2; Fig. 2). There was an effect of location (higher removal away than beneath a conspecific) and no effect of type of diaspore (whole fruit or cleaned seed) on the removal rates (Table 2). All two-way interactions were significant (Table 2). The difference in removal rates between control and exclosure treatments increased from beneath to away from conspecifics (Fig. 2). This difference was driven mainly by higher removal of cleaned seeds compared to whole fruits (Fig. 2). The three-way interaction was not significant. The overall mean difference between the amount of diaspores removed from cage treatments and controls, irrespective of place of deposition, suggests that rodents removed ca. 26.9 % of the diaspores. Overall, post-dispersal seed losses to granivores were estimated as 35.2 %, the sum of losses to rodents (26.9 %) and to A. sexdens (8.3 %).
Removal of diaspores of E. ambiguum by ants and vertebrates on the floor of an Atlantic rainforest in southeastern Brazil. Exclosure treatments were accessible to ants only, whereas the paired open controls were accessible to ants and vertebrates. Two types of diaspores were used: whole fruits (black bars), and cleaned seeds without the fleshy portion (white bars). Data are the number of diaspores removed out of five placed in paired treatments beneath the canopy (a) and outside the canopy (b) in the 2012–2013 fruiting season (n = 26; mean ± SE)
Effects of birds and ants on seed germination
Seed passage through a bird’s gut and handling by ants increased the germination percentage of seeds (both treatments with 100 % of germinated seeds) compared to whole fruits (control 1, 50 %) and hand-cleaned seeds (control 2, 43.8 %) (G = 59.97; df = 3; P < 0.001; Fig. 3). Likewise, bird-swallowed and ant-handled seeds germinated faster than whole fruits or cleaned seeds (Kolmogorov–Smirnov test, both D = 0.87; P < 0.001). The germination speed of bird-dispersed and ant-dispersed seeds did not differ (D = 0.02; P = 0.98). Neither germination percentage (G = 0.25; df = 1; P = 0.62) nor germination speed (D = 0.22; P = 0.23) differed between whole fruits and cleaned seeds (Fig. 3).
Seed germination after treatment by different vectors of dispersal. Data display the cumulative frequency of germination (germination speed) of seeds from E. ambiguum: removed from bird droppings of captive Turdus albicollis and Turdus leucomelas (birds); from refuse dumps of a captive colony of Pachycondyla striata (ants); from whole fruits coated by fruit pulp (control 1); from seeds with the fleshy portion removed manually by us (control 2). Each treatment has n = 32 seeds
Seedling distribution and survival
In the experiment comparing seedling distribution in 0.5 × 0.5-m plots near ant nests and in paired controls we recorded E. ambiguum seedlings growing only on plots set on ant nest mounds (0.57 ± 0.87 seedlings/0.25 m2, n = 23; W = 28; P = 0.020). The survival of these seedlings growing in ant nests was compared to those seedlings found away from nests in the 100 × 2-m quadrats. The 1-year survival rate of seedlings growing on nest mounds (83 %) was higher than that of seedlings growing away from ant nests (63 %) (Peto and Peto’s generalized Wilcoxon, χ 2 = 4; P = 0.046). Among the 105 seedlings sampled away from ant nests, 50 grew beneath the canopy of a conspecific E. ambiguum and had lower survival (44 %) than the 55 seedlings established away from a conspecific canopy (78 %) (Peto and Peto’s logrank test, χ 2 = 14.8, df = 1, P < 0.001). The greater the distance from the parent plant, the larger the probability of seedling survival (logistic regression, χ 2 = 6; df = 1; P = 0.02) as given by the model: Probability of survival = [exp(0.1554 + 0.3442 × x)]/{1 + [exp(0.1554 + 0.3442 × x)]}, where x is the distance from nearest reproductive plant in m.
Contribution of dispersal phase 1 and phase 2 to SDE
We found no difference between the relative importance of birds (phase 1) and ants (phase 2) in the number of seeds dispersed per E. ambiguum tree (Mann–Whitney U test, Z = 0.689; n = 11; P = 0.49), the quantitative component of SDE (Table 1). A summary of the contribution of birds and ants to plant regeneration up to 1-year-old seedlings is shown in Fig. 4. Considering 100 seeds produced by an E. ambiguum and excluding the effect of phase 2, birds (phase 1) could potentially contribute to the production of up to 12.8 one-year-old seedlings (considering only seeds dispersed away). This figure increases to 14.3 seedlings if seeds dropped by birds beneath parental plants are included. Ants (phase 2) would add 12.2 one-year-old seedlings produced from seeds dispersed away in phase 1. This number increases to 18.7 seedlings when considering the effect of the rescuing of fallen/bird-dropped seeds under parental fruiting plants. Overall, the joint contribution of birds and ants to SDE enabled the production of ca. 20.3 one-year-old seedlings per 100 seeds produced (Fig. 4). Therefore, diplochory enabled an increase of six seedlings to each 100 seeds produced, corresponding to a 42 % increment in the number of seedlings produced in relation to what could be attained if seed dispersal was performed only by phase 1.
The contribution of phase 1 and phase 2 of dispersal performed by birds and ants, respectively, to 1-year-old seedling recruitment of E. ambiguum in an Atlantic rainforest site in southeastern Brazil. The starting point is 100 % of the crop size in the canopy, from which on average 25.9 % is removed by birds, 9.5 % is dropped by birds beneath the plant crown and 57.4 % fall to the ground as ripe fruit. The numbers of seeds and seedlings that reach each subsequent stage are depicted inside boxes based on a hypothetical crop size of 100 seeds. Numbers outside boxes indicate transition probabilities between subsequent stages based on observations in the field and experiments. Plant regeneration stages more directly influenced by birds and ants are in blue and pink boxes, respectively. There may be small differences in the sum of values due to rounding up. See Table 1 and text for details
Discussion
This study provides quantitative evidence that diplochory substantially increases the SDE of Erythroxylum ambiguum by covering several consecutive stages of dispersal in the same system. Considering broadly each vector of dispersal, birds (phase 1) seem to be more consistent in their effects on SDE than ants (phase 2). For instance, most bird-diaspore interactions in a plant crown provide a reliable chance of primary seed dispersal away from plant canopy: 11 (92 %) out of 12 bird species consistently carried away most fruit they interacted with (Fig. 1). In contrast, only five (25 %) out of 20 ant species carried fallen diaspores to ant nests, a behavior that provides the best set of benefits to plant regeneration (Giladi 2006; Rico-Gray and Oliveira 2007; Warren and Giladi 2014). Most ant-diaspore interactions resulted in the collection of liquids and removal of the fleshy portion, with the seed remaining at the spot (Fig. 1). These types of interactions are common between ants and fallen non-myrmecochorous diaspores, in which there are few opportunities for resource monopolization by dominant ants and a small subset of the ant community provides most benefits to the plants (Christianini et al. 2012 and references therein).
The costs of phase 1 dispersal seem to be small because birds do not prey on seeds, and most bird visitors are effective dispersers by removing seeds. Moreover, seeds that naturally fall or are dropped by birds under parents are not wasted and may be rescued by ants (Christianini and Oliveira 2009). Partially eaten fruits are more attractive to seed-rescuing ants than are intact fruits (Bieber et al. 2013), which enhances the chance of subsequent removal by ants and decreases the potential costs of phase 1. Although seed removal by granivorous ants (such as Atta sexdens) was quite low, the costs of phase 2 are likely higher than the costs of phase 1 because not all ants provide displacement of seeds, and seeds cleaned at the spot attracted more vertebrate seed predators than did fruits. Higher predation of cleaned seeds of E. ambiguum by rodents may be favored by the release of toxic compounds in fruit pulp that deter the consumption of pulp-embedded seeds by mammals (Colodel et al. 2004). The presence of toxic compounds in fruit pulp may explain the absence of fruit removal by frugivorous mammals during phase 1 dispersal. Fruit pulp often plays more roles than attraction and reward for frugivorous animals that perform seed dispersal, such as serving as physical and chemical barriers against predators and pathogens (Fedriani and Delibes 2013 and references therein). Nevertheless, the participation of ants in a further step of dispersal clearly surpasses the costs of increased granivory of cleaned seeds, as indicated by the higher number of seedlings produced after two subsequent phases of dispersal.
It is uncommon to find plant species producing fleshy fruits with high lipid content in the pulp (Jordano 2000), but high lipid content is correlated with higher seed removal by birds (Stiles 1993). The chemical composition of lipid-rich bird-dispersed diaspores resembles that of the elaiosomes from true myrmecochores (Pizo and Oliveira 2001). Fallen diaspores of non-myrmecochorous plants producing lipid-rich fruits, similar to that found in E. ambiguum, often benefit from an enhanced chance of subsequent interactions with a high-quality seed-dispersing ant guild represented by carnivore/scavenger poneromorphs that track lipid-rich food (Pizo and Oliveira 2001; Passos and Oliveira 2002; Christianini and Oliveira 2010; Christianini et al. 2012; Leal et al. 2014; Warren and Giladi 2014). These large ants forage individually, quickly discovering and retrieving seeds to their nests, traits that qualify them as effective dispersers (Warren and Giladi 2014). Four of the five ants that removed the fallen diaspores of E. ambiguum at CBSP were poneromorphs. Secondary seed dispersal by these ants often increases the likelihood of seed survival and germination (Christianini et al. 2012 and references therein). From the ant’s perspective, the development of ant larvae is enhanced when ant colonies are fed with lipid-rich bird-dispersed diaspores (Bottcher and Oliveira 2014). Thus, high lipid content in fruit pulp may facilitate the inclusion of ants in a second step of dispersal because it prompts subsequent interactions between certain plant and ant taxa following bird-plant interactions. In a scenario like CBSP, a plant lineage that acquires traits that encourage dispersal by ants in phase 2 would present a selective advantage over lineages relying only on phase 1 (Fig. 4). The presentation of lipid-rich diaspores may thus be an evolutionary adaptation to both primary and secondary dispersers.
Contrary to expectations based on the morphological traits of the diaspore, birds and ants had the same quantitative importance in the seed dispersal of E. ambiguum. Detailed studies have found similar results for other plant species producing lipid-rich diaspores in the Atlantic Forest (Clusia criuva) and cerrado (Xylopia aromatica) in Brazil (Passos and Oliveira 2002; Christianini and Oliveira 2010) and in deciduous forests in Madagascar (Commiphora guillaumini) (Böhning-Gaese et al. 1999). Taken together these results highlight an overlooked quantitative role of ants in the seed dispersal of lipid-rich non-myrmecochorous plants in these tropical biomes. The participation of birds and ants in subsequent steps of dispersal enables an increase of virtually 100 % in the number of E. ambiguum seeds dispersed away compared to the amount attained if only birds participated. This should decrease dispersal limitation, a common constraint for plant recruitment (Clark et al. 2007). Large animals often have more important roles in long-distance dispersal than small animals (Jordano et al. 2007). In our system birds are able to deliver seeds at much larger spatial scales than ants. Seed dispersal away from conspecifics clearly enhances E. ambiguum seedling survival. The benefits of phase 1 seem to be concentrated in providing seeds with a higher chance of escaping from mortality near the parental plant and the ability to colonize new sites, as predicted by Vander Wall and Longland (2004). Ants may be more important in short-scale processes that operate within habitats and populations after seeds reach the ground, such as directed dispersal (Wenny 2001).
Although birds and ants provide different treatments to the seed, the effect on seed germination was remarkably similar because birds and ants truly enhanced the germination of E. ambiguum compared to controls. The results of the experiments could not be explained by the release from inhibitors of germination and high osmotic pressure in the fruit pulp that would prevent germination (Samuels and Levey 2005). Passage through the bird gut may trigger germination after physical or chemical abrasion of the seed coat (Traveset et al. 2001), whereas seeds manipulated by ants may benefit from a lower risk of pathogen attack due to the antibiotic properties of the ant’s cuticular glands (Ohkawara and Akino 2005). We do not know if ants are able to scarify the seeds of E. ambiguum while removing fruit pulp. Anyway, higher and faster germination is likely advantageous in that it decreases seed exposure to predators and pathogens.
The benefits to seedling establishment provided by ants are probably not derived from the higher nutrient content of the soils in ant nests because there was no difference in the macro- and micronutrient content in the soils from the ant nests and controls in CBSP (P. H. S. A. Camargo, unpublished data). However, large poneromorph ants are known to decrease the insect herbivory of seedlings growing around their nests (Passos and Oliveira 2004), which may explain the higher survival of seedlings near ant nests. Herbivory is one of the main factors that drives seedling mortality worldwide (Moles and Westoby 2004). Nests of large-sized poneromorphs last longer than those of smaller ants (A. V. Christianini, personal observation), which may allow for the long-term protection of seedlings against insect herbivores. Beneficial effects on seedling emergence and establishment seem to be the usual contribution of phase 2 dispersal, as seen in studies in which beetles (Culot et al. 2015; Pérez-Ramos et al. 2013), rodents (Vander Wall et al. 2005; Vander Wall 2008; Briggs et al. 2009) and ants (Passos and Oliveira 2002; Christianini and Oliveira 2010; but see Böhning-Gaese et al. 1999) participate in phase 2. Therefore, phase 2 would provide a fine-tuned dispersal mechanism after the coarse long-distance dispersal in phase 1, targeting seeds to specific microsites where recruitment is more likely to be successful, i.e., directed dispersal (Vander Wall and Longland 2004; Briggs et al. 2009; Christianini and Oliveira 2010; Leal et al. 2014).
Birds and ants clearly play complementary roles in the successive stages of seed dispersal and plant regeneration of E. ambiguum (see also Christianini and Oliveira 2010). However, the massive defaunation of vertebrate frugivores around the world (Dirzo et al. 2014) suggests that the relative importance of ants is likely to increase for the regeneration of many plants that share vertebrates and ants as seed dispersal vectors (Christianini et al. 2014). Although ants undoubtedly improve the SDE of E. ambiguum, if the plant relies only on ants for regeneration, one should expect severe decreases in SDE and truncation in seedling regeneration toward short distances from parental plants. This would be a clear disadvantage compared to a double-step dispersal process (diplochory) with birds. However, the role of ants in rescuing fallen seeds below parental plants may buffer to some extent those diplochorous plants against the negative consequences of decreases of phase 1 seed dispersers.
References
Andersen E, Feer F (2005) The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In: Forget PM, Lambert J, Hulme P, Vander Wall S (eds) Seed fate: predation, dispersal and seedling establishment. CABI International, Wallingford, pp 331–349
Bieber AGD, Silva PSD, Oliveira PS (2013) Attractiveness of fallen fleshy fruits to ants depends on previous handling by frugivores. Écoscience 20:85–89. doi:10.2980/20-1-3573
Böhning-Gaese K, Gaese BH, Rabemanantsoa SB (1999) Importance of primary and secondary seed dispersal in the Malagasy tree Commiphora guillaumini. Ecology 80:821–832. doi:10.1890/0012-9658(1999)080[0821:IOPASS]2.0.CO;2
Bottcher C, Oliveira PS (2014) Consumption of lipid-rich seed arils improves larval development in a Neotropical primarily carnivorous ant, Odontomachus chelifer (Ponerinae). J Trop Ecol 30:621–624. doi:10.1017/S0266467414000479
Briggs JS, Vander Wall SB, Jenkins SH (2009) Forest rodents provide directed dispersal of Jeffrey pine seeds. Ecology 90:675–687. doi:10.1890/07-0542.1
Calviño-Cancela M, Martín-Herrero J (2009) Effectiveness of a varied assemblage of seed dispersers of a fleshy-fruited plant. Ecology 90:3503–3515. doi:10.1890/08-1629.1
Christianini AV, Oliveira PS (2009) The relevance of ants as seed rescuers of a primarily bird-dispersed tree in Neotropical cerrado savanna. Oecologia 160:735–745. doi:10.1007/s00442-009-1349-2
Christianini AV, Oliveira PS (2010) Birds and ants provide complementary seed dispersal in a Neotropical savanna. J Ecol 98:573–582. doi:10.1111/j.1365-2745.2010.01653.x
Christianini AV, Mayhé-Nunes AJ, Oliveira PS (2012) Exploitation of fallen diaspores by ants: are there ant–plant partner choices? Biotropica 44:360–367. doi:10.1111/j.1744-7429.2011.00822.x
Christianini AV, Oliveira PS, Bruna EM, Vasconcelos HL (2014) Fauna in decline: meek shall inherit. Science 345:1129. doi:10.1126/science.345.6201.1129-a
Clark CJ, Poulsen JR, Levey DJ, Osenberg CW (2007) Are plant populations seed limited? A critique and meta-analysis of seed addition experiments. Am Nat 170:128–142. doi:10.1086/518565
Colodel EM, Seitz AL, Schmitz M, Borba MR, Raymundo DL, Driemeier D (2004) Intoxicação por Eryhtroxylum deciduum (Erythroxylaceae) em ovinos. Pesq Vet Bras 24:165–168. doi:10.1590/S0100-736X2004000300009
Connell JH (1971) On the role of natural enemies in preventing competitive exclusion in some marine animal and in rainforest trees. In: Den Boen PJ, Gradwell PR (eds) Dynamics of populations. Pudoc, Wageningen, pp 298–312
Culot L, Huynen MC, Heymann EW (2015) Partitioning the relative contribution of one-phase and two-phase seed dispersal when evaluating seed dispersal effectiveness. Methods Ecol Evol 6:178–186. doi:10.1111/2041-210X.12317
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the Anthropocene. Science 345:401–406. doi:10.1126/science.1251817
Fedriani JM, Delibes M (2013) Pulp feeders alter plant interactions with subsequent animal associates. J Ecol 101:1581–1588. doi:10.1111/1365-2745.12146
Forget PM, Lambert JE, Hulme PE, Vander Wall SB (2004) Seed fate: predation, dispersal and seedling establishment. CABI International, Wallingford
Fundação Florestal (2008) Parque Estadual Carlos Botelho: Plano de manejo. Fundação Florestal, São Paulo
Giladi I (2006) Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112:481–492. doi:10.1111/j.0030-1299.2006.14258.x
González-Castro A, Calviño-Cancela M, Nogales M (2015) Comparing seed dispersal effectiveness by frugivores at community level. Ecology 96:808–818. doi:10.1890/14-0655.1
Gotelli NJ, Ellison AM (2011) Princípios de estatística em ecologia. Artmed, Porto Alegre
Gove AD, Majer JD, Dunn RR (2007) A keystone ant species promotes seed dispersal in “diffuse” mutualism. Oecologia 153:687–697. doi:10.1007/s00442-007-0756-5
Hampe A (2004) Extensive hydrocory uncouples spatiotemporal patterns of seedfall and seedling recruitment in a ‘bird-dispersed’ riparian tree. J Ecol 92:797–807. doi:10.1111/j.0022-0477.2004.00918.x
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Syst 13:201–228. doi:10.1146/annurev.es.13.110182.001221
Janzen DH (1970) Herbivores and the number of trees species in tropical forests. Am Nat 104:501–528. doi:10.1086/282687
Jordano P (2000) Fruits and Frugivory. In: Fenner M (ed) Seeds: the ecology of regeneration in plant communities. CABI International, Wallingford, pp 125–166
Jordano P, Schupp EW (2000) Seed disperser effectiveness: the quantity component and patterns of seed rain for Prunus mahaleb. Ecol Monogr 70:591–615. doi:10.1890/0012-9615(2000)070[0591:SDETQC]2.0.CO;2
Jordano P, García C, Godoy JA, García-Castaño JL (2007) Differential contribution of frugivores to complex seed dispersal patterns. Proc Natl Acad Sci USA 104:3278–3282. doi:10.1073/pnas.0606793104
Leal LC, Lima Neto MC, Oliveira AFM, Andersen AN, Leal IR (2014) Myrmecochores can target high-quality disperser ants: variation in elaiosome traits and ant preferences for myrmecochorous Euphorbiaceae in Brazilian Caatinga. Oecologia 174:493–500. doi:10.1007/s00442-013-2789-2
Lengyel S, Gove AD, Latimer AM, Majer JD, Dunn RR (2009) Ants sow the seeds of global diversification in flowering plants. PLoS One 4:e5480. doi:10.1371/journal.pone.0005480
Lima RAF, Dittrich VAO, Souza VC, Salino A, Breier TB, Aguiar OT (2011) Flora vascular do Parque Estadual Carlos Botelho, São Paulo, Brasil. Biota Neotrop 11:173–214. doi:10.1590/S1676-06032011000400018
Lomáscolo SB, Levey DJ, Kimball RT, Bolker BM, Alborn HT (2010) Dispersers shape fruit diversity in Ficus (Moraceae). Proc Natl Acad Sci USA 107:14668–14672. doi:10.1073/pnas.1008773107
Moles AT, Westoby M (2004) What do seedlings die from and what are the implications for the evolution of seed size? Oikos 106:193–199. doi:10.1111/j.0030-1299.2004.13101.x
Nathan R (2006) Long-distance dispersal of plants. Science 313:786–788. doi:10.1126/science.1124975
Ohkawara K, Akino R (2005) Seed cleaning behavior by tropical ants and its anti-fungal effect. J Ethol 23:93–98. doi:10.1007/s10164-004-0132-4
Passos L, Oliveira PS (2002) Ants affect the distribution and performance of seedlings of Clusia criuva, a primarily bird-dispersed rain forest tree. J Ecol 90:517–528. doi:10.1046/j.1365-2745.2002.00687.x
Passos L, Oliveira PS (2004) Interaction between ants and fruits of Guapira opposita (Nyctaginaceae) in a Brazilian sandy plain rainforest: ant effects on seeds and seedlings. Oecologia 139:376–382. doi:10.1007/s00442-004-1531-5
Pérez-Ramos IM, Verdú JR, Numa C, Marañón T, Lobo JM (2013) The comparative effectiveness of rodents and dung beetles as local seed dispersers in Mediterranean oak forests. PLoS One 8:e77197. doi:10.1371/journal.pone.0077197
Pizo MA, Oliveira PS (2001) Size and lipid content of nonmyrmecochorous diaspores: effects on the interaction with litter-foraging ants in the Atlantic rain forest of Brazil. Plant Ecol 157:37–52. doi:10.1023/A:1013735305100
Pyke DA, Thompson JN (1986) Statistical analysis of survival and removal rate experiments. Ecology 67:240–245. doi:10.2307/1938523
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna. http://www.R-project.org/> Accessed 4 May 2013
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant–plant interactions. University of Chicago Press, Chicago
Samuels IA, Levey DJ (2005) Effects of gut passage on seed germination: do experiments answer the questions they ask? Funct Ecol 19:365–368. doi:10.1111/j.1365-2435.2005.00973.x
Schupp EW (1993) Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio 107(108):15–29. doi:10.1007/BF00052209
Schupp EW, Jordano P, Gómez JM (2010) Seed dispersal effectiveness revisited: a conceptual review. New Phytol 188:333–353. doi:10.1111/j.1469-8137.2010.03402.x
Stiles EW (1993) The influence of pulp lipids on fruit preference by birds. Vegetatio 107/108:227–235. doi:10.1007/BF00052225
Traveset A, Riera N, Mas RE (2001) Passage through bird guts causes interspecific differences in seed germination characteristics. Funct Ecol 15:669–675. doi:10.1046/j.0269-8463.2001.00561.x
Vander Wall SB (2008) On the relative contributions of wind vs. animals to seed dispersal of four sierra Nevada Pines. Ecology 89:1837–1849. doi:10.1890/07-0409.1
Vander Wall SB, Longland WS (2004) Diplochory: are two seed dispersers better than one? Trends Ecol Evol 19:155–161. doi:10.1016/j.tree.2003.12.004
Vander Wall SB, Kuhn KM, Gworek JR (2005) Two-phase seed dispersal: linking the effects of frugivorous birds and seed-caching rodents. Oecologia 145:282–287. doi:10.1007/s00442-005-0125-1
Vieira EM, Pizo MA, Izar P (2003) Fruit and seed exploitation by small rodents of the Brazilian Atlantic forest. Mammalia 67:533–539. doi:10.1515/mamm-2003-0407
Wang BC, Smith TB (2002) Closing the seed dispersal loop. Trends Ecol Evol 17:379–385. doi:10.1016/S0169-5347(02)02541-7
Warren RJ II, Giladi I (2014) Ant-mediated seed dispersal: a few ant species (Hymenoptera: Formicidae) benefit many plants. Myrmecol News 20:129–140
Wenny DG (2001) Advantages of seed dispersal: a re-evaluation of directed dispersal. Evol Ecol Res 3:51–74
Acknowledgments
This article is part of the thesis of P. H. S. A. C. funded by the Conselho Nacional de Pesquisa (CNPq 478938/2011-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. We thank M. A. Pizo and A. J. Piratelli for criticism. Comments from E. W. Schupp and an anonymous referee helped us to improve the manuscript. We thank S. M. Rodrigues and A. J. Piratelli for sharing data on bird frugivory, F. F. M. Capelo for plant identification, A. J. Raszl for chemical analysis, and R. F. Mantezi, A. S. F. Silva and the Zoológico Municipal de Sorocaba for the facilities to perform the bird feeding trials. We thank the Fundação Florestal de São Paulo and the staff of CBSP for the research license and logistic support. We are grateful to B. J. Lopes, D. J. Moreno, J. P. Line, J. Lima, L. M. S. A. Camargo, S. Z. T. Amancio and V. M. Silva for help during fieldwork.
Author contribution statement
A. V. C., M. M. M. and P. H. S. A. C. designed the experiments. P. H. S. A. C. performed the experiments. R. M. F. identified the ants. P. H. S. A. C. and A. V. C. analyzed the data. A. V. C., P. H. S. A. C., M. M. M. and R. M. F. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Communicated by Moshe Inbar.
Rights and permissions
About this article
Cite this article
Camargo, P.H.S.A., Martins, M.M., Feitosa, R.M. et al. Bird and ant synergy increases the seed dispersal effectiveness of an ornithochoric shrub. Oecologia 181, 507–518 (2016). https://doi.org/10.1007/s00442-016-3571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3571-z