Abstract
Frugivorous birds disperse the seeds of many fruit-bearing plants, but the fate of seeds after defecation or regurgitation is often unknown. Some rodents gather and scatter hoard seeds, and some of these may be overlooked, germinate, and establish plants. We show that these two disparate modes of seed dispersal are linked in some plants. Rodents removed large (>25 mg) seeds from simulated bird feces (pseudofeces) at rates of 8–50%/day and scatter hoarded them in soil. Ants (Formica sibylla) also harvested some seeds and carried them to their nests. Rodents carried seeds 2.5±3.2 m to cache sites (maximum 12 m) and buried seeds at 8±7 mm depth. Enclosure studies suggest that yellow pine chipmunks (Tamias amoenus) and deer mice (Peromyscus maniculatus) made the caches. In spring, some seeds germinated from rodent caches and established seedlings, but no seedlings established directly from pseudofeces. This form of two-phase seed dispersal is important because each phase offers different benefits to plants. Frugivory by birds permits relatively long-range dispersal and potential colonization of new sites, whereas rodent caching moves seeds from exposed, low-quality sites (bird feces on the ground surface) to a soil environment that may help maintain seed viability and promote successful seedling establishment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Frugivory by birds coupled with elimination of seeds in feces is a well-known means of plant dispersal (Wenny and Levey 1998; Loiselle and Blake 1999; Herrera 2002), but postdispersal fate of seeds in bird feces has received little study (e.g., Herrera et al. 1994; Wang and Smith 2002). Fecal deposits are often unfavorable establishment sites for seedlings because seeds can be deposited on an inhospitable surface, exposed to seed predators, fungi, or harsh abiotic conditions (desiccation, variation in temperature, ultraviolet light, etc.; De Steven and Putz 1984; Sallabanks and Courtney 1992; Normah et al. 1997; Lambert 2002). Feces are often rich in seeds, so if germination occurs, competition can be intense (Howe 1989). Successful establishment of seedlings is often associated with the movement of seeds away from the point of deposition, either down into the soil or horizontally across the soil surface (Sheldon 1974; van Tooren 1988). It is generally assumed that successful seeds are those that avoid detection by animals (Janzen 1982a, b; LoGiudice and Ostfeld 2002; Moles et al. 2003) and get buried in soil or plant litter by abiotic processes (van Tooren 1988; Chambers et al. 1991).
In the tropics, animals are important secondary dispersers of seeds in feces. Ants remove seeds from bird feces and carry them to their nutrient-rich nests where some seeds germinate and establish plants (Roberts and Heithaus 1986; Davidson 1988; Kaufmann et al. 1991; Kaspari 1993; Levey and Byrne 1993; Bohning-Gaese et al. 1999; Passos and Oliveira 2002; Pizo et al. 2005). In recent years, dung beetles have been found to move mammal dung and its embedded seeds for short distances to nesting or feeding sites (Estrada and Coates-Estrada 1991; Andresen 2001, 2002). A portion of this dung is buried at a depth that permits seedling emergence and establishment. And rodents are known to remove seeds from mammal feces and scatter hoard them in soil (Forget and Milleron 1991; Wenny 1999; Feer and Forget 2002). These secondary seed movements often result in patterns of seedling recruitment that are quite different from the patterns of primary seed dispersal generated by frugivorous animals (Schupp 1995; Rey and Alcantara 2000).
In temperate ecosystems, secondary movement of seeds from feces has not received much attention. Those that have monitored seed removal often assume that removed seeds had been consumed (e.g., LoGiudice and Ostfeld 2002). We tested the idea that rodents in temperate forests secondarily disperse seeds initially ingested and dispersed by birds (primary dispersers).
Bird feces are amenable to experimental manipulation to follow the harvest and secondary movements of seeds. The feces of omnivorous, frugivorous birds, such as the American robin (Turdus migratorius), is a combination of partially digested food (often containing viable seeds) from the bird’s digestive tract and nitrogenous wastes (primarily uric acid) from the urinary system (Singer 2003). These two components of feces merge in the cloaca before elimination. The uric acid forms a white precipitate that partially encloses the dark-colored material from the digestive tract. In this study, we created pseudofeces by placing seeds labeled with the radioisotope sandium-46 in a suspension of uric acid. The pseudofeces had an appearance and chemical composition similar to that of real bird feces, and the radioisotope permitted us to monitor the presence of seeds in feces and track seed movements away from fecal deposits.
We tested five hypotheses regarding the behavior of rodents that encounter bird feces that contain seeds. First, rodents remove seeds from bird feces. Second, rodents cache some of the seeds removed from bird feces (i.e., removed seeds are secondarily dispersed). Third, large seeds are more likely to be secondarily dispersed than smaller seeds. Fourth, white bird feces attract foraging rodents. And fifth, the rate of seed removal from feces is independent of the availability of alternative foods.
2 Materials and methods
We conducted this study in the Whittell Forest, a facility of the University of Nevada System, in the Carson Range ≈30 km south of Reno, NV (39°15′35′′N, 119°52′35′′W, 1,970 m). The main study site is a 0.25 ha plot of open Jeffrey pine (Pinus jeffreyi) with an understory of antelope bitterbrush (Purshia tridentata) on decomposed granitic soils. The climate is semiarid with hot, dry summers and winters with snow accumulations of ≈1 m.
We assumed that seed size would play an important role in determining whether rodents would remove seeds from feces, so we selected fruit with a broad range of seed sizes. We gathered the fruits of seven common plants that grow on the east slope of the Sierra Nevada that are eaten by songbirds (Table 1), and we extracted and cleaned the seeds. We labeled the seeds with scandium-46, a gamma-emitting radionuclide (half-life of 84.5 day) (Vander Wall 2000). In the field, we established 140 stations in a 10 × 14 grid with 3-m spacing. Twenty stations were assigned to each seed species in a randomized block design. We created pseudofeces by placing seeds on ≈0.5 ml of uric acid (Sigma Chemical Inc.) suspension on the ground, and adding an additional 0.5 ml of uric acid to cover the seeds. Most seeds were totally obscured by the uric acid. Number of seeds per fecal deposit varied with seed size such that the mass of seeds per deposit was similar: Prunus emarginatus (2 seeds), Rhamnus rubra (2 seeds), Cornus stolonifera (3 seeds), Amelanchier pallida (4 seeds), Rosa woodsii (10 seeds), Ribes nevadense (20 seeds), Sambucus cerulea (20 seeds). The number of seeds per fecal deposit was probably within the range seen in the feces of wild, frugivorous birds, but sometimes large seeds (e.g., Prunus) are regurgitated rather than passed through the digestive tract. We established the grid on 15 August 2003 and checked it eight times at irregular intervals until 18 October. During each visit, we checked all pseudofeces and searched for caches using a Geiger counter on the grid and ≈5 m zone around the grid. On 2 and 19 May 2004, we resurveyed all fecal deposits and cache sites to locate emergent seedlings.
To determine whether uric acid influenced the foraging activity of rodents, we established a seed removal transect (Vander Wall 1994) of 100 stations (50 with uric acid pseudofeces alternating with 50 without uric acid) with 5-m spacing using Rhamnus seeds. We established the transect at a nearby site, monitored it over 3 days (31 August–2 September 2003) and recorded seed removal. Three weeks later (20–22 September), we repeated the study in the midst of a large Jeffrey pine seed fall to test the effect of background seed density on the rate of seed removal from pseudofeces.
During July 2004, we tested the response of isolated yellow pine chipmunks (Tamias amoenus; n=7) and deer mice (Peromyscus maniculatus; n=3) to fecal deposits containing either Rhamnus or Prunus seeds inside 10×10 m rodent-proof enclosures. The enclosure had wire mesh walls ≈70 cm high topped with aluminum flashing. The wire mesh extended ≈50 cm into the ground to prevent rodents from burrowing in or out of the enclosure. We placed 16 pseudofeces containing two radioactive seeds (8 fecal deposits for each species) identical to those used in 2003 in a 4×4 grid with ≈2-m spacing. After 24 h, we removed the rodent and surveyed the enclosure to locate cached and eaten seeds. We provided water but not food to rodents during trials.
We used survival analysis (Proc Lifereg in SAS, 2000, Alison 1995) to test for differences in the rates of removal of different species of seeds from pseudofeces. We used interval censoring and a Weibull distribution (Allison 1995). We used fecal deposits (not seeds) as the response variable, and required that at least half of the seeds be taken from a deposit before considering that fecal deposit to be depleted by animals.
3 Results
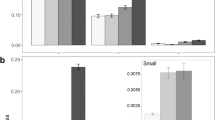
Rodents removed the four largest (> 25 mg; Table 1) seed species from bird pseudofeces (Fig. 1). Rhamnus seeds disappeared the fastest (50.0%/day) while the three other large-seeded species were removed at rates between 8.2%/day and 11.7%/day. Survival analysis showed that rodents removed Rhamnus seeds 6.9 times faster than Prunus seeds (X 2=35.82, P<0.0001) and that rodents removed Amelanchier seeds 1.9 times faster than Cornus seeds (X 2=6.64, P<0.01). Prunus seeds disappeared at rates similar to Cornus and Amelanchier seeds (P>0.25 for both comparisons). The three smallest species of seeds (<12 mg; Table 1) were not gathered from pseudofeces by rodents, but rodents appeared to have examined >60% of pseudofeces containing these small seeds.
Seeds within pseudofeces were not always taken at the same time. Prunus stones in a fecal deposit were taken during different visits at least 54.3% of the time. The percentages for Cornus, Rhamnus, and Amelanchier were at least 41.3%, 30.0%, and 8.1%, respectively. This suggests that rodents made repeated visits to fecal deposits to find seeds.
We found 38 rodent caches containing 52 seeds (1.4 seeds/cache), accounting for 27.1% of the 192 large seeds taken from feces. Caches were 2.5±3.2 m (mean±1 SD) away from the nearest fecal deposit of that seed species (minimum secondary dispersal distances; Fig. 2). Burial depths were 7.8±7.0 mm (range=1–40 mm), probably within the range of depths suitable for germination and emergence. We found 14 Prunus caches (48.6% of seeds taken from feces), eight Rhamnus caches (22.5%), 13 Cornus caches (44.4%) and three Amelanchier caches (9.6%).
To determine which species of rodents were making caches, we offered Prunus and Rhamnus seeds embedded in pseudofeces to yellow pine chipmunks and deer mice isolated in field enclosures. One chipmunk and two mice removed seeds from pseudofeces during these short trials. The chipmunk made two Prunus and three Rhamnus caches, and the two deer mice made four Prunus and four Rhamnus caches. These caches were similar to those made in the field experiment where rodents had free access to pseudofeces.
Ants (Formica sibylla) removed seeds from at least 14 fecal deposits. Seeds taken by ants included eight Rhamnus and several of the smaller species: two Amelanchier, six Rosa, and two Sambucus. Most of these seeds were carried deep into ant nests, but one Sambucus and two Amelanchier seeds were found discarded and partially buried just outside nests. None of them successfully germinated the following spring.
Autumn rains eroded pseudofeces and exposed small seeds. Some of the Rosa (31 seeds; 15.5% of those in feces), Ribes (29 seeds, 7.3%), and Sambucus (12 seeds; 3.0%) seeds were scattered up to 10 cm (mean±1 SD=2.1±1.5 cm) from the fecal deposit by rain drops. Soil disturbances (e.g., digging by rodents) near 33 of 60 fecal deposits with small seeds further scattered seeds and partially buried seeds and feces. By spring, we could not relocate any of these seeds, suggesting that they had become buried, eaten by seed predators, or dispersed farther away from the pseudofeces.
Uric acid precipitate in bird feces is conspicuous and may act as a signal of potential seed availability to foraging rodents. Alternatively, uric acid is a mild irritant and may deter foraging rodents. We tested for these effects along transects using Rhamnus seeds with and without pseudofeces. Animals removed Rhamnus seeds on the ground (without pseudofeces) at a rate similar to seeds in pseudofeces (51%/day vs. 54%/day, respectively; X 2=1.82, P=0.117), indicating that the uric acid neither discourages nor attracts foragers.
Availability of alternative foods did not influence rate of removal of seeds from pseudofeces. Rodents removed Rhamnus seeds from pseudofeces at a similar rate (61%/day vs. 54%/day, respectively; X 2=0.18, P=0.671) whether or not there was a rich supply of highly preferred Jeffrey pine (Pinus jeffreyi) seeds (Vander Wall 1995) available. This suggests that animals do not resort to removing seeds from feces only when other foods are unavailable.
By spring, most uric acid had dissolved and pseudofeces were difficult to distinguish. Among the 60 fecal deposits that contained seeds in autumn, only one produced a single Sambucus seedling, which died within several days. However, three Rhamnus caches (37.5%) produced germinants. In addition, 15 caches (39%; seven Prunus caches, six Cornus caches and two Rhamnus caches) contained dormant, apparently viable seeds.
4 Discussion
Rodents removed relatively large seeds (>25 mg) from simulated bird feces quickly, and they cached many of those seeds in soil. Using field enclosures, we demonstrated that yellow pine chipmunks and deer mice remove seeds from pseudofeces and cache them in a manner similar to that observed in the field experiment. Furthermore, the caches that rodents made in the field experiment were similar to those made by yellow pine chipmunks and deer mice in earlier studies (Vander Wall 2000; Vander Wall et al. 2001). Because these and related species of Tamias and Peromyscus are common throughout a large portion of temperate North America, we suspect that this interaction between frugivorous birds and seed-caching rodents is probably far more common than currently appreciated.
Similar patterns of seed removal have been found in other studies. Kollmann et al. (1998) monitored the removal of 12 seed species from Petri dishes by rodents in Germany and southern England and found that Prunus spp. seeds disappeared faster than Cornus sanguinea and Rosa fruticosus seeds, and that Sambucus nigra seeds were among the species with the slowest removal rates. However, they assumed that the removed seed had been consumed even though the larger seeds (Prunus and Cornus) might have been scatter-hoarded.
Many birds regurgitate or spit out large seeds rather than pass them through their digestive tract (Meyer and Witmer 1998; Witmer 1998). Our result from the seed removal transects reveals that the rate of removal of seeds in pseudofeces is not different from the seeds unassociated with uric acid, suggesting that seeds regurgitated by birds are likely to be removed from the ground surface just as quickly as those that are defecated by birds. Feer and Forget (2002) also found that mammal feces did not influence the rate of seed removal by rodents.
Rodents ignored small species of seeds. Abiotic factors (rain and wind) moved Rosa, Ribes, and Sambucus seeds a few centimeters across the ground. In addition, animals inadvertently disturbed the ground near pseudofeces, causing burial of many seeds. Sheldon (1974) and van Tooren (1988) have made similar observations. Ants moved some of the smaller seeds short distances, but we did not collect sufficient data to determine whether they might have a positive impact on seedling recruitment.
In this study, recruitment was poor for all seed species whether or not they were removed from pseudofeces by animals. One reason for this was that our study plot was a poor habitat for five of the seven plant species tested (only Rosa woodsii grew on our study plot and Rhamnus rubra occurred in similar habitat). The other five plant species occur in more mesic habitats in shaded lodgepole pine forests or along riparian corridors. If we had conducted our study in a more mesic habitat, we probably would have had more seeds germinate from pseudofeces and rodent caches.
This study demonstrates that some of the larger seeds in bird feces can experience two distinct phases of seed dispersal (i.e., diplochory). Each of these phases can offer different benefits to plants (Vander Wall and Longland 2004). Phase one or primary dispersal by frugivorous birds has the potential to colonize new areas (Willson 1993; Gibson and Wheelwright 1995) and helps seeds escape potentially high density-dependent seed mortality near the parent plant (Janzen 1970). Birds typically damage or destroy only a small fraction of the seeds they ingest, and passage of the seeds through the bird’s digestive tract can facilitate germination (Yagihashi et al. 1998; Traveset et al. 2001). However, seeds are deposited on the ground surface where they are vulnerable to postdispersal seed predators, extreme physical conditions, and where germination failure can be high. Rodents are important postdispersal predators of seeds, but they also scatter hoard many seeds (phase-two dispersal). Caching by rodents does not move seeds far relative to that achieved by bird dispersal, so the colonization of new patches is unlikely to be an important benefit of phase-two dispersal. However, caching places seeds in conditions that often favor seedling establishment. Once cached, seeds are relatively safe from other sources of seed mortality such as ants, beetles, and birds that act as seed predators. Two-phase seed dispersal has the potential to increase the overall effectiveness of seed dispersal over any single means of seed dispersal (Vander Wall and Longland 2004).
A measure of the complexity of ecological communities is the nature and frequency of species interactions. The two-phase seed dispersal syndrome described here is a novel form of species interaction that will probably prove to be more common in temperate regions than currently recognized, and, once identified and understood, these species interactions are likely to help explain the patterns of species diversity and how species persist in nature. For example, realizing that some plant propagules are dispersed in two or more discrete phases may help us understand how seeds survive and produce seedlings once they reach a suitable habitat. A deeper appreciation of complex seed dispersal systems will help us understand better the functioning of ecological communities and the selective forces (e.g., how differing selection by primary dispersers, secondary dispersers, and the abiotic environment interact to influence the evolution of seed size) acting on fruit and seed characteristics.
References
Allison PD (1995) Survival analysis using the SAS system: a practical guide. SAS Institute Inc, Cary
Andresen E (2001) Effects of dung presence, dung amount and secondary dispersal by dung beetles on the fate of Micropholis guyanensis (Sapotaceae) seeds in Central Amazonia. J Trop Ecol 17:61–78
Andresen E (2002) Dung beetles in a Central Amazonian rainforest and their ecological role as secondary seed dispersers. Ecol Ent 27:257–270
Bohning-Gaese K, Gaese BH, Rabemanantsoa SB (1999) Importance of primary and secondary seed dispersal in the Malagasy tree Commiphora guillaumini. Ecology 80:821–832
Chambers JC, MacMahon JA, Haefner JH (1991) Seed entrapment in alpine ecosystems: effects of soil particle size and diaspore morphology. Ecology 72:1668–1677
Davidson DW (1988) Ecological studies of neotropical ant gardens. Ecology 69:1138–1152
De Steven D, Putz FE (1984) Impact of mammals on early recruitment of a tropical canopy tree, Dipteryx panamensis, in Panama. Oikos 43:207–216
Estrada A, Coates-Estrada R (1991) Howler monkey (Alouatta palliata), dung beetles (Scarabaeidae) and seed dispersal: ecological interactions in the tropcial rain forest of Los Tuxtlas, Mexico. J Trop Ecol 7:459–474
Feer F, Forget P-M (2002) Spatio-temperal variation in post-dispersal seed fate. Biotropica 34:555–566
Forget P-M, Milleron T (1991) Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87:596–599
Gibson JP, Wheelwright NT (1995) Genetic structure in a population of a tropical tree Ocotea tenera (Lauraceae): influence of avian seed dispersal. Oecologia 103:49–54
Herrera CM (2002) Seed dispersal by vertebrates. Plant–animal interaction: an evolutionary approach. Blackwell, Padstow, Cornwall, pp 185–208
Herrera CM, Jordano P, Lopez-Soria L, Amat JA (1994) Recruitment of a mast-fruiting, bird-dispersed tree: bridging frugivore activity and seedling establishment. Ecol Monogr 64:315–344
Howe HF (1989) Scatter- and clump-dispersal and seedling demography: hypothesis and implications. Oecologia 79:417–426
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Janzen DH (1982a) Attraction of Liomys mice to horse dung and the extinction of this response. Anim Behav 30:483–489
Janzen DH (1982b) Removal of seeds from horse dung by tropical rodents: influence of habitat and amount of dung. Ecology 63:1887–1900
Jensen SP (1993) Temporal changes in food preferences of wood mice (Apodemus sylvaticus L). Oecologia 94:76–82
Kaspari M (1993) Removal of seeds from neotropical frugivore droppings: ant responses to seed number. Oecologia 95:81–88
Kaufmann S, McKey DB, Hossaert-McKey M, Horvitz CC (1991) Adaptations for a two-phase seed dispersal system involving vertebrates and ants in a hemiepiphytic fig (Ficus microcarpa: Moraceae). Am J Bot 78:971–977
Kollmann J, Coomes DA, White SM (1998) Consistencies in post-dispersal seed predation of temperate fleshy-fruited species among seasons, years and sites. Funct Ecol 12:683–690
Lambert JE (2002) Exploring the link between animal frugivory and plant strategies: the case of primate fruit-processing and post-dispersal seed fate. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CAB International, Wallingford, pp 365–379
Levey DJ, Byrne MM (1993) Complex ant-plant interactions: rain forest ants as secondary dispersers and post-dispersal seed predators. Ecology 74:1802–1812
LoGiudice K, Ostfeld RS (2002) Interactions between mammals and trees: predation on mammal-dispersed seeds and the effect of ambient food. Oecologia 130:420–425
Loiselle BA, Blake JG (1999) Dispersal of Melastome seeds by fruit-eating birds of tropical forest understory. Ecology 80:330–336
Meyer GA, Witmer MC (1998) Influence of seed processing by frugivorous birds on germination success of three North American shrubs. Am Midl Nat 140:129–139
Moles AT, Warton DI, Westoby M (2003) Do small-seeded species have higher survival through seed predation than large-seeded species? Ecology 84:3148–3161
Normah MN, Ramiya SD, Gintangga M (1997) Desiccation sensitivity of recalcitrant seeds - A study on tropical fruit species. Seed Sci Res 7:179–183
Passos L, Oliveira PS (2002) Ants affect the distribution and performance of seedlings of Clusia criuva, a primarily bird-dispersed rain forest tree. J Ecol 90:517–528
Pizo MA, Passos L, Oliveira PS (2005) Ants as secondary seed dispersers of vertebrate-dispersed diaspores in Brazilian Atlantic forests. In: Forget P-M, Lambert J, Hulme P, Vander Wall SB (eds) Seed fates: predation, dispersal and seedling establishment. CABI Publishing, Wallingford
Rey PJ, Alcantara JM (2000) Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. J Ecol 88:622–633
Roberts JT, Heithaus ER (1986) Ants rearrange the vertebrate-generated seed shadow of a Neotropical fig tree. Ecology 67:1046–1051
Sallabanks R, Courtney SP (1992) Frugivory, seed predation, and insect-vertebrate interactions. Annu Rev Entomol 37:377–400
Schupp EW (1995) Seed-seedling conflicts, habitat choice, and patterns of plant recruitment. Am J Bot 82:399–409
Sheldon JC (1974) The behavior of seeds in soil: III The influence of seed morphology and the behaviour of seedlings on the establishment of plants from surface-lying seeds. J Ecol 62:47–66
Singer, MA (2003) Dietary protein-induced changes in excretory function: a general animal design feature. Comp Biochem Physiol B Biochem Mol Biol 136:785–801
van Tooren BF (1988) The fate of seeds after dispersal in chalk grassland: the role of the bryophyte layer. Oikos 53:41–48
Traveset A, Riera N, Mas RE (2001) Passage through bird guts causes interspecific differences in seed germination characteristics. Funct Ecol 15:669–675
Vander Wall SB (1994) Removal of wind-dispersed pine seeds by ground-foraging vertebrates. Oikos 69:125–132
Vander Wall SB (1995) The effects of seed value on the caching behavior of yellow pine chipmunks. Oikos 74:533–537
Vander Wall SB (2000) The influence of environmental conditions on cache recovery and cache pilferage by yellow pine chipmunks (Tamias amoenus) and deer mice (Peromyscus maniculatus). Behav Ecol 11:544–549
Vander Wall SB, Longland WS (2004) Diplochory: are two seed dispersers better than one. Trends Ecol Evol 19:155–161
Vander Wall SB, Thayer TC, Hodge JS, Beck MJ, Roth JK (2001) Scatter-hoarding behavior of deer mice (Peromyscus maniculatus). West N Am Nat 61:109–113
Wang BC, Smith TB (2002) Closing the seed dispersal loop. Trends Ecol Evol 17:379–385
Wenny DG (1999) Two-stage dispersal of Guarea glabra and G kunthiana (Meliaceae) in Monteverde, Costa Rica. J Trop Ecol 15:481–496
Wenny DG, Levey DJ (1998) Directed seed dispersal by bellbirds in a tropical cloud forest. Proc Natl Acad Sci USA 95:6204–6207
Willson MF (1993) Dispersal mode, seed shadows, and colonization patterns. Vegetatio 107/108:261–280
Witmer MC (1998) Do seeds hinder digestive processing of fruit pulp? Implications for plant/frugivore mutualisms. Auk 115:319–326
Yagihashi T, Hayashida M, Miyamoto T (1998) Effects of bird ingestion on seed germination of Sorbus commixta. Oecologia 114:209–212
Acknowledgements
We thank S. Agosta and E. Meyer for their help in the field. J. Briggs and M. Beck helped us with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Craig Osenberg
Rights and permissions
About this article
Cite this article
Vander Wall, S.B., Kuhn, K.M. & Gworek, J.R. Two-phase seed dispersal: linking the effects of frugivorous birds and seed-caching rodents. Oecologia 145, 281–286 (2005). https://doi.org/10.1007/s00442-005-0125-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0125-1