Abstract
Successful reproduction in a seasonal environment can be accomplished with resources that are stored before use (“capital resources”) or resources that are used immediately (“income resources”). Research examining capital versus income resource usage during reproduction has primarily focused on assigning species to positions along a capital–income gradient. Here, we examine the causes and reproductive consequences of among and within-year variation in hoarded capital versus income resource usage by female North American red squirrels (Tamiasciurus hudsonicus) during mid-lactation in a highly seasonal environment. Among years, the proportion of feeding events that were on capital resources (PROPCAP) averaged 39 % during the yearly median mid-lactation periods, but ranged widely between 2 and 100 %. In years with earlier parturition dates, females primarily used hoarded capital resources during mid-lactation, whereas in years with later parturition dates, females primarily used income resources during mid-lactation. Within years, PROPCAP during mid-lactation tended to be greater in early-breeding females than in late-breeding females. Rates of water flux in females during mid-lactation provided further evidence that late-breeding females used more water-rich income resources. The proportion of litters that were partially or completely lost, and the litter mass that lactating females supported, was not influenced by the large among-year differences in hoarded capital resource usage. Red squirrels appear to delay reproduction following years with low cone production to time peak reproductive demands to be late enough to be supported by income resources that only become available later in the season. In conclusion, our results offer a rare example of the capacity of a food-hoarding mammal to support reproduction exploiting a wide range of capital and income resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful reproduction requires nutrient (reviewed in Meijer and Drent 1999; Picciano 2003) and energy resources (Millar 1978; Gittleman and Thompson 1988; Kenagy et al. 1989; Hammond and Diamond 1992; Speakman and McQueenie 1996). Generally, there are two types of resources that can be used to support reproduction—“capital” and “income” resources (Jönsson 1997; Bonnet et al. 1998; Stephens et al. 2009). In the case of capital resources, there is a delay between resource acquisition and use. During the delay, the acquired resources are stored either as on-body reserves (e.g. Diller and Wallace 1984; Costa et al. 1986; Sénéchal et al. 2011a) or as food hoards (Yosef and Pinshow 1989; Vander Wall 1990; Wauters et al. 1995). In the case of income resources, acquired resources are used immediately to support reproduction (Boyd 1998; Meijer and Drent 1999; Henry et al. 2002). Not surprisingly, the extent to which animals support reproduction on capital versus income resources is linked to life-history traits, including the annual initiation and the timing of breeding (Jönsson 1997; Bonnet et al. 1998; Boyd 2000; Alerstam 2006), and aspects of parental investment and care (Schulz and Bowen 2005; Houston et al. 2007).
Many studies have focused on defining whether species are capital versus income breeders. Among ectotherms, capital breeding may reflect the rule rather than the exception (Bonnet et al. 1998; but see Warner et al. 2008). Among endotherms, marine mammals that fast on land throughout lactation are generally considered to be the only pure capital breeders (Bonner 1984; Boness and Bowen 1996; Schulz and Bowen 2005). Common Eiders (Somateria mollissima) and other arctic breeding birds were previously considered to be pure capital breeders relying on capital resources accumulated from their previous habitats to support egg production (Ryder 1970; Ankney and MacInnes 1978; Drent and Daan 1980; Parker and Holm 1990; Meijer and Drent 1999). However, more recent research has demonstrated that these species also rely on income resources for egg production (Klaassen et al. 2001; Gauthier et al. 2003; Sénéchal et al. 2011a). It is now clear that pure capital breeders are rare, and thus most species that rely to some extent on capital resources during reproduction in fact use mixed-strategies relying on a combination of capital and income resources (Mainguy and Thomas 1985; Choinière and Gauthier 1995; Ganter and Cooke 1996; Meijer and Drent 1999; Bowen et al. 2001; Gauthier et al. 2003).
Continued research on species using mixed-strategies is demonstrating that it is difficult to define capital versus income resource usage within species because the capital–income mix varies among and within years. Examinations of within-species variation in capital versus income reliance have focused more on within-year rather than among-year variation (Gauthier et al. 2003; Morrison and Hobson 2004; Warner et al. 2008; Wheatley et al. 2008; Sénéchal et al. 2011a). Moreover, these studies have almost exclusively examined species that store capital as on-body reserves, whereas there are few studies on species that store capital as hoarded food (but see Samelius et al. 2007 for a non-reproductive example).
The reproductive success of species breeding in seasonal environments is influenced by the mix of capital and income resources used to support reproduction. In seasonal environments, early breeding is generally associated with increased reproductive success (the majority of this research has been on birds; e.g. Klomp 1970; Perrins 1970; Lepage et al. 2000; Drent 2006; but see McAdam and Boutin 2003; Réale et al. 2003 for research on North American red squirrels, Tamiasciurus hudsonicus; henceforth, squirrels). Theoretical models and empirical studies using indirect estimates of capital accumulation in birds suggest that large capital reserves allow individuals to breed earlier, prior to the appearance of seasonal resources (Drent and Daan 1980; Rowe et al. 1994; Bêty et al. 2003; Sénéchal et al. 2011b; reviewed in Drent 2006; see Varpe et al. 2009 for a non-avian model). This body of research also suggests that individuals with smaller capital reserves must either reduce their reproductive investment and/or delay reproduction to when income resources become seasonally available. Interestingly, studies that have assessed the diet of arctic nesting birds have found, counterintuitively, that late-breeding individuals have a higher proportion of capital resources in their eggs than early-breeding individuals (Gauthier et al. 2003; Sénéchal et al. 2011a). Overall, there is a lack of research on non-avian species that incorporates the causes and reproductive consequences of within-species variation in capital versus income resource usage during reproduction.
At first glance, squirrels in south-western Yukon, Canada, are capital breeders that support reproduction on hoarded white spruce (Picea glauca) seeds (Humphries and Boutin 2000; Boutin et al. 2006; McAdam et al. 2007). Squirrels at this site hoard white spruce cones on their year-round food-based territories in late summer and autumn for later consumption of the seed over winter (squirrels are non-hibernators) and the following spring/summer (Fletcher et al. 2010). White spruce cone production in autumn of a given year is strongly correlated with the timing of squirrel reproduction the following spring, with large cone crops correlated with earlier reproduction the following year (Boutin et al. 2006). This suggests that squirrels are capital breeders that depend on accumulated cone hoards to support reproduction. However, squirrels also hoard mushrooms and feed on a variety of income resources, including spruce buds in late-winter/spring, as well as fresh cones, fresh mushrooms, hypogeous fungi (truffles), berries, flowers, tree catkins, snowshoe hare (Lepus americanus) leverets, and birds’ eggs in spring/summer (Layne 1954; Smith 1968; O’Donoghue 1994; Steele 1998; Willson et al. 2003). Moreover, estimates of the amount of cones that squirrels hoard in our population suggest that squirrels must consume a large proportion of their hoarded resources over winter, prior to breeding (Fletcher et al. 2010).
Here, we examined the causes and reproductive consequences of within- and among-year variation in capital–income reliance by squirrels during mid-lactation in a highly seasonal environment. Specifically, we examined whether the contribution of capital resources during mid-lactation differs among and within years according to variation in the timing of reproduction. If squirrels are strict capital breeders, the diets of lactating females should be dominated by hoarded food in all years, independent of reproductive timing. On the other hand, if squirrels are facultative capital breeders, in years following low cone production when reproduction is delayed (Boutin et al. 2006), lactating squirrels should rely less on capital than in years following high cone production when reproduction is earlier. We also examined correlations between the contribution of capital resources during mid-lactation and reproductive parameters, such as the occurrence of litter loss and the litter mass supported by lactating females.
Three attributes of squirrel natural history made this research possible. First, their dependency on masting conifer species in a highly seasonal environment introduces substantial phenological variation in the timing of reproduction (Boutin et al. 2006; Fletcher et al. 2010). Second, squirrels are highly visible while foraging, which allows direct and simultaneous observations of income acquisition (i.e. consumption of fresh non-hoarded food) and capital depletion (i.e. hoard consumption). Third, our ability to count, mark, and track the growth of juveniles prior to weaning allows us to address questions related to reproductive parameters.
Materials and methods
Study population
We examined a free-ranging population of squirrels between 1990 and 2010, in the Kluane region of south-western Yukon, Canada (60°57′N, 138°2′W). Our study area was in a glacial valley composed of boreal forest dominated by white spruce (P. glauca), with an understory of willow (Salix spp.), interspersed with stands of trembling aspen (Populus tremuloides) and some open meadows (Krebs and Boonstra 2001). Snowmelt typically occurs in early-May and the growing season is from mid-May through mid-August (Krebs and Boonstra 2001). This population has been the subject of long-term research, and further details of the study sites, research methodology, and this population’s ecology and evolutionary biology are published elsewhere (Boutin et al. 2006; McAdam et al. 2007). Briefly, squirrels were examined on two ~40-ha study sites, bisected by the Alaska Highway, where all individuals were individually marked with ear-tags (Monel #1). Immigrant juvenile and adult squirrels to our population were also ear-tagged during systematic live-trapping censuses.
Squirrel reproduction
We tracked the reproduction of adult females following standardized protocols (Boutin et al. 2006; McAdam et al. 2007). All females on the two study sites were repeatedly live-trapped to track the development of pregnancy. Radio-telemetry was used to locate each female’s nest as soon as she gave birth, and all the pups were temporarily removed, sexed, weighed, and individually marked by ear-notches. The parturition date of each litter was based on the relationship between mass and age derived from known-aged pups (S. Boutin, A.G. McAdam, and M.M. Humphries, unpublished data). Litters were removed from our analyses if the pups had grown to be too large to obtain an accurate litter parturition date estimate (i.e. one pup >25 g). Pups were temporarily removed from their nest a second time at ~25 days postpartum to be ear-tagged (Monel #1) and re-weighed to calculate pup growth rates. The period between parturition and 25 days postpartum is an important survival bottleneck in this population (McAdam et al. 2007). We estimated the mass of each pup at 25 days postpartum based on its growth rate and the amount of days between when the pup would have been 25 days postpartum and when it was removed from its natal nest for the second time. Then, we summed the estimated masses of all pups at 25 days postpartum to obtain an estimate of female reproductive investment. Litters were removed from the analysis examining litter mass at 25 days postpartum if we could not accurately estimate the growth rate of each pup in the litter. This occurred for three possible reasons: (1) not all pups were found when pups were first removed from their nest, (2) not all pups survived until when they were removed from their nest the second time, (3) the interval between the first and second time pups were removed from their nest was <5 days. A litter was completely lost when all juveniles died prior to ear-tagging (McAdam et al. 2007), whereas a litter was partially lost when at least one juvenile in the litter was missing from the nest, and thus was assumed dead, at ~25 days postpartum. Litters were removed from the complete and partial litter loss analyses if the lactating female died prior to when the pups were temporarily removed from their nest a second time.
Capital resource availability
Squirrels feed on the seeds within white spruce cones in the year that they are produced and also hoard spruce cones in the autumn as capital resources to be used in the subsequent winter and during the spring/summer of the following year (Steele 1998; Fletcher et al. 2010). We quantified white spruce cone availability each year before squirrels began hoarding cones (McAdam et al. 2007; Fletcher et al. 2010). We counted all cones visible on one side of the top 3 m of approximately 80 regularly spaced trees on each of the two study sites, ln(x + 1) transformed the counts for each tree, and averaged the ln(x + 1) transformed values to determine an index of cone availability on each study site (henceforth cone index; Boutin et al. 2006; LaMontagne and Boutin 2007, 2009).
Squirrels also feed on and hoard epigeous and hypogeous fungi (Smith 1968; henceforth mushrooms). At our study site, mushrooms become available for squirrels in late-June. We quantified the yearly availability of mushrooms on one of the study sites in all years between 1993 and 2010 prior to when squirrels started hoarding mushrooms. The index of mushroom availability was the ln(x + 1) transformed average of all the mushroom biomass (g wet/10 m2) within a 3-m radius of ≥80 regularly spaced stakes in the core of the study site (henceforth mushroom index; Krebs et al. 2008). Past research at this site suggests that the mushroom index from this one study area also reflects mushroom production throughout the study region because mushroom production is spatially autocorrelated over large distances in this region (Krebs et al. 2008; Fletcher et al. 2010).
Feeding events and food items
We recorded the items that individual non-lactating and lactating female squirrels fed on during 17,882 feeding events in all years between 1990 and 2010 (excluding 1998, see below). The items squirrels feed on can be visually identified because squirrels habituate to observation (Smith 1968) and the tree canopy is low and open at our study site. The average number of feeding events within each year was 894.1 ± 122.8 (SE). We excluded 1998 from the analysis because only 27 feeding events were observed in this year. Overall, we observed feeding events over a period of 29 weeks, between 1 March and 26 September, but 82 % of feeding events were observed between 10 May and 15 August. All feeding events were associated with known individuals, of known reproductive statuses, because all squirrels had unique combinations of colored wires or discs attached to their ear-tags that could be seen using binoculars even if squirrels were feeding in the trees. Overall, we recorded 6,957 feeding events from lactating females (between parturition and 70 days postpartum) and 10,925 feeding events from non-lactating females (including females of all ages). Feeding events were obtained from opportunistic encounters with individually marked squirrels (33 %) and from focused observation periods on specific individual squirrels when all feeding events were recorded (67 %; see also Dantzer et al. 2010). The significance of all relationships and the qualitative interpretations remained unchanged if we only analysed feeding events from opportunistic encounters with individually marked squirrels or from focused observation periods on specific individual squirrels when all feeding events were recorded (results not shown).
The items that individual squirrels fed on were grouped into seven categories. Category details and tallies of feeding events on these items from non-lactating and lactating females are presented in Table S1. The first two categories included feeding on spruce seeds coming from: (1) cones produced in the current year (henceforth, fresh cones), and (2) cones that were produced in previous years that were hoarded by squirrels (henceforth, hoarded cones). The difference between fresh and hoarded cones can be distinguished in the field based on cone color (Nienstaedt and Zasada 1990). The third and fourth categories included feeding on: (1) mushrooms produced in the current year (henceforth, fresh mushrooms), and (2) mushrooms that were produced in previous years that were hoarded by squirrels (henceforth, hoarded mushrooms). Unfortunately, we did not differentiate between feeding on fresh versus hoarded mushrooms in our long-term field protocol. Instead, we differentiated between feeding on fresh and hoarded mushrooms in our analyses based on the transition of feeding on these two types of mushrooms in a subset of observations in 2009 when feeding on fresh and hoarded mushrooms were recorded separately (n = 195, 18 March–13 August). In 2009, we did not observe feeding on fresh mushrooms prior to 18 June, but following this date, 96 % of the mushroom feeding events were on fresh mushrooms (88/92; Fig. S1). To reflect this transition, we fit a logistic function to the 2009 data (x axis: date; y axis: hoarded mushrooms = 0 and fresh mushrooms = 1). Then, we used the logistic line of best fit, which reflects the proportion of mushroom feeding events on a given day that were on fresh mushrooms, to assign the mushroom feeding events in all study years to be on either hoarded or fresh mushrooms (see Fig. S1 for further details). Henceforth, hoarded cones and hoarded mushrooms will be referred to as capital resources, whereas all other food items are considered income resources. The fifth category was feeding on vegetative as well as undifferentiated and differentiated reproductive white spruce buds (vegetative and reproductive buds were not differentiated in the field). The sixth category was feeding on white spruce witches broom rust caused by the fungus Chrysomyxa arctostaphyli. A seventh category of food items called “other” incorporated all remaining food items. This category included nine food items outlined in Table S1.
In 2009, we collected samples of most primary food items consumed by squirrels to quantify percent water content, crude protein content (% dry weight), and energy content (kcal/g dry weight).
Diets during mid-lactation
Although feeding events from non-lactating and lactating females are presented in Fig. 1, the main focus of our study was the feeding behaviour of females experiencing mid-lactation. Squirrels wean offspring at 70 days postpartum (S. Boutin, A.G. McAdam, M.M., Humphries, unpublished data; McAdam et al. 2007), thus the mid-lactation date is 35 days postpartum. In order for a feeding event to be considered to be from a female experiencing mid-lactation, the feeding event needed to be collected 15 days on either side of the females mid-lactation date (between 20 and 50 days postpartum). This selection criteria excludes early lactation when energy demands are less (Johnson et al. 2001) and late lactation when juveniles start supplementing their diets with fresh foods (S. Boutin, A.G. McAdam, M.M., Humphries, unpublished data). Of the 6,957 feeding events we recorded on lactating females, 4,216 were recorded from females experiencing mid-lactation and 2,741 were recorded from females outside of mid-lactation (see Table S1). All analyses below include only the 4,216 feeding events recorded from females experiencing mid-lactation.
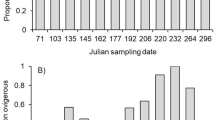
The proportion of feeding events on seven dietary items within weekly intervals pooling non-lactating and lactating female red squirrels (Tamiasciurus hudsonicus) across all study years (lower panel). In the lower panel, the white-filled bars are capital resources (hoarded cones and mushrooms) and the top grey-filled bars are income resources. The upper panel histogram reflects the number of females reaching mid-lactation during the same weekly intervals as in the lower panel in all study years. The upper panel line represents weekly climate normal average temperatures (1967–2008) measured at the nearest Environment Canada weather station (Burwash Landing, Yukon Territory; 61°22′N, 139°03′W)
We determined the diets of females during yearly median mid-lactation periods and for early- and late-breeders within each year. Yearly median mid-lactation periods (yearly median mid-lactation date ±15 days) were calculated using the mid-lactation dates of all females that attempted a litter on our study sites. The diet during the yearly median mid-lactation periods included all feeding events from all females experiencing mid-lactation during this period. The diet of early- and late-breeders within each year included all feeding events prior to, or after, the yearly median mid-lactation date, respectively. The diet during the yearly median mid-lactation periods included feeding events from early- and late-breeders; however, the non-independence of these periods was not problematic for our analyses because the diets during the yearly median mid-lactation period and the diets of early- and late-breeders were examined in separate analyses. To be clear, the diet during the yearly median mid-lactation periods were never compared to the diets of either early- and late-breeders in the same analysis; only the diets of early- and late-breeders were compared in the same analysis. We omitted years from the analysis if we observed <20 feeding events during the yearly median mid-lactation period, or from either early- or late-breeders. On average, the diets of females during the yearly median mid-lactation periods were based on 110 ± 19 feeding events, whereas the diets of early- and late-breeders were based on 124 ± 29 and 167 ± 56 feeding events, respectively.
We used a linear mixed-model analysis to examine how the proportion of feeding events on capital resources, henceforth PROPCAP (arcsine-transformed), was affected by within- and among-year variation in reproductive timing. The within-year effect of reproductive timing on PROPCAP was assessed with a categorical variable specifying early- versus late-breeder feeding events. The among-year effect of reproductive timing on PROPCAP was examined by including the yearly median mid-lactation date (days since 1 January) as a continuous variable. We also included the interaction between the within- and among-year terms to test whether the effect of within-year variation in reproductive timing on PROPCAP was influenced by changes in the among-year mid-lactation date. A random effect of year was included in the model to account for the non-independence of early and late mid-lactation PROPCAP within the same year.
We also used three linear-mixed model analyses to examine how PROPCAP (arcsine-transformed) and within-year variation in reproductive timing (categorical variable: early- versus late-breeder), were correlated with: (1) the proportion of litters that were partially lost (arcsine-transformed), (2) the proportion of litters that were completely lost (arcsine-transformed), and (3) the litter mass at 25 days postpartum. In this analysis, we also included the interaction between PROPCAP and the timing of breeding (i.e. early- versus late-breeder) to determine if differences between early- and late-breeders depended on PROPCAP. Similar to above, a random effect of year was included in these analyses. The significance of fixed effects was tested using likelihood ratio tests comparing nested models (models estimated using maximum likelihood estimation, χ 2 distribution, df = difference in the df between the two nested models). Unless otherwise noted, non-significant interactions and main effects were sequentially removed from the models.
Using water flux to examine seasonal dietary changes
We used the doubly-labelled water (DLW) technique (Lifson et al. 1955; Nagy 1983; Speakman 1997) to quantify the water flux of lactating females near their mid-lactation dates. Briefly, water flux was calculated based on the washout of the 2H isotope over the DLW interval. Blood samples were taken from unlabelled squirrels at the same site to evaluate the background levels of the 2H (Speakman and Racey 1987: method C). Water flux values (mL/day) were calculated as the product of k d (rate constant of the exponential decline of 2H over DLW interval, units = day−1) and the individual’s body water at the initial blood sample based on the dilution of 2H isotope following the DLW injection (i.e. N d as a percent of squirrel mass × squirrel mass prior to DLW injection, units = mL; Lifson and McClintock 1966). We determined the water flux of 145 lactating females between 2003 and 2009. The DLW technique was initiated in lactating females that were 33–47 days postpartum (35–40 days postpartum for 87 % of females) and the duration of the DLW interval ranged between 2 and 5 days. We performed the DLW technique on these individuals primarily to obtain estimates of daily energy expenditure. The daily energy expenditure measures of 90 lactating females in this study are presented in Fletcher et al. (2012). Ninety-six percent of final blood samples were obtained within 2 h of a 24-h interval from the initial blood sample, which controlled for circadian rhythms of activity (Speakman and Racey 1988). Additional DLW field and laboratory techniques are published in Fletcher et al. (2012).
Water flux values generally reflect dietary water content (Kam et al. 1987; Mutze et al. 1991; Degen et al. 1997), but may also reflect water flux resulting from milk export in lactating females (Kurta et al. 1990; Oswald et al. 1993; Degen et al. 2004). To test if levels of water flux in lactating females were correlated with milk export to their pups, we used a linear mixed model to test whether lactating female water flux was correlated with litter mass at 25 days postpartum. In this analysis, we also included random effects of female ID and year, in addition to a fixed effect of lactating female mass (average mass just prior to the DLW injection and at the final blood sample). This analysis included water flux values from a subset of lactating females for which we could calculate litter mass at 25 days postpartum (n = 105).
All analyses were performed using R (v.2.13.1; R Development Core Team 2011). The lme4 library was used for linear-mixed model analyses (Bates and Maechler 2009). The model coefficients (±standard error) presented in the text and tables reflect analyses including transformed independent and dependent variables, whereas raw values are presented in the figures. Alpha was set to 0.05.
Results
Based on all feeding events from non-lactating and lactating females, squirrels were most often observed consuming buds and seeds from cones of white spruce trees, followed by mushrooms, and a variety of other plant and animal foods (Fig. 1; Table S1). The diet of squirrels transitioned from extensive usage of capital resources early in the season, to a predominance of income resources associated with plant growth and reproduction later in the season (Fig. 1). Across all years of observation, the earliest reproducing females reached their mid-lactation dates when capital resources predominated, the latest reproducing females reached their mid-lactation dates when income resources predominated, and the majority of females reached their mid-lactation dates during the capital to income transition (Fig. 1).
Indices of cone and mushroom production in the previous year were strongly predictive of their respective dietary importance during yearly median mid-lactation periods the following year. Specifically, the previous year’s cone index was positively correlated with feeding on hoarded cones during yearly median mid-lactation periods the following year (Table 1a; Fig. 2). In addition, the previous year’s mushroom index was also positively correlated with feeding on hoarded mushrooms during yearly median mid-lactation periods the following year (Table 1b; Fig. 2). The previous year’s mushroom index had no effect on hoarded cone feeding the following year (Table 1a), and the previous year’s cone index had no effect on hoarded mushroom feeding the following year (Table 1b). The indices of these two food items in the previous year also did not interact to influence either cone or mushroom feeding the following year (Table 1b).
The relationship between cone and mushroom indices in the previous year (available to be hoarded by squirrels) and the proportion of feeding events on hoarded cones and hoarded mushrooms, respectively, the following year during the yearly median mid-lactation periods. Lines of best fit were generated from two separate linear regressions between the untransformed proportion of feeding events and the respective indices of food source availability (this differs from Table 1, where proportions were arcsine-transformed)
PROCAP during the yearly median mid-lactation periods averaged 39 % and ranged between 2 and 100 % (individual points not shown, but the dashed line-of-best-fit in Fig. 3 is fitted to these values). In years with later median mid-lactation dates, PROPCAP was reduced (coef. = −1.1e−2 ± 2.0e−3, \(\chi_{1}^{2}\) = 14.9, P = 0.0001; Fig. 3). Moreover, there was a within-year trend for less PROPCAP in late-breeders as compared to early-breeders (\(\chi_{1}^{2}\) = 2.9, P = 0.09; Fig. 3). The difference between late- and early-breeders in PROPCAP was not influenced by among-year variation in median mid-lactation dates (i.e. the between-year and within-year timing variables did not interact: \(\chi_{1}^{2}\) = 0.52, P = 0.47; interaction removed from model).
The relationship between the timing of mid-lactation and the proportion of feeding events on capital resources (i.e. hoarded cones and mushrooms) in the diets of early- and late-breeding squirrels. Each pair of points connected by a solid line represents one study year. Early- and late-breeding squirrels reached mid-lactation (35 days postpartum) prior to, or after, the yearly median mid-lactation date, respectively. The dashed line reflects a linear regression between the yearly median mid-lactation date and the proportion of feeding events on capital resources during the yearly median mid-lactation period
The water, crude protein, and energy content of the main food items consumed by squirrels are presented in Table 2. Spruce seeds and hoarded mushrooms had low water contents (≤15 %), whereas all fresh food items had much higher water contents (fresh mushrooms and spruce needle buds; ≥68 %). Although all these water content values were based on samples collected within 1 year, and there is likely annual variation in water content, we assumed that relative differences between these items (i.e. spruce seed and hoarded mushrooms are drier than fresh mushrooms and spruce needle buds) were consistent across years.
Water flux during mid-lactation increased over the breeding season (Fig. 4; \(\chi_{1}^{2}\) = 13.0, P = 0.0003, including a significant fixed effect of mass: 0.57 ± 0.13; \(\chi_{1}^{2}\) = 17.3, P < 0.0001, and ID and year as random effects). Water flux was unrelated to litter mass at 25 days postpartum (1.38e−2 ± 6.93e−2, \(\chi_{1}^{2}\) = 3.4e−2, P = 0.85; model included fixed effect of mass and random effects of ID and year).
Seasonal change in the water flux of females during mid-lactation calculated using the doubly-labelled water technique. The seven open symbols that appear as outliers were collected on lactating females during a mast year (2005) when late breeding squirrels relied extensively on fresh cones that have low water content relative to other income resources (Table 2). The dashed line of best fit includes all points, whereas the solid line excludes the points from the mast year (i.e. includes only the solid points). The significance of the relationship reported in the text is improved by excluding the points from the mast year (dashed line; \(\chi_{1}^{2}\) = 27.1, P < 0.0001; including mass as a fixed effect and ID and year as random effects)
PROPCAP during mid-lactation was not related to the proportion of litters that were partially lost (\(\chi_{1}^{2}\) = 1.2e−1, P = 0.72; Fig. 5a) or completely lost (\(\chi_{1}^{2}\) = 1.4, P = 0.23; Fig. 5b). Complete litter loss was greater in early-breeders than in late-breeders (coef. = 7.6e−2 ± 3.7e−2, \(\chi_{1}^{2}\) = 3.9, P = 0.0475; Fig. 5b); however, there was no difference between early- and late-breeders in partial litter loss (\(\chi_{1}^{2}\) = 2.3e−1, P = 0.63; Fig. 5a). In both of these analyses, the interactions between PROPCAP and the timing of breeding were non-significant (P > 0.15; interactions removed from the models).
The relationship between the proportion of capital in the diets of early- and late-breeding squirrels during mid-lactation and the a proportion of litters partially lost (at least one juvenile dead prior to ~25 days postpartum), b proportion of litters completely lost (all juveniles dead prior to ~25 days postpartum), and c litter mass at 25 days postpartum. Each pair of points connected by a solid line represents one study year. Early- and late-breeding squirrels reached mid-lactation (35 days postpartum) prior to, or after, the yearly median mid-lactation date, respectively
In the analysis examining litter mass at 25 days postpartum, there was a significant interaction between PROPCAP and the timing of breeding (coef. = −48.6 ± 11.8, \(\chi_{1}^{2}\) = 11.2, P = 0.0008; Fig. 5c). In two separate linear-mixed regression models, PROPCAP was not related to litter mass (\(\chi_{1}^{2}\) = 8.4e−1, P = 0.36) and late-breeders had significantly greater litter masses than early-breeders (coef. = 11.4 ± 5.4, \(\chi_{1}^{2}\) = 3.9, P = 0.049). The interpretation of the significant interaction between PROPCAP and the timing of breeding is that for early- and late-breeders with high levels of PROPCAP, the amount by which the litter mass of late-breeders exceeded that of early-breeders was reduced as compared to early- and late-breeders with low levels of PROPCAP.
Discussion
At the most basic level, our results highlight that squirrels can meet the energetic demands of mid-lactation in response to massively variable environmental conditions. The earliest breeders reached mid-lactation during the week of 5 April, when deep snow would have covered the study site and daily average temperature was −4 °C, whereas median-timed breeders reached mid-lactation during the week of 7 June, when snow was completely absent from the study site for more than a month, and daily average temperature was 14 °C warmer. Much of the variation in reproductive timing was among years, with years following large cone crops characterised by much earlier reproduction than years following small cone crops (Boutin et al. 2006). Nevertheless, within-year variation in reproductive timing was also pronounced—the average difference between the mid-lactation date of the 25th and 75th percentile of breeders averaging all years was 25 days.
Across all years, the diet of females followed the seasonal transition of available resources. During March, approximately three-quarters of females’ diets were hoarded cones and mushrooms, with the remaining quarter consisting of spruce buds. In May, the proportion of spruce buds in the diet of females started to increase to a maximum in early-June. This increase in bud consumption corresponds with the period when the vegetative and reproductive buds of white spruce end their dormancy, vegetative buds have their most rapid elongation, reproductive structures are formed, and pollination occurs (Owens et al. 1977; Owens and Molder 1979). The subsequent June to July decline in white spruce bud consumption coincides with the flushing of white spruce shoots and the initiation of embryo development (Owens et al. 1977; Owens and Molder 1979). Near the peak of bud consumption, squirrels began feeding on additional income resources, such as witches brooms, fresh mushrooms, and fresh cones. Given the water content of income resources far exceeds the water content of hoarded mushrooms and seed from hoarded cones (Table 2), the pronounced increase in the water flux of females during mid-lactation from early May to mid-summer provides additional evidence that the diet of late-breeders included more income and less capital than early-breeders.
The proportion of feeding events on capital resources (i.e. PROPCAP) by squirrels during mid-lactation was correlated with reproductive timing. Within years, early-breeders tended to have higher levels of capital feeding than late-breeders. However, the majority of the variation in reproductive timing and capital feeding was among years, with years with earlier breeding characterised by more capital feeding, as compared to years with later breeding with less capital feeding. We also found that feeding on hoarded cones and mushrooms were positively correlated with indices of cone and mushroom production in the previous year, respectively.
Previous research has generally assumed that species fall at a point along the capital–income continuum (literature reviewed in Stephens et al. 2009). Our finding that PROCAP during the yearly median mid-lactation periods ranged between 2 and 100 % potentially suggests that where a species or a population fits along the capital–income continuum may be more flexible than previously thought. Future research examining among-year variation in capital resource usage during reproduction in both species that store resources as on-body reserves or as food hoards is currently required (e.g. see Gauthier et al. 2003). These data will provide insight regarding whether large variation in capital resource usage among-years is a peculiarity of red squirrels or reflects interesting differences between species that store resources in on-body reserves or in food hoards.
It is important to note that our estimate of feeding on hoarded mushrooms, and thus our estimates of PROPCAP, may have been influenced by how mushroom feeding events were assigned. We assigned feeding events on mushrooms to be either on fresh or hoarded mushrooms in all years based on data from 2009 when these two types of mushrooms were recorded as separate items. Only one mushroom crop is produced during the short growing season at our study site (Krebs and Boonstra 2001; Krebs et al. 2008). Mushrooms generally start becoming available in late-June and they reach their peak availability in mid-July to early-August (C.J. Krebs, personal communication). As a result, we are confident that hoarded rather than fresh mushrooms were primarily consumed prior to mid-July. Based on the water-flux data, we are also confident that the overall proportion of all income sources in the diet of lactating squirrels increased over the breeding season. We are less confident that the transition from feeding on fresh to hoarded mushrooms that we observed in 2009 occurred in all years, potentially causing us to underestimate the proportion of hoarded mushrooms in the diet of squirrels breeding later in the season. However, given that the vast majority of females in our study reached mid-lactation prior to the peak availability of mushrooms suggested by Krebs (mid-July to early August), we feel that our methodology of assigning mushrooms feeding events likely had a negligible effect on our conclusions.
Similar to species that accumulate capital resources as on-body reserves, our results suggest that species that accumulate capital in food hoards delay reproduction to when income resources become seasonally available. In years following large cone crops, squirrels breed earlier and support mid-lactation primarily on hoarded resources because fresh income resources only become available later in the season. Supporting mid-lactation almost entirely on hoarded resources is possible following large cone crops because squirrels hoard more cones in response to larger cone crops (Fletcher et al. 2010), and thus, following winter, individuals have sufficient hoarded resources remaining to support reproduction. In years following small cone crops, when the hoard size of squirrels is more likely to be insufficient to support early reproduction, squirrels reproduce late enough to support reproduction on fresh income resources that only become available later in the season. Warmer air temperatures, leading to reduced thermoregulatory demands and relaxed nest attendance constraints, may be an additional advantage of late season breeding (Becker 1993; Studd 2012). Either or both of these advantages of late breeding presumably operates in opposition to benefits of early breeding related to offspring settlement (McAdam and Boutin 2003; Réale et al. 2003; C.T. Williams, J.E. Lane, M.M. Humphries, A.G. McAdam, and S. Boutin, unpublished data), as well as post-reproductive recovery and capital accumulation by parents for the following year (Archibald et al. in press). Overall, it is clear that squirrels adjust reproductive timing based on cone crop production in the previous year (Boutin et al. 2006). However, interesting and unresolved questions relate to whether squirrels, and presumably other hoarding capital breeders, adjust their reproductive timing based on the size of their hoard or on other environmental cues.
The pronounced differences in the diets of squirrels among-years indicates that lactating females can satisfy the energy, protein, and micronutrient requirements of reproduction from divergent diets. Although early breeding females in this squirrel population have increased reproductive success (Réale et al. 1999; McAdam and Boutin 2003; Williams et al., unpublished data), our results are counter to the hypothesis that capital feeding is required to support reproduction. Specifically, the proportion of litters that were partially or completely lost, and the litter mass that lactating females supported, was not influenced by large among-year differences in capital feeding. White (2007, 2011) has made the opposite argument suggesting that immature plant reproductive structures, coming from fresh income resources, are critical for providing the nutritional requirements of reproducing vertebrates. Our documentation of a substantial seasonal switch from stored white spruce seed and mushrooms to newly available spruce buds (vegetative and reproductive) and fresh cones (containing unripe and ripe seeds) is consistent with White’s argument. However, contrary to White’s argument, our results demonstrate that these income food resources are not required to support reproduction in squirrels at our study site. This likely results because spruce seed, which was the principal capital resource, has high levels of crude protein (Table 2; for comparative purposes, laboratory mice and rats support lactation on a commercial diet with ~24 % crude protein; Rao and Knapka 1987). Overall, future experimental work should be conducted to determine the extent to which reproductive parameters are determined by seasonal changes in diet versus other seasonal environmental changes that occur in concert.
Because of the generally high water content and lower energy content of most income food items (Table 2), satisfying the high energy requirements of lactation (Fletcher et al. 2012) later in the breeding season may require a feeding strategy that results in a dietary surplus of water. Thus, the substantial increase in water flux we observed may be related to both the increased water content of income resources and a need for females to eat more income resources than they would have to eat capital resources because of the lower energy content of income resources. This possibility applies directly to feeding on fresh mushrooms, which were a common dietary item later in the breeding season. Past research on golden-mantled ground squirrels (Spermophilus saturatus) suggests that nutritional quality of mushrooms is poor due to digestive constraints (Cork and Kenagy 1989). However, it is also thought that the high prevalence of mushrooms in the diet of rodents results because their high abundance and detectability allows a high yield of energy and nutrients in relation to foraging effort (Cork and Kenagy 1989).
Overall, our results emphasise that the diets of reproductive animals can vary drastically throughout the breeding season, while underscoring that vastly different diets can support reproduction. There is well-warranted concern that environmental change will lead to a mismatch between reproductive timing and the seasonal availability of resources required to support reproduction (Visser et al. 1998; Jones and Cresswell 2010). While our research was not intended to directly address this issue, and our study system is complicated by a mixed reliance on capital and income resources, it does offer an example of the capacity of wild mammals to support reproduction while flexibly exploiting a wide range of seasonally available resources.
References
Alerstam T (2006) Strategies for the transition to breeding in time-selected bird migration. Ardea 94:347–357
Ankney CD, MacInnes CD (1978) Nutrient reserves and reproductive performance of female Lesser Snow Geese. Auk 95:459–471
Archibald DW, Fletcher QE, Boutin S, McAdam AG, Speakman JR, Humphries MM (in press) Sex-specific hoarding behavior in North American red squirrels (Tamiasciurus hudsonicus). J Mamm
Bates D, Maechler M (2009) lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-31
Becker CD (1993) Environmental cues of estrus in the North American red squirrel (Tamiasciurus hudsonicus Bangs). Can J Zool 71:1326–1333
Bêty J, Gauthier G, Giroux J (2003) Body condition, migration, and timing of reproduction in snow geese: a test of the condition-dependent model of optimal clutch size. Am Nat 162:110–121
Boness DJ, Bowen WD (1996) The evolution of maternal care in pinnipeds. Bioscience 46:645–654
Bonner W (1984) Lactation strategies in pinnipeds: problems for a marine mammalian group. Symp Zool Soc Lond 51:253–272
Bonnet X, Bradshaw D, Shine R (1998) Capital versus income breeding: an ectothermic perspective. Oikos 83:333–342
Boutin S, Wauters LA, McAdam AG, Humphries MM, Tosi G, Dhondt AA (2006) Anticipatory reproduction and population growth in seed predators. Science 314:1928–1930
Bowen WD, Iverson SJ, Boness DJ, Oftedal OT (2001) Foraging effort, food intake and lactation performance depend on maternal mass in a small phocid seal. Funct Ecol 15:325–334
Boyd IL (1998) Time and energy constraints in pinniped lactation. Am Nat 152:717–728
Boyd IL (2000) State-dependent fertility in pinnipeds: contrasting capital and income breeders. Funct Ecol 14:623–630
Carrier DJ, Kendall EJ, Bock CA, Cunningham JE, Dunstan DI (1999) Water content, lipid deposition, and (+)-abscisic acid content in developing white spruce seeds. J Exp Bot 50:1359–1364
Choinière L, Gauthier G (1995) Energetics of reproduction in female and male greater snow geese. Oecologia 103:379–389
Cork SJ, Kenagy GJ (1989) Nutritional value of hypogeous fungus for a forest-dwelling ground squirrel. Ecology 70:577–586
Costa DP, Le Boeuf BJ, Huntley AC, Ortiz CL (1986) The energetics of lactation in the Northern elephant seal, Mirounga angustirostris. J Zool 209:21–33
Dantzer B, McAdam AG, Palme R, Humphries MM, Boutin S, Boonstra R (2010) Maternal androgens and behaviour in free-ranging North American red squirrels. Anim Behav 81:469–479
Degen AA, Khokhlova IS, Kam M, Nagy KA (1997) Body size, granivory and seasonal dietary shifts in desert gerbilline rodents. Funct Ecol 11:53–59
Degen AA, Khokhlova IS, Kam M, Snider I (2004) Water budget during reproduction in female common spiny mice (Acomys cahirinus). J Mammal 85:1106–1110
Diller LV, Wallace RL (1984) Reproductive biology of the northern pacific rattlesnake (Crotalus viridis oreganus) in northern Idaho. Herpetologica 40:182–193
Drent RH (2006) The timing of birds’ breeding seasons: the Perrins hypothesis revisited especially for migrants. Ardea 94:305–322
Drent RH, Daan S (1980) The prudent parent—energetic adjustments in avian breeding. Ardea 68:225–252
Fletcher QE, Boutin S, Lane JE, LaMontagne JM, McAdam AG, Krebs CJ, Humphries MM (2010) The functional response of a hoarding seed predator to mast seeding. Ecology 91:2673–2683
Fletcher QE, Speakman JR, Boutin S, McAdam AG, Woods SB, Humphries MM (2012) Seasonal stage differences overwhelm environmental and individual factors as determinants of energy expenditure in free-ranging red squirrels. Funct Ecol 26:677–687
Ganter B, Cooke F (1996) Pre-incubation feeding activities and energy budgets of Snow Geese: can food on the breeding grounds influence fecundity? Oecologia 106:153–165
Gauthier G, Bêty J, Hobson KA (2003) Are greater snow geese capital breeders? New evidence from a stable-isotope model. Ecology 84:3250–3264
Gittleman JL, Thompson SD (1988) Energy allocation in mammalian reproduction. Am Zool 28:863–875
Grodziński W, Sawicka-Kapusta K (1970) Energy values of tree-seeds eaten by small mammals. Oikos 21:52–58
Hammond KA, Diamond J (1992) An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiol Zool 65:952–977
Henry M, Thomas DW, Vaudry R, Carrier M (2002) Foraging distances and home range of pregnant and lactating little brown bats (Myotis lucifugus). J Mammal 83:767–774
Houston AI, Stephens PA, Boyd IL, Harding KC, McNamara JM (2007) Capital or income breeding? A theoretical model of female reproductive strategies. Behav Ecol 18:241–250
Humphries MM, Boutin S (2000) The determinants of optimal litter size in free-ranging red squirrels. Ecology 81:2867–2877
Johnson MS, Thomson SC, Speakman JR (2001) Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J Exp Biol 204:1925–1935
Jones T, Cresswell W (2010) The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? J Anim Ecol 79:98–108
Jönsson KI (1997) Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66
Kam M, Degen AA, Nagy KA (1987) Seasonal energy, water, and food consumption of Negev chukars and sand partridges. Ecology 68:1029–1037
Kenagy GJ, Sharbaugh SM, Nagy KA (1989) Annual cycle of energy and time expenditure in a golden-mantled ground squirrel population. Oecologia 78:269–282
Klaassen M, Lindströem A, Meltofte H, Piersma T (2001) Arctic waders are not capital breeders. Nature 413:794
Klomp H (1970) The determination of clutch size in birds: a review. Ardea 58:1–124
Krebs CJ, Boonstra R (2001) The Kluane region. In: Krebs CJ, Boutin S, Boonstra R (eds) Ecosystem dynamics of the boreal forest. Oxford University Press, New York
Krebs CJ, Carrier P, Boutin S, Boonstra R, Hofer E (2008) Mushroom crops in relation to weather in the southwestern Yukon. Botany 86:1497–1502
Kurta A, Kunz TH, Nagy KA (1990) Energetics and water flux of free-ranging big brown bats (Eptesicus fuscus) during pregnancy and lactation. J Mammal 71:59–65
LaMontagne JM, Boutin S (2007) Local-scale synchrony and variability in mast seed production patterns of Picea glauca. J Ecol 95:991–1000
LaMontagne JM, Boutin S (2009) Quantitative methods for defining mast-seeding years across species and studies. J Veg Sci 20:745–753
Layne JN (1954) The biology of the red squirrel, Tamiasciurus hudsonicus loquax (Bangs), in central New York. Ecol Monogr 24:227–268
Lepage D, Gauthier G, Menu S (2000) Reproductive consequences of egg-laying decisions in snow geese. J Anim Ecol 69:414–427
Lifson N, McClintock R (1966) Theory of use of the turnover rates of body water for measuring energy and material balance. J Theor Biol 12:46–74
Lifson N, Gordon GB, McClintock R (1955) Measurement of total carbon dioxide production by means of D2O18. J Appl Physiol 7:704–710
Lobo N, Millar JS (2011) The efficacy of conifer seeds as major food resources to deer mice (Peromyscus maniculatus) and southern red-backed voles (Myodes gapperi). Mammal Biol 76:274–284
Mainguy SK, Thomas VG (1985) Comparisons of body reserve buildup and use in several groups of Canada Geese. Can J Zool 63:1765–1772
McAdam AG, Boutin S (2003) Variation in viability selection among cohorts of juvenile red squirrels (Tamiasciurus hudsonicus). Evolution 57:1689–1697
McAdam AG, Boutin S, Sykes AK, Humphries MM (2007) Life histories of female red squirrels and their contributions to population growth and lifetime fitness. Écoscience 14:362–369
Meijer T, Drent R (1999) Re-examination of the capital and income dichotomy in breeding birds. Ibis 141:399–414
Millar JS (1978) Energetics of reproduction in Peromyscus leucopus—cost of lactation. Ecology 59:1055–1061
Morrison RIG, Hobson KA (2004) Use of body stores in shorebirds after arrival on high-arctic breeding grounds. Auk 121:333–344
Mutze GJ, Green B, Newgrain K (1991) Water flux and energy use in wild house mice (Mus domesticus) and the impact of seasonal aridity on breeding and population levels. Oecologia 88:529–538
Nagy KA (1983) The doubly labeled water (3HH18O) method: a guide to its use. University of California, Los Angeles Publication No. 12-1417, University of California, Los Angeles
Nienstaedt H, Zasada JC (1990) Picea glauca (Moench) Voss white spruce. In: Silvics of North America, vol. 1. Conifers. U.S. Department of Agriculture, Washington, DC
O’Donoghue M (1994) Early survival of juvenile snowshoe hares. Ecology 75:1582–1592
Oswald C, Fonken P, Atkinson D, Palladino M (1993) Lactational water balance and recycling in white-footed mice, red-backed voles, and gerbils. J Mammal 74:963–970
Owens JN, Molder M (1979) Sexual reproduction of white spruce (Picea glauca). Can J Bot 57:152–169
Owens JN, Molder M, Langer H (1977) Bud development in Picea glauca. I. Annual growth cycle of vegetative buds and shoot elongation as they relate to date and temperature sums. Can J Bot 55:2728–2745
Parker H, Holm H (1990) Patterns of nutrient and energy expenditure in female Common Eiders nesting in the high arctic. Auk 107:660–668
Pendergast BA, Boag DA (1971) Nutritional aspects of the diet of spruce grouse in central Alberta. Condor 73:437–443
Perrins CM (1970) The timing of birds’ breeding seasons. Ibis 112:242–255
Picciano MF (2003) Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr 133:1997S–2002S
R Development Core Team (2011) R: a language and environment for statistical computing. v. 2.13.1. R Foundation for Statistical Computing, Vienna, Austria
Rao GN, Knapka JJ (1987) Contaminant and nutrient concentrations of natural ingredient rat and mouse diet used in chemical toxicology studies. Fundam Appl Toxicol 9:329–338
Réale D, Festa-Bianchet M, Jorgenson JT (1999) Heritability of body mass varies with age and season in wild bighorn sheep. Heredity 83:526–532
Réale D, Berteaux D, McAdam AG, Boutin S (2003) Lifetime selection on heritable life-history traits in a natural population of red squirrels. Evolution 57:2416–2423
Rowe L, Ludwig D, Schluter D (1994) Time, condition, and the seasonal decline of avian clutch size. Am Nat 143:698–722
Ryder JP (1970) A possible factor in the evolution of clutch size in Ross’ Goose. Wilson Bull 82:5–13
Samelius G, Alisauskas RT, Hobson KA, Larivière S (2007) Prolonging the arctic pulse: long-term exploitation of cached eggs by arctic foxes when lemmings are scarce. J Anim Ecol 76:873–880
Schulz TM, Bowen WD (2005) The evolution of lactation strategies in pinnipeds: a phylogenetic analysis. Ecol Monogr 75:159–177
Sénéchal É, Bêty J, Gilchrist HG (2011a) Interactions between lay date, clutch size, and postlaying energetic needs in a capital breeder. Behav Ecol 22:162–168
Sénéchal É, Bêty J, Gilchrist HG, Hobson KA, Jamieson SE (2011b) Do purely capital layers exist among flying birds? Evidence of exogenous contribution to arctic-nesting common eider eggs. Oecologia 165:593–604
Smith CC (1968) Adaptive nature of social organization in genus of three [sic] squirrels Tamiasciurus. Ecol Monogr 38:31–64
Speakman JR (1997) Doubly-labelled water: theory and practice. Chapman and Hall, London
Speakman JR, McQueenie J (1996) Limits to sustained metabolic rate: the link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol Zool 69:746–769
Speakman JR, Racey PA (1987) The equilibrium concentration of O-18 in body-water—implications for the accuracy of the doubly-labeled water technique and a potential new method of measuring RQ in free-living animals. J Theor Biol 127:79–95
Speakman JR, Racey PA (1988) Consequences of non-steady-state CO2 production for accuracy of the doubly labeled water technique—the importance of recapture interval. Comp Biochem Physiol A 90:337–340
Steele MA (1998) Tamiasciurus hudsonicus. Mamm Species 586:1–9
Stephens PA, Boyd IL, McNamara JM, Houston AI (2009) Capital breeding and income breeding: their meaning, measurement, and worth. Ecology 90:2057–2067
Studd EK (2012) Environmental and biological correlates of maternal investment in red squirrels. MSc thesis, McGill University, Montreal
Vander Wall SB (1990) Food hoarding in animals. University of Chicago Press, Chicago
Varpe Ø, Jørgensen C, Tarling GA, Fiksen Ø (2009) The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 118:363–370
Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc R Soc Lond B 265:1867–1870
Warner DA, Bonnet X, Hobson KA, Shine R (2008) Lizards combine stored energy and recently acquired nutrients flexibly to fuel reproduction. J Anim Ecol 77:1242–1249
Wauters LA, Suhonen J, Dhondt AA (1995) Fitness consequences of hoarding behaviour in the Eurasian red squirrel. Proc R Soc Lond B 262:277–281
Wheatley KE, Bradshaw CJA, Harcourt RG, Hindell MA (2008) Feast or famine: evidence for mixed capital–income breeding strategies in Weddell seals. Oecologia 155:11–20
White TCR (2007) Mast seeding and mammal breeding: can a bonanza food supply be anticipated? NZ J Zool 34:179–183
White TCR (2011) The significance of unripe seeds and animal tissues in the protein nutrition of herbivores. Biol Rev 86:217–224
Willson MF, De Santo TL, Sieving KE (2003) Red squirrels and predation risk to bird nests in northern forests. Can J Zool 81:1202–1208
Yosef R, Pinshow B (1989) Cache size in shrikes influences female mate choice and reproductive success. Auk 106:418–421
Acknowledgments
We thank all squirrelers, especially Maura Forest for her field data collection and initial work on this project. We are grateful to Ainsley Sykes and Elizabeth Anderson for their assistance with field data preparation. Thanks also to Paula Redman and Peter Thomson for technical assistance in isotope analyses for the DLW work. We thank the Champagne and Aishihik First Nations for allowing us to do research on their lands. We thank Agnes Moose and her family for access to her traditional trapping area. Research support was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC), the National Science Foundation (Grant DEB-0515973), and Northern Scientific Training Program Grants to S.B., A.G.M. and M.M.H., and Q.E.F. An NSERC Postgraduate Graduate Scholarship provided personal support to Q.E.F. This is paper number 70 of the Kluane Red Squirrel Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pawel Koteja.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fletcher, Q.E., Landry-Cuerrier, M., Boutin, S. et al. Reproductive timing and reliance on hoarded capital resources by lactating red squirrels. Oecologia 173, 1203–1215 (2013). https://doi.org/10.1007/s00442-013-2699-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2699-3