Abstract
The strategy of relying extensively on stored resources for reproduction has been termed capital breeding and is in contrast to income breeding, where needs of reproduction are satisfied by exogenous (dietary) resources. Most species likely fall somewhere between these two extremes, and the position of an organism along this gradient can influence several key life-history traits. Common eiders (Somateria mollissima) are the only flying birds that are still typically considered pure capital breeders, suggesting that they depend exclusively on endogenous reserves to form their eggs and incubate. We investigated the annual and seasonal variation in contributions of endogenous and exogenous resources to egg formation in eiders breeding at the East Bay colony in the Canadian Arctic. We collected prey items along with females and their eggs during various stages of breeding and used two complementary analytical approaches: body reserve dynamics and stable isotope [δ13C, δ15N] mixing models. Indices of protein reserves remained stable from pre-laying to post-laying stages, while lipid reserves declined significantly during laying. Similarly, stable isotope analyses indicated that (1) exogenous nutrients derived from marine invertebrates strongly contributed to the formation of lipid-free egg constituents, and (2) yolk lipids were constituted mostly from endogenous lipids. We also found evidence of seasonal variation in the use of body reserves, with early breeders using proportionally more exogenous proteins to form each egg than late breeders. Based on these results, we reject the hypothesis that eiders are pure capital layers. In these flying birds, the fitness costs of a strict capital breeding strategy, such as temporary loss of flight capability and limitation of clutch and egg size, may outweigh benefits such as a reduction in egg predation rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The availability of endogenous and exogenous resources to breeding organisms can influence several key life-history traits such as timing of breeding (Rowe et al. 1994; Bêty et al. 2003), number and quality of offspring (Brown and Shine 2002; Jardine et al. 2008), and degree of parental care (Lewis and Kappeler 2005), as well as reproductive investment and lifespan (Chastel et al. 1995; Festa-Bianchet et al. 1998). Individuals should allocate available resources in order to maximize their fitness (Stearns 1992), and determining how and when organisms acquire resources needed for reproduction is crucial to understand their life-history strategies.
Animals breeding in environments where food is seasonally limited could increase their fitness by relying on stored resources acquired prior to breeding. Alternatively, organisms living in environments with reliable food resources could increase their reproductive success by rapidly responding to increases in local food supply (Drent and Daan 1980; Jönsson 1997). These two strategies are respectively called “capital” (i.e., financed by stored capital) and “income” (i.e., based on concurrent food intake) breeding, and can be considered opposite ends of a continuum (Meijer and Drent 1999; Klaassen et al. 2006; Stephens et al. 2009).
Capital breeding has several potential energetic and survival costs which can be counterbalanced by various benefits, such as breeding when the value of offspring peaks and increasing the offspring production rate (Varpe et al. 2009). Several migratory birds that nest in northern regions were first thought to rely extensively on endogenous reserves to breed successfully, given the unpredictability of nutrient abundance at breeding sites and the short breeding period. However, recent studies have shown that birds previously thought to be capital breeders rely considerably on exogenous resources during egg formation and incubation (e.g., Gauthier et al. 2003; Hobson et al. 2004; Gorman et al. 2008).
The common eider (Somateria mollissima, hereafter eider) is the only flight-capable bird species that is still generally considered a pure capital breeder. Indeed, resources required for both producing and incubating clutches in this species are generally assumed to be drawn entirely from endogenous reserves (Parker and Holm 1990; Meijer and Drent 1999). However, females have been observed feeding during the pre-breeding and laying periods (Christensen 2000; Guillemette 2001; Hario and Hollmén 2004), making it unclear whether females allocate exogenous nutrients to egg production or if they accumulate them for use later during the breeding period.
Using two complementary approaches, we investigated the annual and seasonal variation in contributions of endogenous and exogenous resources to egg formation in eiders nesting in the Canadian Arctic. We first described body reserve dynamics from pre-breeding to post-laying reproductive stages. We then used stable isotope analyses to quantify the relative contribution of body reserves and prey species to egg formation (Hobson 2006). Combining these methods provides a better assessment of the energetic reproductive strategy of individuals. Body reserves dynamics provides information on resources mobilization, and stable isotopes help to trace sources of specific macronutrients invested in different egg components.
Materials and methods
Study site
Our study was conducted on Mitivik Island (0.24 km2) in the East Bay Migratory Bird Sanctuary, Southampton Island, Nunavut, Canada (64°02′N, 81°47′W), which supports the largest known eider breeding colony in the Canadian Arctic (up to 8,000 pairs annually). Eiders from East Bay spend the winter in south-west Greenland or Labrador and migrate through Hudson Strait (Mosbech et al. 2006). Females are present in the vicinity of the nesting island up to 1 month prior to laying (Mosbech et al. 2006; Bêty and Gilchrist, unpublished data), and forage in river mouths and ice leads before and during laying (prey items found in the digestive tract; Bêty and Gilchrist, unpublished data; see also Abraham and Ankney 1986). The exact geographical source of endogenous reserves used by breeding females is unknown, but they are likely accumulated towards the end of the spring migration and in proximity to breeding areas (Jamieson 2003; Mosbech et al. 2006). Eiders lay one egg per 28 h (Watson et al. 1993) and complete egg laying in 2–7 days at our study site (average clutch size 4, range 2–6; Sénéchal et al. 2011). Like most precocial birds, female eiders tend to increase the time spent at the nest with the progression of the laying period (Swennen et al. 1993; Hanssen et al. 2002).
Females and eggs collection
Pre-breeding, pre-laying, and laying adult eider females (see below for definitions) were shot at foraging and resting sites located within 5 km from the nesting colony (from 11 June to 11 July; n = 15 in 2002, 15 in 2003, and 14 in 2004). Post-laying females (i.e., sitting on a full clutch for 24–48 h) and a few additional laying females (1 in 2002, 1 in 2003 and 2 in 2004) were captured on their nest using nest traps, and euthanized using halothane (from June 18 to July 22; total n = 15, 17 and 19 in 2002, 2003, and 2004, respectively). Immediately after collecting, females were field-dissected and endogenous reserves were estimated by weighing wet right breast muscle (pectoralis and supra-coracoid), abdominal fat, total right leg (including all muscles that originate or insert in the femur or tibiotarsus bones) and total body (excluding food items in gizzards and eggs in the oviducts; see Jamieson et al. 2006 for details on dissections and correlations between proxies and total body reserves). Breeding stages of females were confirmed by inspecting follicles: females without developing or post-ovulatory follicles were considered in the pre-breeding stage (which could also include non-breeding birds); the presence of only developing follicles was associated with the pre-laying stage; females with both developing and post-ovulatory follicles were considered laying; while post-laying females had post-ovulatory follicles only.
Prior to nest-trapping, we visited the nest every 1 or 2 days during laying to mark the eggs and determine the laying sequence. First and last laid eggs were collected at the same time as the female (only the first egg was collected for laying females). Frequently, the number of post-ovulatory follicles was higher than the number of eggs found in nests (33 cases out of 52). In those cases, we assumed that missing eggs were the first ones, given the low nest attendance of females at the beginning of laying and the high predation rate of first eggs (Andersson and Waldeck 2006). In only two cases, the number of eggs found in the nest was higher than the number of post-ovulatory follicles, which indicated nest parasitism. Eggs (boiled), breast muscle, abdominal fat and liver samples were kept frozen at −20°C for subsequent laboratory analyses.
Prey collection
Prey species consumed by pre-laying and laying eiders were identified by examination of feces and gizzards, as well as behavioral observations (see also Abraham and Ankney 1986). These included bivalves (Hiatella arctica, Serripes spp.), gasteropods (Acmea testudinalis), and amphipods (Gammarus spp.). We collected prey items by scuba-diving at 56 locations within East Bay (1–30 m deep) in mid-July 2007 (0–20 km from the nesting colony). Diving sites were associated with heavily used eider feeding areas identified by previous telemetry surveys (Bêty and Gilchrist, unpublished data). As prey species and female eiders (and their eggs) were not collected the same year, and as prey were not collected during the eider egg formation period, we investigated potential annual and seasonal variations in prey isotopic ratios by collecting amphipods throughout three breeding seasons (2003, 2007 and 2008). We found no evidence of such variation and are confident that our sampling design did not generate bias in our analyses (see Online Resource 1). All prey samples were kept frozen at −20°C, except for a few small organisms that were preserved in 70% ethanol, before laboratory analyses.
Laboratory analyses
Breast muscle and liver samples, as well as egg components (albumen and yolk) and prey species (without exoskeleton for bivalves) were oven-dried (60°C for 48 h) and ground to powder. Abdominal fat samples were immersed in 2:1 chloroform:methanol for 24 h and dissolved lipids were collected. Because endogenous and exogenous lipids and proteins can be allocated differently to egg formation, we separated our samples into lipid and lipid-free components. Lipids were extracted from prey, yolk, liver and breast muscle samples with successive rinses of the 2:1 chloroform:methanol. Lipid extracts were conserved for every tissue except for breast muscle. Solvent was evaporated completely within a fume hood and the remaining lipid residue was stored frozen. Carbonates were also extracted from lipid-free marine organism samples by treating them with drops of 0.1 N HCL without rinsing (Carabel et al. 2006). Those samples were then oven-dried (60°C for 24 h) and powdered with a mortar and pestle.
We loaded 1.00 ± 0.01 mg of each sample in a tin cup and combusted them in a Robo-Prep elemental analyzer (Europa Scientific, Crewe, UK). Resultant gases were delivered, using continuous-flow isotope ratio mass spectrometry (CFIRMS), to a Europa 20:20 mass spectrometer (Europa Scientific) for stable-carbon and nitrogen isotope ratio determination. Stable isotope ratios are expressed in delta (δ) notation relative to the Pee Dee Belemnite or AIR standards for carbon and nitrogen, respectively (see Hobson 1995). Based on replicate within-run analyses of a keratin (BWB II) and egg albumen laboratory standards, analytical error was estimated to be ±0.3‰ for δ15N and 0.1‰ for δ13C.
Isotope mixing model calculations
We calculated relative endogenous and exogenous protein contributions to egg components using the Bayesian-based MixSIR model (version 1.0.4; Moore and Semmens 2008; Semmens et al. 2009) based upon δ13C and δ15N values of prey items, egg components and eider tissues. Six protein sources were included in these models: breast muscle (endogenous source) and five prey types (exogenous sources), all lipid-free. Protein models were generated for both lipid-free yolk and albumen samples. A number of isotope models are now available to calculate relative contributions of n + 1 sources when measuring n isotopes. Thus, we compared our results obtained with the recent Bayesian MixSIR model to the outcomes generated by the well-known non-Bayesian IsoSource model (Phillips and Gregg 2003) and the Bayesian SIAR model (Jackson et al. 2009; see Online Resource 2).
The relative contribution of endogenous and exogenous lipids to yolk-lipid was calculated only from δ13C measurements, given the low nitrogen levels in lipids. However, as endogenous lipid δ13C values overlapped those of prey items (see “Results”) we could not adequately quantify the specific contribution of all potential sources to yolk-lipid. We therefore included only two sources in lipid models, abdominal fat and liver lipids, to estimate the relative contributions of endogenous and exogenous lipids, respectively, and used IsoError linear mixing model (Phillips 2001). Carbon isotope values of liver lipids can be used as an integrative indicator of exogenous lipid ratios, as they provide short-term dietary information (Hobson and Clark 1992). However, liver is also an organ where lipid catabolism occurs and where yolk precursors are synthesized. Yolk lipids and abdominal fat δ13C values were expected to differ only if exogenous resources contribute to egg lipids (assuming no fractionation between abdominal fat and yolk lipids; see Gauthier et al. 2003). In this case, a large difference between δ13C values in fat reserves and those in yolk lipids would indicate a stronger contribution of prey items consumed during egg formation (see also “Discussion”).
Through the process of isotopic discrimination, stable isotope values in egg components are expected to differ from those in contributing (endogenous or exogenous) nutrient pools. Isotopic discrimination factors between food and egg components have been determined experimentally in few bird species. We used values (see below) from the carnivore model of Hobson (1995). However, discrimination between body reserves and eggs is much harder to estimate, and consequently has not been determined experimentally. Therefore, we used the approach proposed by Gauthier et al. (2003) to trace egg nutrient sources in waterfowl. They suggested using fractionation values wherein a bird forms egg components from a diet consisting of meat proteins and lipids (see Hobson 1995), such that the bird uses nutrients from its own body tissues to produce its eggs. In terms of fractionation associated with δ15N for instance, the breakdown of endogenous muscles to produce eggs is presumably metabolically similar to the breakdown of ingested muscles for the same purpose (see Gauthier et al. 2003 for thorough discussion of this issue). We thus assumed that lipid-free sources (prey items and breast muscle, see above) discriminate (1) from lipid-free yolk by +3.50 ± 0.35‰ for δ15N and 0 ± 0.5‰ for δ13C, and (2) from albumen by +3.10 ± 0.35‰ for δ15N and +0.9 ± 0.5‰ for δ13C (Hobson 1995). Given that yolk lipids are likely to be derived without discrimination either from the diet or from lipid stores (see Hobson 1995), and that lipids from every source have to pass through the liver before their transport to yolk precursors (Stevens 2000), we also assumed no δ13C isotopic discrimination between lipids of sources (liver and abdominal fat, see above) and the yolk (see also Gauthier et al. 2003).
Statistical analyses
Endogenous reserves dynamics
We adjusted, when required, endogenous reserve estimates to body size using principal component analysis (Sedinger et al. 1997). We used measurements (±1 mm) of the right wing chord length, right tarsus length, and total head length to establish the first principal component (PC1), which explained 47% of the variation observed in the original data. All eigenvectors ranged from 0.18 to 0.83 and were positive, suggesting that each measure increased with body size. PC1 scores were thus an appropriate index of structural size. Body and leg masses were significantly related with PC1 scores (general linear regressions: body mass: P = 0.007, R 2 = 0.08; leg mass: P = 0.004, R 2 = 0.09), so we used the residuals from these models in subsequent analyses. Body and leg masses are, hereafter, presented in size-corrected form.
Variation in endogenous reserves between breeding stages and years were analyzed with general linear models and Tukey–Kramer multiple comparisons tests. The interaction terms between year and breeding stage were non-significant (all P > 0.05) and were removed from models. Within a given stage, we performed Pearson correlation tests to examine relationships between body reserves and number of follicles. We did not detect strong violations of assumptions of residual normality (Shapiro–Wilk test, all P > 0.05) and variance homogeneity (Levene’s test, all P > 0.05 except for abdominal fat with P = 0.005). As non-parametric test (Kruskal–Wallis one-way ANOVA) provided similar results (not shown), we used parametric tests in all cases.
Stable isotopes
Inter-annual variation in δ13C and δ15N values of endogenous reserves and eggs were analyzed with one-way ANOVAs. We did not detect strong violations of the assumptions required for these analyses (Levene’s test for homogeneity of variances, all P > 0.05 except for albumen δ13C of first eggs with P = 0.02; Shapiro–Wilk test of normality, all P > 0.05 except for yolk δ13C of first and last eggs with P = 0.002 and 0.03, respectively). As non-parametric tests (Kruskal–Wallis one-way ANOVA) provided similar results (not shown), we used parametric tests in all cases. Tukey–Kramer multiple comparisons tests were performed when inter-annual variations were significant.
We performed a MANOVA on the δ13C and δ15N values of potential egg protein sources, which were grouped in six categories: Serripes spp., Hiatella sp., Acmea testudinalis, amphipods collected at river mouths, amphipods collected offshore, and breast muscles. They all showed significantly different values (Wilks λ = 0.08, F 10,184 = 47.17, P < 0.001), thus we used them as independent sources in isotopic models. Likewise, a one-way ANOVA on δ13C of lipid sources showed that they were statistically different (mean ± 1SD: liver lipid, −21.22 ± 0.78, and abdominal fat, −21.97 ± 0.99; F 1,101 = 15.2, P < 0.001).
To detect inter-annual variation in protein allocation to eggs, we ran MixSIR models separately for each of the 3 years. Similarly, to determine if there was seasonal variation in protein allocation to eggs, we grouped females according to their relative lay date: early (≤5 days before the yearly median; n = 14), mid (yearly median ±4 days; n = 23), and late breeders (≥5 days after yearly median; n = 15), and ran separate models. Laying dates of females were back-calculated according to the number of post-ovulatory follicles (assuming a 28-h laying interval; Watson et al. 1993). In order to avoid pseudo-replication, we included only one collected egg per clutch (the earliest in the laying sequence) in the models.
Finally, to determine if the source of nutrients changed within the laying sequence, we calculated the difference in δ13C and δ15N values between the last and first egg collected in each clutch. The average position in the laying sequence of the first egg collected was 1.8, and 4 for last eggs (hereafter called first and last eggs). Using general linear models, we determined, for every isotope ratio, if the intra-clutch difference varied with the relative lay date.
All statistical analyses were performed using SAS V 9.1 (SAS Institute) with a critical level of significance of 0.05. Standard errors (±1SE) are presented unless otherwise stated.
Results
Endogenous reserve dynamics
Endogenous reserve estimates varied significantly between reproductive stages and years (all P ≤ 0.02; Table 1). After controlling for reproductive stages, we found that females were on average heavier and had higher lipid reserves in 2003, while the lowest protein reserves were observed in 2002. Body reserves were generally higher for females which had started follicle development (pre-laying) than for pre-breeding ones (which were in similar condition than post-laying females; Fig. 1). From pre-laying to post-laying, significant differences were detected in total body weight and abdominal fat, indicating a considerable mobilization of fat reserves during the laying period (average loss of 16% body mass and 53% fat reserves). Conversely, index of protein reserves (breast muscles and leg muscles) did not vary significantly from the start of egg formation to the onset of incubation (Fig. 1). This indicates that endogenous protein mobilization during egg formation was absent or weak (the observed non-statistically significant decrease in breast muscle mass was 5% on average). Within-stage correlations between endogenous reserve estimates and number of follicles (developing or post-ovulatory) were not significant (P > 0.05), except for leg mass of laying females (r = 0.59, P = 0.03), and post-laying females’ total body mass (r = −0.38, P = 0.005) and breast muscle mass (r = −0.30, P = 0.03).
Year-corrected masses of common eider (Somateria mollissima) females’ endogenous reserves from pre-breeding to post-laying stages, Mitivik Island, East Bay Migratory Bird Sanctuary, Nunavut, Canada. Dashed and dotted lines represent mean ± SE for each stage. Stages accompanied by different letters differed significantly (α = 0.0083, Bonferroni correction)
Isotopic composition of eider somatic tissues and eggs
Carbon and nitrogen isotope ratios of eider somatic tissues were similar between years (δ15N: breast muscles, F 2,49 = 0.66, and liver lipid-free, F 2,46 = 1.82; δ13C: breast muscles, F 2,49 = 2.39, liver lipid-free, F 2,46 = 0.26, liver lipids, F 2,48 = 1.72 and abdominal fat, F 2,33 = 0.97; all P > 0.1).
The δ13C values of lipid-free yolk differed between years (F 2,47 = 5.15, P = 0.01 and F 2,44 = 4.45, P = 0.02 for first and last eggs, respectively), both were significantly higher in 2003 (Table 1). However, δ13C of albumen and yolk-lipid did not significantly vary between years (all P ≥ 0.35). Stable nitrogen isotope ratios varied annually only for lipid-free yolk of the first egg, with the highest δ15N values observed in 2003, and the lowest in 2004 (F 2,47 = 3.66, P = 0.03; P > 0.18 in other cases; Table 1).
Relative contribution of endogenous and exogenous sources to egg formation
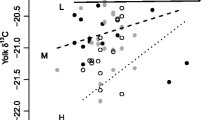
Based on isotopic models, protein allocation to eggs was derived mainly from local exogenous resources. Indeed, endogenous contributions to lipid-free yolk and albumen did not exceed 16 and 11%, respectively (except for lipid-free yolk in 2003; Table 2). Interestingly, the main exogenous protein source appeared to vary annually for the yolk but not for the albumen (always primarily derived from the clam Hiatella arctica; Table 2; Fig. 2). Hiatella arctica contributed the most to lipid-free yolk in 2002 and 2004 but very little in 2003, when Gammarus sp. were the main exogenous source.
Lipid-free stable isotope ratios (‰ mean ± SD) of egg components of the first egg collected in each nest, endogenous reserves, and prey items of common eiders. Year of egg collection is indicated in each panel (a–c). Sample sizes of each food item range from 5 to 22. In some cases, only positive or negative standard errors are shown to avoid overlaps. Only egg component ratios are corrected for discrimination. Therefore, it is not appropriate to interpret prey contributions to body reserves from this figure
Body fat apparently contributed largely to egg lipids, reaching 74.0% (±0.4) in 2003 and 67.0% (±0.5) in 2004 (the model was unable to generate estimates for 2002, given that the δ13C of yolk lipids was higher than both abdominal fat and liver-lipid δ13C values: Fig. 3; see “Discussion”). Results from paired t tests also suggested a strong contribution of endogenous lipids. In all years, individual abdominal fat δ13C values did not significantly differ from their yolk lipids δ13C values (mean absolute differences in 2002: 0.85‰, df = 13, t = 1.63, P = 0.13; 2003: 0.39‰, df = 9, t = −0.57, P = 0.58; 2004: 0.72‰, df = 10, t = −1.76, P = 0.11; all years combined: 0.04‰, df = 34, t = 0.25, P = 0.80). Conversely, individual liver lipids δ13C values significantly differed from their yolk lipids δ13C values in 2003 and 2004, but not in 2002 (mean absolute differences observed in 2002: 0.57‰, df = 13, t = 0.03, P = 0.97; 2003: 1.83‰, df = 15, t = −3.34, P = 0.005; 2004: 0.76‰, df = 18, t = −2.88, P = 0.01; all years combined: −0.39‰, df = 48, t = −3.34, P < 0.001).
Lipid δ13C (‰ mean ± SD) of egg components (first egg collected in each nest), endogenous reserves, and liver of common eiders (a–c). Prey lipid ratios did not allow discriminating endogenous reserves and exogenous resources (d). Sample sizes of each food item range from 5 to 22, except for Serripes spp. (n = 1). In some cases, only positive or negative standard errors are shown to avoid overlaps
Seasonal variations of source contributions
Distributions of possible endogenous protein contributions to the first egg of early and mid breeders were similar and very low (most likely endogenous contribution in lipid-free yolk and albumen ranged from 0.9 to 4.3%; Fig. 4). However, late breeders apparently mobilized a higher proportion of endogenous proteins to form each egg (most likely contribution of 23.3 and 9.6% for lipid-free yolk and albumen, respectively; Fig. 4). Given that δ13C values of abdominal fat overlapped those of exogenous resources (see Fig. 3), it was not possible to investigate seasonal variation in the relative contribution of endogenous lipids.
The difference between albumen δ13C and δ15N values of first and last laid eggs collected within a clutch did not vary with timing of breeding (P ≥ 0.66). However, within a clutch, δ15N values tended to be slightly higher in albumen of last eggs compared to first eggs (average of 0.58‰; P = 0.08), which may suggest a higher contribution of endogenous reserves at the end of the laying sequence (i.e., last egg values are closer to breast muscle δ15N values; Fig. 2). We also found some evidence for seasonal variation in the differences of lipid-free yolk isotope values between last and first eggs (δ13C: F 1,45 = 3.12, P = 0.08; δ15N: F 1,45 = 8.16, P = 0.007; Fig. 5). This likely reflects seasonal changes in the accessibility of prey in the vicinity of the eider colony and suggests that early and late nesters feed on different prey items and partly change their diet during clutch formation (see “Discussion”).
Lipid-free yolk δ13C and δ15N (‰) differences between last and first eggs laid in the laying sequence, according to the relative lay date of females. Dotted lines separate early, mid and late breeders (see “Materials and methods”)
Discussion
Our results derived from both body reserve dynamics and stable isotope analyses indicate that common eiders nesting at the East Bay colony in the Canadian Arctic produce their eggs primarily from body fat and exogenous proteins. We thus reject the hypothesis that common eiders are pure capital laying birds (Parker and Holm 1990; Meijer and Drent 1999). Like other well-studied bird species (e.g., Gauthier et al. 2003; Hobson et al. 2004, 2005; Bond et al. 2007; Gorman et al. 2008), and several other organisms (Casas et al. 2005; Stephens et al. 2009), eiders in this population use a mixed capital–income breeding strategy. Relying exclusively on stored resources is likely non-adaptive in arctic-nesting eiders. In these flying birds, the fitness costs of being strict capital layer (e.g., temporary loss of flight capability and limitation of clutch or egg size) likely outweigh the potential benefits (e.g., reduction in egg predation rate; see below).
Determining to what extent endogenous reserves are used for reproduction involves the placement of a species (Stephens et al. 2009), and individuals within species (Gauthier et al. 2003; Varpe et al. 2009), along the capital–income continuum. Given that mixed strategies are difficult to quantify, it is especially challenging to compare animals studied using different methods. Based on detailed stable isotope studies, Greater snow geese (Chen caerulescens atlantica; Gauthier et al. 2003) and Barrow’s goldeneye (Bucephala islandica; Hobson et al. 2005) are the waterfowl species closest to the capital end of the capital–income continuum, while redhead (Aythya americana; Hobson et al. 2004) and harlequin ducks (Histrionicus histrionicus; Bond et al. 2007) were found to be mostly income layers. Considering egg formation and incubation strategies of arctic-nesting common eiders (which fast during the entire incubation; Bottitta et al. 2003), it appears that they remain the most extreme known capital breeders among studied flying birds. Yet, they cannot be considered pure capital layers due to the unequivocal contribution of exogenous macronutrients in egg formation, for which the lipid-free components are higher than those observed in other bird species (e.g., Gauthier et al. 2003). Detailed investigations conducted in other common eider nesting colonies would strengthen our conclusions as food availability, bird density or predation risk on the breeding grounds could potentially affect their optimal breeding strategy.
Stable isotope models and uncertainty issues
Our stable isotope approach involves uncertainty. For instance, discrimination factors (Wolf et al. 2009), sources included in models (Phillips and Gregg 2003), pre-treatment of samples in laboratories (Soreide et al. 2006), and inherent variability among samples (Barnes et al. 2008) all represent potential sources of bias which could be unintentionally included in models and affect conclusions. To strengthen our conclusions, we used Bayesian mixing models, which include uncertainty (e.g., they incorporate standard deviations of source isotope ratios and take into account the variability among mixture data points: Inger and Bearhop 2008; Moore and Semmens 2008). We also carefully chose the diet sources included in models (i.e., based on direct observations). Better estimates of discrimination factors for eiders (between food and egg, and body reserves and egg) are needed to reduce uncertainty in source contribution estimates. However, no researchers have been able to induce captive birds to produce and lay eggs while fasting. Therefore, isotopic fractionation from body stores to egg components will be, if possible, extremely difficult to obtain experimentally. To our knowledge, the approach we used to estimate these fractionation factors is the best option available to approximate the relative contribution of endogenous reserves to egg macronutrients (see Gauthier et al. 2003). Overall, we are confident in our main conclusions (i.e., strong contribution of exogenous proteins to egg formation and significant intra-population variations in body reserve contributions) because (1) our results were supported by data on endogenous reserve dynamics, and (2) the same discrimination factors were applied to all individuals.
Variations in source contribution to egg formation
Inter-annual variation
Sea-ice extent can affect foraging conditions of marine birds and their prey selection, particularly during spring migration and prior to egg formation (Gaston et al. 2005; Chaulk et al. 2007). Our stable isotope models showed that yolk proteins were derived principally from clams in 2002 and 2004, but from amphipods in 2003, a year characterized by late ice break-up (Love et al. 2010). Moreover, in 2003, females were heavier upon return to the nesting colony and showed an increase in their endogenous protein contribution to yolk (Table 2).
Yolk lipids were derived principally from body fat in 2003 and 2004, but the relative contribution could not be calculated in 2002 using stable isotopes. In fact, stable carbon isotope ratios of yolk lipids of the first egg did not fall between those of liver lipids and abdominal fat δ13C values in that year (Fig. 3a) but did for the last egg (Table 1). Given that females laid their first egg 4–5 days before they were collected, and that the half-life of carbon (turnover rate) in liver is ~2–3 days (Hobson and Clark 1992), it is possible that liver δ13C values changed between the formation of the first egg and the time the female was collected (i.e., a prey switch during egg formation).
Inter-individual variation
In seasonal environments, early born offspring generally have higher post-hatching survival than late born, and this typically leads to a seasonal decline in egg value (e.g., Lepage et al. 2000; Love et al. 2010). Birds can, however, benefit from a delay in laying to acquire more endogenous resources prior to breeding (Bêty et al. 2003). In this context, the optimization models suggest that, in order to maximize their expected reproductive success, birds arriving late or in poor body condition on their breeding grounds should lay fewer eggs later (Rowe et al. 1994). We found that the endogenous protein contribution per egg increased in late breeders, which also tended to lay fewer eggs (seasonal decline in clutch size based on post-ovulatory follicles: r = −0.26, P = 0.07). This suggests that, by laying fewer eggs, late breeders can afford to slightly increase the proportion of endogenous nutrients invested in each egg and, therefore, breed as early as possible (when offspring value is still high enough to successfully reproduce; Lepage et al. 2000; see also Varpe et al. 2009). Similar observations have been reported in arctic-nesting snow geese (Gauthier et al. 2003). On the other hand, females that laid fewer eggs apparently had extra protein reserves available at the end of laying (i.e., negative correlation between post-laying breast muscles mass and number of eggs laid). Females that produce small clutches may thus have started egg production with similar protein reserves compared to large clutch females, which allowed them to finish laying in slightly better condition (see Sénéchal et al. 2011 for detailed analyses and thorough discussion of this topic).
Intra-clutch variation
The seasonal variation in the difference between isotope values of the first and last laid egg may reflect prey switching during egg formation. Rapid follicular growth takes about 9 days in common eiders (Robertson 1995); nearly 2 weeks can separate the recruitment of the first follicle and the ovulation of the last one in a four-egg clutch. Due to ice cover, early nesting eiders at our study site are likely forced to feed about 20 km off the nesting island while forming their first egg. As their follicle development or laying period progresses in mid/late June, open shorelines become available in the bay and females can start feeding on amphipods, the only abundant prey available within the periphery of the nesting island and in river mouths. This potential switch is consistent with the differences in δ13C and δ15N values of lipid-free yolk between last and first eggs (Figs. 2 and 5). Late nesters showed the opposite trend in both δ13C and δ15N values (Fig. 5), indicating a reverse switch. In fact, late breeders may start follicle development while feeding on amphipods in the vicinity of the nesting site and then switch to clams, which become available in the bay in late June/early July when ice starts breaking up. An alternative explanation is that these females may allocate more endogenous reserves to yolk formation as the development of follicles progresses. Finally, the systematic enrichment in albumen δ15N during the laying sequence (regardless of the timing of breeding) could be explained by a higher endogenous investment in the last egg. Indeed, albumen of the last egg is formed 18–24 h before clutch completion (Williams 1999), and females tend to spend more time on the nest at the end of laying in order to protect the clutch against predators (Swennen et al. 1993; Hanssen et al. 2002). Further investigations are needed to tease apart these alternative explanations.
Capital breeding: trade-offs in flying birds
The acquisition of nutrient reserves prior to reproduction may be a selected trait, especially for species breeding in unpredictable environments. Endogenous reserves can enable females to start clutch production regardless of local resource availability, and in turn increase their fecundity (Varpe et al. 2009). Moreover, egg predation is a major source of breeding failure in birds, and increasing nest attentiveness can strongly reduce predation risk (Bêty et al. 2002). From this perspective, there should be a strong selection pressure to reduce the time spent foraging away from the nest. Eider ducks typically increase nest attendance as laying progresses (Hanssen et al. 2002), which reduces egg predation risk and time available for feeding. Females can stay close to their nests during laying (E. Sénéchal and J. Bêty, personal observations) and are able to adjust the start of embryonic development in their clutch (Hanssen et al. 2002). They can thus modulate the exposure of eggs to predators while avoiding potential costs of hatching asynchrony (Hanssen et al. 2002). A pure capital breeding strategy would likely allow females to nearly eliminate nest predation from avian predators.
However, in order to finance laying and incubation exclusively from stored capital, females would have to further delay breeding (i.e., acquire more body reserves before laying the first egg; Bêty et al. 2003) and such delay would generate a decrease in egg value (Lepage et al. 2000; Love et al. 2010). Alternatively, females could produce smaller clutches, which would also decrease the fitness value of the clutch (Lepage et al. 2000). Heavier endogenous reserves increase wing loading, and the loss of flight capabilities in several insect species permits females to allocate more resources to egg production (Zera et al. 1998; Socha and Sula 2008). Pre-laying eiders are known to be at the limit of flight capabilities, with high energetic costs of flight (Guillemette and Ouellet 2005a, b). At times, pectoral and flight muscle masses of pre-laying eider females can barely sustain flight due to the increase in body mass associated with body reserves and egg formation (Guillemette and Ouellet 2005b; Ouellet et al. 2008). The effects of an increase in wing loading and a decrease in relative flight muscle mass can lead to a temporary loss in flight capability among pre-laying females, an extreme case of impaired flight ability associated with reproduction (Guillemette and Ouellet 2005a). In these circumstances, pre-laying females can be highly vulnerable to attacks by predators, such as raptors and foxes, which can lead to injuries or death (J. Bêty, personal observations).
Pure capital breeding does not appear to be selected for in arctic-nesting eider ducks. The trade-off between maximizing the likelihood of successfully producing a large clutch early enough in a seasonal environment and minimizing the time period when females are flightless may partly explain why females do not rely exclusively on endogenous reserves to produce their eggs. Given the potential survival costs associated with temporary flightlessness and the reproductive costs of producing smaller clutches or eggs, a strict capital strategy is likely not optimal for eiders.
In birds, pure capital laying may be optimal for non-flying species only, such as penguins (Meijer and Drent 1999). The use of a single framework incorporating breeding time and effort (clutch and egg size), as well as foraging and energetic strategies used during egg formation and laying (which modulate predation risk of adults and eggs) would significantly aid our understanding of optimal breeding strategies in birds.
References
Abraham KF, Ankney CD (1986) Summer birds of East Bay, Southampton Island, Northwest Territories. Canadian Field-Nat 100:180–185
Andersson M, Waldeck P (2006) Reproductive tactics under severe egg predation: an eider’s dilemma. Oecologia 148:350–355
Barnes C, Jennings S, Polunin NVC, Lancaster JE (2008) The importance of quantifying inherent variability when interpreting stable isotope field data. Oecologia 155:227–235
Bêty J, Gauthier G, Korpimäki E, Giroux JF (2002) Shared predators and indirect trophic interactions: lemming cycle and arctic-nesting geese. J Anim Ecol 71:88–98
Bêty J, Gauthier G, Giroux JF (2003) Body condition, migration, and timing of reproduction in snow geese: a test of the condition-dependent model of optimal clutch size. Am Nat 162:110–121
Bond JC, Esler D, Hobson KA (2007) Isotopic evidence for sources of nutrients allocated to clutch formation by harlequin ducks. Condor 109:698–704
Bottitta GE, Nol E, Gilchrist HG (2003) Effects of experimental manipulation of incubation length on behavior and body mass of common eiders in the Canadian Arctic. Waterbirds 26:100–107
Brown GP, Shine R (2002) Reproductive ecology of a tropical natricine snake, Tropidonophis mairii (Colubridae). J Zool 258:63–72
Carabel S, Godinez-Dominguez E, Verisimo P, Fernandez L, Freire J (2006) An assessment of sample processing methods for stable isotope analyses of marine food webs. J Exp Mar Biol Ecol 336:254–261
Casas J, Pincebourde S, Mandon N, Vannier F, Poujol R, Giron D (2005) Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 86:545–554
Chastel O, Weimerskirch H, Jouventin P (1995) Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76:2240–2246
Chaulk KG, Robertson GJ, Montevecchi WA (2007) Landscape features and sea ice influence nesting common eider abundance and dispersion. Can J Zool 85:301–309
Christensen TK (2000) Female pre-nesting foraging and male vigilance in common eider Somateria mollissima. Bird Study 47:311–319
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252
Festa-Bianchet M, Gaillard JM, Jorgenson JT (1998) Mass- and density-dependent reproductive success and reproductive costs in a capital breeder. Am Nat 152:367–379
Gaston AJ, Gilchrist HG, Hipfner JM (2005) Climate change ice conditions and reproduction in an Arctic nesting marine bird: Brunnich’s guillemot (Uria lomvia L.). J Anim Ecol 74:832–841
Gauthier G, Bety J, Hobson KA (2003) Are greater snow geese capital breeders? New evidence from a stable-isotope model. Ecology 84:3250–3264
Gorman KB, Esler D, Flint PL, Williams TD (2008) Nutrient-reserve dynamics during egg production by female greater scaup (Aythya marila): relationships with timing of reproduction. Auk 125:384–394
Guillemette M (2001) Foraging before spring migration and before breeding in common eiders: Does hyperphagia occur? Condor 103:633–638
Guillemette M, Ouellet JF (2005a) Temporary flightlessness as a potential cost of reproduction in pre-laying common eiders Somateria mollissima. Ibis 147:301–306
Guillemette M, Ouellet JF (2005b) Temporary flightlessness in pre-laying common eiders Somateria mollissima: are females constrained by excessive wing-loading or by minimal flight muscle ratio? Ibis 147:293–300
Hanssen SA, Engebretsen H, Erikstad KE (2002) Incubation start and egg size in relation to body reserves in the common eider. Behav Ecol Sociobiol 52:282–288
Hario M, Hollmén TE (2004) The role of male mate-guarding in pre-laying common eiders Somateria m. mollissima in the northern Baltic Sea. Ornis Fennica 81:119–127
Hobson KA (1995) Reconstructing avian diets using stable-carbon and nitrogen isotope analysis of egg components––patterns of isotopic fractionation and turnover. Condor 97:752–762
Hobson KA (2006) Using stable isotopes to quantitatively track endogenous and exogenous nutrient allocations to eggs of birds that travel to breed. Ardea 94:359–369
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes.1. Turnover of C-13 in tissues. Condor 94:181–188
Hobson KA, Atwell L, Wassenaar LI, Yerkes T (2004) Estimating endogenous nutrient allocations to reproduction in redhead ducks: a dual isotope approach using delta D and delta C-13 measurements of female and egg tissues. Funct Ecol 18:737–745
Hobson KA, Thompson JE, Evans MR, Boyd S (2005) Tracing nutrient allocation to reproduction in Barrow’s goldeneye. J Wildl Manag 69:1221–1228
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Jackson AL, Inger R, Bearhop S, Parnell A (2009) Erroneous behaviour of MixSIR, a recently published Bayesian isotope mixing model: a discussion of Moore & Semmens (2008). Ecol Lett 12:E1–E5
Jamieson SE (2003) Endogenous reserve dynamics of northern common eiders (Somateria mollissiam borealis) wintering in Greenland. Masters thesis, University of New Brunswick, Canada
Jamieson SE, Gilchrist HG, Merkel FR, Falk K, Diamond AW (2006) An evaluation of methods used to estimate carcass composition of common eiders Somateria mollissima. Wildl Biol 12:219–226
Jardine TD, Chernoff E, Curry RA (2008) Maternal transfer of carbon and nitrogen to progeny of sea-run and resident brook trout (Salvelinus fontinalis). Can J Fish Aquat Sci 65:2201–2210
Jönsson KI (1997) Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66
Klaassen M, Abraham KF, Jefferies RL, Vrtiska M (2006) Factors affecting the site of investment, and the reliance on savings for arctic breeders: the capital-income dichotomy revisited. Ardea 94:371–384
Lepage D, Gauthier G, Menu S (2000) Reproductive consequences of egg-laying decisions in snow geese. J Anim Ecol 69:414–427
Lewis RJ, Kappeler PM (2005) Are Kirindy Sifaka capital or income breeders? It depends. Am J Primatol 67:365–369
Love OP, Gilchrist HG, Descamps S, Semeniuk CAD, Bêty J (2010) Pre-laying climatic cues can time reproduction to optimally match offspring hatching and ice conditions in an Arctic marine bird. Oecologia 164:277–286
Meijer T, Drent R (1999) Re-examination of the capital and income dichotomy in breeding birds. Ibis 141:399–414
Moore JW, Semmens BX (2008) Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett 11:470–480
Mosbech A, Gilchrist G, Merkel F, Sonne C, Flagstad A, Nyegaard H (2006) Year-round movements of Northern common eiders Somateria mollissima borealis breeding in Arctic Canada and West Greenland followed by satellite telemetry. Ardea 94:651–665
Ouellet J-F, Guillemette M, Blier PU (2008) Morphological and physiological aspects of takeoff aptitudes of female common eiders (Somateria mollissima) during the pre-laying period. Can J Zool 86:462–469
Parker H, Holm H (1990) Patterns of nutrient and energy-expenditure in female common eiders nesting in the high Arctic. Auk 107:660–668
Phillips DL (2001) Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia 127:166–170
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Robertson GJ (1995) Factors affecting nest-site selection and nesting success in the common eider Somateria-Mollissima. Ibis 137:109–115
Rowe L, Ludwig D, Schluter D (1994) Time, condition, and the seasonal decline of avian clutch size. Am Nat 143:698–772
Sedinger JS, Ankney CD, Alisauskas RT (1997) Refined methods for assessment of nutrient reserve use and regulation of clutch size. Condor 99:836–840
Semmens BX, Moore JW, Ward EJ (2009) Improving Bayesian isotope mixing models: a response to Jackson et al. (2009). Ecol Lett 12:E6–E8
Sénéchal E, Bêty J, Gilchrist GH (2011) Interactions between lay date, clutch size and post-laying energetic needs in a capital breeder. Behav Ecol (in press)
Socha R, Sula J (2008) Differential allocation of protein resources to flight muscles and reproductive organs in the flightless wing-polymorphic bug, Pyrrhocoris apterus (L.) (Heteroptera). J Comp Physiol B 178:179–188
Soreide JE, Tamelander T, Hop H, Hobson KA, Johansen I (2006) Sample preparation effects on stable C and N isotope values: a comparison of methods in Arctic marine food web studies. Mar Ecol Prog Ser 328:17–28
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stephens PA, Boyd IL, McNamara JM, Houston AI (2009) Capital breeding and income breeding: their meaning, measurement, and worth. Ecology 90:2057–2067
Stevens L (2000) Lipids and their metabolism. In: Avian biochemistry and molecular biology. Cambridge University Press, London, pp 54–60
Swennen C, Ursem JCH, Duiven P (1993) Determinate laying and egg attendance in common eiders. Ornis Scandinavica 24:48–52
Varpe O, Jorgensen C, Tarling GA, Fiksen O (2009) The adaptive value of energy storage and capital breeding in seasonal environments. Oikos 118:363–370
Watson MD, Robertson GJ, Cooke F (1993) Egg-laying time and laying interval in the common eider. Condor 95:869–878
Williams TD (1999) Avian reproduction, overview. In: Encyclopedia of reproduction, vol 1. Academic Press, Toronto, pp 325–336
Wolf N, Carleton SA, Martinez del Rio C (2009) Ten years of experimental animal isotopic ecology. Funct Ecol 23:17–26
Zera AJ, Potts J, Kobus K (1998) The physiology of life-history trade-offs: experimental analysis of a hormonally induced life-history trade-off in Gryllus assimilis. Am Nat 152:7–23
Acknowledgments
This study was supported by (alphabetical order): Canadian Wildlife Service (Environment Canada), Fonds Québécois de la Recherche sur la Nature et les Technologies, Natural Sciences and Engineering Research Council of Canada, Network of Centers of Excellence of Canada ArcticNet, Northern Scientific Training Program (Indian and Northern Affairs Canada), Nunavut Wildlife Management Board, Polar Continental Shelf Project (PCSP), and Université du Québec à Rimouski. We also thank the East Bay crews for their valuable work in the field, F. Hartog and O. D’Amours for their diving expertise, and B. Boucher and R. Chabot for their help in marine invertebrate identification. B.X. Mora-Alvarez assisted with preparation of stable isotope samples which were analyzed by M. Stocki at the Department of Soil Science, University of Saskatchewan. M. Fast provided useful comments on the final version of the manuscript. The Canadian Council on Animal Care has approved the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Markku Orell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sénéchal, É., Bêty, J., Gilchrist, H.G. et al. Do purely capital layers exist among flying birds? Evidence of exogenous contribution to arctic-nesting common eider eggs. Oecologia 165, 593–604 (2011). https://doi.org/10.1007/s00442-010-1853-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1853-4