Abstract
Evolved patterns of resource expenditure for reproduction have resulted in a life history continuum across species. A strictly capital-breeding strategy relies extensively on stored energy for reproduction, whereas income breeding uses energy acquired throughout the reproductive period. However, facultative income breeding has been shown in some classically capital-breeding animals, and was originally thought to provide a nutritional refuge for smaller females incapable of securing sufficient reserves during pre-partum foraging. We examined milk composition and milk output for the Weddell seal to determine to what degree lactation was aided by food intake, and what factors contributed to its manifestation. Milk composition was independent of maternal post-partum mass and condition, but did change over lactation. Changes were most likely in response to energetic and nutritional demands of the pup at different stages of development. During early lactation, females fasted and devoted 54.9% of total energy loss to milk production. Later in lactation 30.5% more energy was devoted to milk production and evidence suggested that larger females fed more during lactation than smaller females. It appears that Weddell seals may exhibit a flexible strategy to adjust reproductive investment to local resource levels by taking advantage of periods when prey are occasionally abundant, although it is restricted to larger females possessing the physiological capacity to dive for longer and exploit different resources during lactation. This supports the assumption that although body mass and phylogenetic history explain most of the variation in lactation patterns (20–69%), the remaining variation has likely resulted from physiological adaptations to local environmental conditions. Our study confirms that Weddell seals use a mixed capital–income breeding strategy, and that considerable intraspecific variation exists. Questions remain as to the amount of energy gain derived from the income strategy, and the consequences for pup condition and survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life history strategies reflect variation in the allocation of an individual’s resources (i.e., time, effort and energy expenditure) to competing life functions such as growth, survival and reproduction. The expenditure for reproduction can be broadly classified according to the temporal distribution of energy acquisition and expenditure. At one end of the continuum, capital breeding relies extensively on stored energy for reproduction, while at the other, income breeding uses energy acquired throughout the course of the reproductive period (Jönsson 1997; Stearns 1992). In mammals, larger species tend to employ the capital-breeding strategy, while smaller species generally rely on income breeding to fuel reproductive costs (Boyd 2000; Trillmich and Weissing 2006). However, various other life history characteristics of a species will determine the degree to which an animal is likely to rely on stored “capital” for breeding (Bonnet et al. 1998), and the position of an organism along the capital–income gradient will in turn be influenced by the particular evolutionary context in which life history traits, such as lactation length and size of offspring, develop.
Lactation is one of the major defining characteristics of mammals and is an essential component of their reproductive strategies. The physiology of lactation is intertwined with that of reproduction itself, and the “lactational capacity” of an individual, or species, relies on the interaction between several physiological and environmental factors (e.g., food availability, prey acquisition efficiency) that determine maternal body condition during foetal development and after parturition (Jenness 1986). Although lactation imposes energetic stress on mothers, it allows offspring to devote a higher proportion of energy (from milk) to growth rather than maintenance (Pond 1977). The proportions of nutritive constituents in milk differ greatly among species and vary during lactation according to the particular reproductive strategy (e.g., rapid development to offspring dependence vs. extended parental care) and growth patterns of the offspring (Jenness 1986).
Species within the Suborder Pinnipedia (order Carnivora) typify the extremes of the capital–income breeding continuum, with females of the Family Phocidae (“true seals”) generally following the capital strategy, while females of the Family Otariidae (fur seals and sea lions) follow the income strategy (Boyd 2000). Typically, phocids have a spatiotemporal separation between foraging and reproduction, resulting in short, intense lactation periods where milk is derived from maternal reserves and has high lipid content (e.g., at times exceeding 50% in some species—Hindell et al. 1994; Iverson et al. 1995a, b). Thus, lactation allows prepartum foraging success to support pup provisioning. Phocid provisioning is limited by these maternal reserves, and fluctuation in energy acquisition prior to the breeding season can influence both the duration and magnitude of maternal expenditure, so that females that forage successfully prior to birth will be able to transfer more energy to offspring during lactation. This in turn affects pup condition and survival (de Little et al. 2007; Hall et al. 2001; McMahon et al. 2000).
As a whole, phocids are categorized as capital breeders. Despite this, some phocid species engage in feeding during the lactation period (Bowen et al. 2001; Eisert et al. 2005; Lydersen and Kovacs 1999), suggesting that these species fall somewhere between the two extremes of the continuum, and leading to the hypothesis that food intake during this time provides an optional supplementation to body reserves for nutritionally challenged individuals. If true, the hypothesis predicts that income-like foraging should occur when the physiological state of the largely capital-investing mother is insufficient to see her pup through to independence. Moreover, this raises questions regarding the magnitude of this putative contribution, its benefits for pup growth, body condition and post-weaning survival, and the environmental contexts in which income supplementation becomes more probable.
Weddell seals (Leptonychotes weddellii Lesson) offer a unique opportunity to test predictions of a capital-breeding species’ ability to adopt income-like reproductive behavior through measures of lactation behavior and energetic expenditure. They are one of the largest phocid species, and given their extensive maternal reserves, it is generally assumed that females should be able to sustain lactation entirely from body reserves. However, the 5–6 week lactation period (Wheatley et al. 2006a) is one of the longest recorded for a phocid, and so increases the energetic demands on the fasting female relative to other phocids that have shorter lactation periods (see Trillmich 1996 for a review). There is evidence that some females feed during lactation (Eisert et al. 2005; Hindell et al. 2002), so we can directly test hypotheses related to the magnitude and form of the capital–income breeding gradient among individuals. Previous work has hypothesized that a mother’s particular combination of strategies will influence the delivery of energy to her pup and its subsequent wean mass, condition and survival probability (Wheatley et al. 2006a).
To measure the energetics of lactation effectively, the mother and pup must be measured simultaneously to separate energy used for maternal maintenance from that used for production and transfer of milk. The measurement of milk energy output over the course of lactation is the most direct assessment of total energy transfer from mother to pup (Iverson et al. 1993). This, combined with body composition changes, also reveal metabolic requirements of the female during lactation. These measurements should therefore contribute to predicting which females feed during lactation and identifying the causes and consequences of this behavior on pup condition and survival. We therefore examined an individual’s milk composition through time, milk output and the extent to which lactation was fueled by income (food intake) in Weddell seals.
Larger animals need a higher food density in the marine ecosystem to achieve a positive energy balance on short foraging trips (Boyd 1998; Trillmich and Weissing 2006), and travel time between breeding and feeding areas influences most maternal behavior (Trillmich and Weissing 2006). Consequently, we predict that foraging by Weddell seal females during pup rearing makes a relatively small contribution to the total energy budget of lactation. We hypothesized that there were two alternatives to account for feeding in females: (1) similar to some small phocids (Bowen et al. 2001), smaller or “lipid-poor” female Weddell seals will follow a capital breeding strategy at the beginning of lactation and later supplement maternal reserves with income-based provisioning as body reserves are depleted, while larger females sustain lactation entirely through capital reserves; or (2) large-bodied females will be able to engage in foraging because they have attained a certain threshold size that allows them to exploit resources (through increased dive durations) that might not be available to smaller females. We also predict these differences in mean reproductive strategy will have detectable consequences on pup growth rate and condition: (1) if foraging is energetically expensive, then small females will only engage in it when essential for their pup’s development, and pups will be in relatively poorer condition relative to pups of larger females who have enough energy to support the costs of lactation; and (2) if large females forage, then their pups should be in better condition than predicted by maternal reserves alone.
Methods
Field procedures
We studied breeding Weddell seals at Hutton Cliffs, East Antarctica (77°51′S, 166°45′E) during the austral summer (October–December) of 2002 and 2003. Thirty mother–pup pairs in 2002 and a different 25 pairs in 2003 were captured 1–6 (mean ± standard error = 3.8 ± 0.22) days post-parturition (dpp). Individual females were identified by flipper tags attached in previous years and pups born to study females were marked with hind flipper tags soon after birth as part of a long-term tagging study (Hadley et al. 2006). Age was known for 19 females in 2002 and 21 females in 2003 (range 6–21 years). Once captured, each female was restrained and chemically immobilized as described in Wheatley et al. (2006b). Females were weighed to the nearest 1 kg, and body length and axial girth measurements recorded. Each pup was weighed to the nearest 0.5 kg and length and girth measured.
Sample collection
Body composition, water flux rates and milk intake were determined using hydrogen isotope dilution at post-parturition (PP) and end-lactation (EL; 36–38 dpp; mean ± SEM 36.9 ± 0.26). After immobilization of females, a 10-ml blood sample was collected to measure background isotope levels. Immediately after, a pre-weighed dose (to the nearest 0.1 mg) of approximately 222 MBq of tritiated water (HTO) was administered to each female. Due to a shortage of HTO at the beginning of the 2002 field season, ten females were administered a dose of deuterium oxide (HDO, specific concentration 99.8%) at the PP capture while HTO was used for all other females and the EL captures (Wheatley et al. 2006a). In 2003, mother–pup pairs were recaptured at mid-lactation (ML; 21–22 dpp), weighed and measured with blood and milk samples taken (n = 25). At EL, following an initial blood sample, isotope was re-administered to the mother (n = 11) and she was measured and sampled as with the PP capture. Body composition was measured for all pups (n = 25) at PP in 2003 and for 25 and 22 pups at EL in 2002 and 2003, respectively. Approximately 74 MBq of HTO was administered in 2002, and a 10 ml dose of HDO (specific concentration 99.8%) in 2003 to each pup. A second blood sample (10 ml) was taken from all animals 150 min on average after initial injection to determine enrichment level.
Milk samples were collected using a modified 50-ml syringe approximately 10–15 min after an intravenous injection of oxytocin (1 ml, 10 IU ml−1), which was administered during immobilization. All samples were stored at −20°C until laboratory analysis.
Laboratory and data analyses
Plasma samples were analyzed for HTO activity using liquid scintillation spectrometry as described in Wheatley et al. (2006a). Mass spectrometric analysis of deuterium enrichment was done in triplicate using H2 gas and a platinum-on-alumina catalyst according to the method described in Scrimgeour et al. (1993). Milk samples (n = 59) were thawed, homogenized and analyzed for proximate composition (water, protein, and lipid content). Carbohydrate content has been found to be negligible in Weddell seals (Tedman and Green 1987), so its consideration and analysis were regarded as unnecessary. Water content was analyzed in duplicate by drying a weighed subsample (0.5–1.0 g) in an 80°C oven for 96 h. Protein content was determined by spectrophotometry according to the method of Clayton et al. (1988), modified for biological fluid with bovine serum albumin as a standard. Milk lipids were quantitatively extracted using a modified (overnight extraction) Bligh and Dyer (1959) one-phase methanol/chloroform/water extraction (2:1:0.8, v/v/v). Iverson et al. (2001) found a significant difference in lipid extraction between the Bligh and Dyer and the Folch methods; therefore, we repeated extractions on some samples from PP (n = 4) and ML (n = 4) captures using a modified version of the Folch et al. (1957) method for comparison.
Isotope dilution overestimates total body water (TBW) by 4.0% for HTO and 2.8% for HDO in grey seals (Halichoerus grypus—Reilly and Fedak 1990). Therefore, isotope dilution space was corrected for using these values. Body composition was estimated for mothers and pups from body mass and TBW according to the equations of Reilly and Fedak (1990). Total body water at mid-lactation was estimated by interpolation, assuming that the decrease in water pool mass of the female was linearly proportional to her mass loss and that the increase in each pup was proportional to mass gain (Iverson et al. 1993; Mellish et al. 1999). Therefore, TBW values at mid-lactation were restricted by the number of animals captured at end-lactation.
Total water influx (TWI) was calculated using Eq. 6 in Nagy and Costa (1980) based on TBW changing linearly with time. Milk intake (MI) was estimated according to the equation of Oftedal and Iverson (1987):
where L d and P d are the daily fat and protein deposition rates (g day−1), respectively, over the lactation period and W m, L m and P m are the water, lipid and protein contents (%), of milk, respectively. The gross energy contents of tissues and milk were calculated using values of 39.3 and 23.6 MJ kg−1 for lipid and protein, respectively (Blaxter 1989). Although we captured 22 pups at the end of lactation, milk intake estimation was restricted by milk samples (n = 10).
A series of standard generalized linear and mixed-effects models (GLM; GLMM) were constructed to examine intraspecific differences of females and pups. Candidate models were influenced by previous knowledge of relationships (or lack thereof) between some model parameters (e.g., no relationship between age and maternal post-partum mass; Wheatley et al. 2006a). Examination of the residuals for all models determined the statistical error distribution and link function that were most appropriate. Model selection was based on Akaike’s Information Criterion corrected for small samples (AICc, Burnham and Anderson 2002). The information-theoretic weight of evidence (w+ i ) for each predictor was calculated by summing the model AICc weights (w i ) over all models in which each term appeared. However, the w+ i values are relative, not absolute, because they will be >0 even if the predictor has no contextual explanatory importance (Burnham and Anderson 2002). To judge which predictors were relevant to the data at hand, a baseline for comparing relative w+ i across predictors was required, so we randomized the data for each predictor separately, recalculated w+ i , and repeated this procedure 100 times for each predictor. The median of this new randomized w+ i distribution for each predictor was taken as the baseline (null) value (w+0). For each term the absolute weight of evidence (Δw+) was obtained by subtracting w+0 from w+ i , and predictors with Δw+ of zero or less have essentially no explanatory power (Burnham and Anderson 2002). Specific model comparisons were based on the information-theoretic evidence ratio (ER), which is equivalent to the AICc weight (w) of the full model divided by the w of the null (intercept) model (Burnham and Anderson 2002). Higher ER values indicate higher likelihoods of the tested model relative to the null. We also calculated the percent deviance explained (%DE) by each model used as a measure of model goodness-of-fit. All statistical analyses were done using the R Package (ver. 2.3.1; R Development Core Team, 2004). Values are presented as mean ± 1 SE of the mean (SEM) unless otherwise stated. See Table 1 for a list of abbreviated terms.
Results
Milk composition
Milk samples (n = 8, from PP and ML) had an average lipid content of 48.7 ± 3.0% with the modified Folch et al. (1957) method, and 48.1 ± 3.0% with the (overnight extraction) Bligh and Dyer (1959) method. Comparison between the two revealed no evidence for a difference (ER = 0.37).
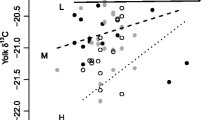
Milk composition changed over the course of lactation (see the table in the “Electronic supplementary material”), and individual variability in composition can be seen at each stage of lactation (Fig. 1). Protein (%P) increased throughout lactation, while lipid (%L) and energy content increased slightly to ML and then decreased again (Fig. 1). The number of days elapsed since parturition (dpp) explained 63.2% of the variation in protein (n = 60). For %L, water (%W) and energy, dpp explained more of the variation than the null model (ER: %L = 5.06 × 1013; %W = 2.55 × 1015; energy = 1.63 × 1021; n = 60); however, the goodness-of-fit of these models was lower (%DE: 1.19, 9.79 and 9.92, respectively). Milk composition at end-lactation from one female (Pu194) was excluded from averages and analyses because there was an indication that weaning may have occurred (%L = 8.2, %P = 18.8, %W = 72.1).
There was no evidence that maternal post-partum mass (MPPM), total body lipid stores (TBLMPPM) or total body protein stores (TBPMPPM) affected milk composition (%L, %P, %W and energy content) at post-partum (Δw+ ≤ 0 for all terms; n = 25).
Milk output and energy flux
For ten females with body and milk composition data at post-parturition, and milk and estimated body composition data at mid-lactation, we calculated total energy loss and total milk energy output to determine the proportion used for metabolism versus milk energy (%ME) to the pup. This was repeated for eight of the females from mid-lactation to end-lactation (Table 2). If the decrease in water pool mass of the female was not linearly proportionally to her mass loss (as assumed), milk intake may be underestimated, affecting pup energy gain calculations.
In early lactation, average %ME was 54.9 ± 0.04% of the total energy lost by the female (n = 10). Between mid-lactation and end-lactation, %ME exceeded that of total energy lost by the female in almost all cases (Table 2). From evidence that females at this location do not feed during the first three weeks of lactation (Eisert et al. 2005), and that %ME (PP–ML) was similar to that of non-feeding southern elephant seals (57.2%; Mirounga leonina, Hindell and Slip 1997) and northern elephant seals (59%; Mirounga angustirostris, Costa et al. 1986), we considered the %ME from post-parturition to mid-lactation to represent that of a non-feeding individual. We assumed that percent milk energy values above this (between ML and EL) represented an external energy source (i.e., mother feeding). We were unable to calculate metabolic rates and therefore could not quantify energy acquired from feeding. Instead, we calculated a “feeding index” for the second half of lactation as:
which represents the number of times that %ME exceeded that of non-feeding individuals (Table 2). This allowed us to rank individuals by the relative amount of feeding. Total energy loss (MJ) was 69.1 ± 0.05% lower in the second half of lactation (n = 8), while total milk energy output (MJ) was 30.5 ± 0.10% higher (n = 8). Therefore, although female energy loss was lower in the second half of lactation, more energy was transferred to the pups.
There was a strong correlation between %ME and energy transfer efficiency (pup energy gain/female energy loss; ER = 284.2; n = 18). This relationship was used to estimate %ME for females captured in 2002, for which there were no milk data (Wheatley et al. 2006a, Table 3). A feeding index for the last half of lactation was also calculated using the average %MEPP − ML for non-feeding animals from 2003 (54.9%, Table 3). Milk intake by pups was also a measure of milk output of mothers. The most-parsimonious GLM testing for the effect of age, MPPM and TBLMPPM on maternal milk output (kg day−1) from post-parturition to mid-lactation included MPPM and age, although only MPPM explained an important component of the variation in this model (Δw+MPPM = 0.216, Δw+TBL = 0.026, Δw+age = 0.000; n = 29), with milk output increasing with MPPM. Daily milk output increased 34.7% from 3.2 ± 0.19 kg day−1 in early lactation to 4.9 ± 0.37 kg day−1 in late lactation (Table 4). This coincided with an increase in daily milk energy output of 35.8%, or 64.4 ± 4.24 MJ day−1 in early lactation and 100.3 ± 7.57 MJ day−1 in late lactation. However, the energy stored per day (as body tissue) by pups decreased by an average of 3.9% between early and late lactation; this equated to a total decrease in energy storage efficiency (energy stored/energy gained) of 59.7% (Table 4).
The most parsimonious model testing for the effect of MPPM, TBLMPPM and year on total %ME included TBLMPPM and year; however, year was the only variable with information-theoretic support (Δw+year = 0.183; Δw+ ≤ 0.000 for all others; n = 29). Model averaging estimated that females in 2002 had 4.1% higher %ME than females in 2003. The most parsimonious model testing for the effect of age, MPPM and year on feeding index included age and MPPM; however, MPPM was largely responsible for driving the relationship (Δw+MPPM = 0.721, Δw+age = −0.022, Δw+year = −0.047; n = 43), indicating that larger females fed more during lactation (Fig. 2). There was also a strong positive relationship between feeding index and daily mass gain (kg day−1) of pups (feeding index = 0.79 × mass gain + 0.85; R 2 = 0.44; n = 43).
Due to the increase in milk protein throughout lactation, we measured whether there was a relationship between total body protein depletion (kg) from the female’s body stores and the feeding index (for both years). We found a positive linear relationship (ER = 10.25, %DE = 20.3%; n = 29), indicating that females who lost more protein had a higher rate of feeding.
Discussion
Although we could not estimate the absolute amount of feeding that occurred (total MJ), we were able to rank females according to their relative feeding frequencies during the latter part of lactation. We found that there was up to a five-fold difference between individuals in the feeding index (Tables 2, 3), and that larger (mass) females fed more frequently, but there was no difference between years. Dive duration increases with body mass (Kooyman 1989; Le Boeuf 1994), so a plausible explanation for the observed trend is that larger females are able to exploit resources in McMurdo Sound more efficiently than their smaller counterparts, especially where population density (Stirling 1969) and intraspecific competition is high (Hindell et al. 2002). In other words, only after attaining a certain threshold body size and condition will the costs of extra foraging during late-lactation be outweighed by the additional energy acquired for milk production. The positive relationship we observed between the maternal feeding index and daily mass gain of pups may support the hypothesis that larger females can deliver relatively more energy to their pup than predicted by their body reserves alone (i.e., the rich getting richer), or be confounded by the observation that large females (with a higher feeding index) have the capacity to deliver more energy to their pup (Wheatley et al. 2006a), regardless of feeding. The lack of an age effect suggests that reproductive experience is not as important as maternal mass per se in determining the delivery of energy to the pup given the lack of correlation between body size and age found previously (Wheatley et al. 2006a). Therefore, smaller females not only had less capital available to pups at the onset of lactation, they were also less capable of supplementing that disadvantage with late-lactation feeding (i.e., the poor remaining poor). We conclude then that the lactation-feeding phenomenon in this mainly capital-breeding mammal does not provide an effective nutritional refuge for smaller females incapable of securing sufficient reserves during their pre-parturition foraging trips; rather, it appears to be a flexible strategy employed by those individuals with the physiological capacity to supplement their capital reserves with income feeding.
Although most (20–69%) variation in pinniped lactation patterns can be explained by phylogenetic history and body size, the remaining variation has likely resulted from adaptations to local environmental conditions (Ferguson 2006). It appears that differences in life history strategies exist within lactating Weddell seals and these have resulted from several interrelated features: (1) relatively precocial pups that are able to swim and dive during lactation, (2) one of the longest lactation periods of any phocid, and (3) milk protein increases throughout lactation, depleting maternal body stores. These factors may influence the extent to which each female relies on stored energy to fuel lactation and the physiological capacity to forage later in lactation. In some respects, the existence of flexible foraging strategies in Weddell seals questions the true nature of the phocid lactation continuum, and it also suggests that other species may also frequently break out of their physiological pigeon-hole to engage in “anomalous” lactation behaviors.
The dichotomy between capital and income breeders is a scheme that is increasingly used to understand the life history strategies of birds (Gauthier et al. 2003; Meijer and Drent 1999), reptiles (Bonnet et al. 1998), parasites (Casas et al. 2005) and pinnipeds (Boyd 1998; Trillmich and Weissing 2006). The demographic cost of reproductive investment (i.e., decreased survival and future reproduction as a function of current reproduction) is a pivotal trade-off around which life histories are thought to evolve (Harshman and Zera 2006; Stearns 1992). Our results provide additional insight into the selective forces driving the evolution of particular strategies (at least, in mammals), and suggest that local environmental conditions may alter evolved behaviors to the point where individuals have some capacity to adapt to temporally variable resource availability beyond that predicted by their phylogenetic origins.
The results of our study therefore assist in our understanding of the trade-offs associated with reproductive strategies and life history theory in general. The pattern of milk composition in Weddell seals that we described is different to that reported by Tedman (1980), Tedman and Green (1987) and to that of other phocid seals. Tedman (1980) found no evidence for milk composition changes throughout lactation; however, his analysis was based on cross-sectional data, low sample sizes (total n = 11), and high variance. Our study was more detailed and longitudinal, revealing overall that milk composition does indeed change over the course of lactation. Lipid content at PP (39.9%) was higher than that reported for southern elephant seals (16.1%; Hindell et al. 1994) but similar to that of harbour seals (40.8%; Phoca vitulina, Lang et al. 2005) and grey seals (34.5%; Mellish et al. 1999). Rather than a gradual increase throughout lactation, we found instead that lipid increased to mid-lactation and then declined toward post-parturition levels. Protein changes (low post-parturition, then tripling) were the least similar to any other seal species, and the large increase and resultant muscle growth may balance increased fat deposition due to higher energy intake in pups early in lactation. Although there was individual variability in milk composition (at parturition, see Fig. 1), it was not related to maternal post-partum mass or body composition. However, maternal post-partum mass did influence milk output (kg day−1), with larger females producing more milk. This was consistent with the previously reported positive relationship between maternal post-partum mass and pup mass again (Wheatley et al. 2006a).
The observed pattern of milk composition changes in Weddell seals helps explain why they have a longer lactation period than similar-sized phocids. For instance, at parturition, mothers must devote resources to producing lipid-rich milk for the pup’s thermoregulatory needs, followed by a later increase in protein required for lean tissue growth. Protein values at mid- and end-lactation were similar to those reported for the income-breeding Antarctic fur seal (Arctocephalus gazella—Arnould and Boyd 1995) late in lactation. Therefore, this need for protein later in lactation may require females to feed rather than deplete their own stores (i.e., muscle), as well as influence total lactation length. Our measures of milk output and storage efficiency were similar to those measured by Tedman and Green (1987) averaged over the entire lactation period; however, we have demonstrated that changes occur during lactation. Milk output increased over lactation, similar to other species (Lydersen and Kovacs 1996; Mellish et al. 1999). In contrast, the rate of pup mass gain decreased. This may be the result of reduced storage efficiency and higher energy expenditure of pups that engage in swimming and diving, a phenomenon not normally observed in pre-weaned pups of most other phocids.
Milk energy represented the largest proportion of female energy expenditure during lactation, and fatter females were able to devote a higher percentage of their energy loss to milk production than their own metabolic requirements. This may result from lower lean body mass, culminating in a lower metabolic overhead that allows for more energy to be devoted to milk production. Through differences in maternal post-partum mass and expenditure during lactation, Wheatley et al. (2006a) provided evidence for environmental variation between the two years of study (2002, 2003). Similar results were found here where the year term was the only variable found to influence milk energy delivery for the entire duration of lactation, with more energy (4.1%) delivered in 2002. This was not related to increased foraging success in one year (2002), but based on energy transfer efficiency rates (Wheatley et al. 2006a). Accordingly, it may be related to females devoting more time foraging in 2003, albeit with little success, as maintenance expenditure (i.e., diving costs) dominated milk production in that year.
We conclude that the income acquired during lactation in this predominantly capital-breeding mammal is relatively nominal because milk lipid values late in lactation were similar to those at parturition, mass loss rates of the females were not different between early and late lactation, and pup mass gain was lower than at the beginning of lactation. Although differences did exist in the amount of feeding occurring among individuals, effects on “lactational capacity” were small and support the hypothesis that large Weddell seal mothers are more opportunistic feeders during lactation when they can add energetic value to their expenditure. This strategy favors fast delivery rates of energy over a short period of time, but provides the behavioral and physiological plasticity to maximize offspring survival via supplementary (income-fueled) expenditure when conditions are suitable.
References
Arnould JPY, Boyd IL (1995) Inter- and intra-annual variation in milk composition in Antarctic fur seals (Arctocephalus gazella). Physiol Zool 68:1164–1180
Blaxter K (1989) Energy metabolism in animals and man. Cambridge University Press, Cambridge
Bligh EG, Dyer WJ (1959) A rapid method of lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bonnet X, Bradshaw D, Shine R (1998) Capital versus income breeding: an ectothermic perspective. Oikos 83:333–342
Bowen WD, Iverson SJ, Boness DJ, Oftedal OT (2001) Foraging effort, food intake and lactation performance depend on maternal mass in a small phocid seal. Funct Ecol 15:325–334
Boyd IL (1998) Time and energy constraints in pinniped lactation. Am Nat 152:717–728
Boyd IL (2000) State-dependent fertility in pinnipeds: contrasting capital and income breeders. Funct Ecol 14:623–630
Burnham KP, Anderson DR (2002) Model selection and multimodal inference: a practical information-theoretic approach. Springer, New York
Casas J, Pincebourde S, Mandon N, Vannier F, Poujol R, Giron D (2005) Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 86:545–554
Clayton JR, Dortch Q, Thoresen SS, Ahmed SI (1988) Evaluation of methods for the separation and analysis of proteins and free amino acids in phytoplankton samples. J Plankton Res 10:341–358
Costa DP, LeBoeuf BJ, Huntley AC, Ortiz CL (1986) The energetics of lactation in the northern elephant seal, Mirounga angustirostris. J Zool (Lond) 209:21–33
de Little SC, Bradshaw CJA, McMahon CR, Hindell MA (2007) Complex interplay between intrinsic and extrinsic drivers of long-term survival trends in southern elephant seals. BMC Ecol 7:3
Eisert R, Oftedal OT, Lever M, Ramdohr S, Breier BH, Barrell GK (2005) Detection of food intake in a marine mammal using marine osmolytes and their analogues as dietary biomarkers. Mar Ecol Prog Ser 300:213–228
Ferguson SH (2006) The influence of environment, mating habitat, and predation on evolution of pinniped lactation strategies. J Mamm Evol 13:63–82
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gauthier G, Bêty J, Hobson KA (2003) Are greater snow geese capital breeders? New evidence from a stable isotope model. Ecology 84:3250–3264
Hadley GL, Rotella JJ, Garrott RA, Nichols JD (2006) Variation in probability of first reproduction of Weddell seals. J Anim Ecol 75:1058–1070
Hall AJ, McConnell BJ, Barker RJ (2001) Factors affecting first-year survival in grey seals and their implications for life history strategy. J Anim Ecol 70:138–149
Harshman LG, Zera AJ (2006) The cost of reproduction: the devil in the details. Trends Ecol Evol 22:80–86
Hindell MA, Slip DJ (1997) The importance of being fat: maternal expenditure in the southern elephant seal Mirounga leonina. In: Hindell MA, Kemper C (eds) Marine mammal research in the southern hemisphere volume 1: status, ecology and medicine, vol 1. Surrey Beatty & Sons, Chipping Norton, Australia, pp 72–77
Hindell MA, Bryden MM, Burton HR (1994) Early growth and milk-composition in southern elephant seals (Mirounga leonina). Aust J Zool 42:723–732
Hindell MA, Harcourt R, Waas JR, Thompson D (2002) Fine-scale three-dimensional spatial use by diving, lactating female Weddell seals Leptonychotes weddellii. Mar Ecol Prog Ser 242:275–284
Iverson SJ, Bowen WD, Boness DJ, Oftedal OT (1993) The effect of maternal size and milk output on pup growth in grey seals (Halichoerus grypus). Physiol Zool 66:61–88
Iverson SJ, Hamosh M, Bowen WD (1995a) Lipoprotein lipase activity and its relationship to high milk fat transfer during lactation in grey seals. J Comp Physiol B 165:384–395
Iverson SJ, Oftedal OT, Bowen WD, Boness DJ, Sampugna J (1995b) Prenatal and postnatal transfer of fatty acids from mother to pup in the hooded seal. J Comp Physiol B 165:1–12
Iverson SJ, Lang SLC, Cooper MH (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36:1283–1287
Jenness R (1986) Symposium: species variation in mammary gland function. J Dairy Sci 69:869–885
Jönsson KI (1997) Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66
Kooyman GL (1989) Diverse divers. Springer, Berlin
Lang SLC, Iverson SJ, Bowen WD (2005) Individual variation in milk composition over lactation in harbour seals (Phoca vitulina) and the potential consequences of intermittent attendance. Can J Zool 83:1525–1531
Le Boeuf BJ (1994) Variation in the diving pattern of northern elephant seals with age, mass, sex, and reproductive condition. In: Le Boeuf BJ, Law RJ (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, CA, pp 237–252
Lydersen C, Kovacs KM (1996) Energetics of lactation in harp seals (Phoca groenlandica) from the Gulf of St. Lawrence, Canada. J Comp Physiol B 166:295–304
Lydersen C, Kovacs KM (1999) Behaviour and energetics of ice-breeding, North Atlantic phocid seals during the lactation period. Mar Ecol Prog Ser 187:265–281
McMahon CR, Burton HR, Bester MN (2000) Weaning mass and the future survival of juvenile southern elephant seals, Mirounga leonina, at Macquarie Island. Antarct Sci 12:149–153
Meijer T, Drent RH (1999) Re-examination of the capital and income dichotomy in breeding birds. Ibis 141:399–414
Mellish J-AE, Iverson SJ, Bowen WD (1999) Variation in milk production and lactation performance in grey seals and consequences for pup growth and weaning characteristics. Physiol Biochem Zool 72:677–690
Nagy KA, Costa DP (1980) Water flux in animals: analysis of potential errors in the tritiated water method. Am J Zool 238:R454–R465
Oftedal OT, Iverson SJ (1987) Hydrogen isotope methodology for measurement of milk intake and energetics of growth in suckling young. In: Huntley AC, Costa DP, Worthy GAJ, Castellini MA (eds) Approaches to marine mammal energetics. Allen Press, Lawrence, KS, pp 67–96
Pond CM (1977) Significance of lactation in evolution of mammals. Evolution 31:177–199
Reilly JR, Fedak MA (1990) Measurement of the body composition of living gray seals by hydrogen isotope dilution. J Appl Physiol 69:885–891
Scrimgeour CM, Rollo MM, Mudambo MKT, Handley LL, Prosser SJ (1993) A simplified method for deuterium/hydrogen isotope ratio measurements on water samples of biological origin. Biol Mass Spectrom 22:383–387
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stirling I (1969) Ecology of the Weddell seal in McMurdo Sound, Antarctica. Ecology 50:574–585
Tedman RA (1980) Lactation in the Weddell seal, Leptonychotes weddellii (Lesson). Department of Anatomy, University of Queensland, Brisbane, Australia, p 305
Tedman RA, Green B (1987) Water and sodium fluxes and lactational energetics in suckling pups of Weddell seals (Leptonychotes weddellii). J Zool (Lond) 212:29–42
Trillmich F (1996) Parental investment in pinnipeds. Adv Study Behav 25:533–577
Trillmich F, Weissing FJ (2006) Lactation patterns of pinnipeds are not explained by optimization of maternal energy delivery rates. Behav Ecol Sociobiol 60:137–149
Wheatley KE, Bradshaw CJA, Davis LS, Harcourt RG, Hindell MA (2006a) Influence of maternal mass and condition on energy transfer in Weddell seals. J Anim Ecol 75:724–733
Wheatley KE, Bradshaw CJA, Harcourt RG, Davis LS, Hindell MA (2006b) Chemical immobilization of adult female Weddell seals with tiletamine and zolazepam: effects of age, condition and stage of lactation. BMC Vet Res 2:8
Acknowledgments
We thank P. Brewin, M. Brock, W. Cook, L. Davis, D. Geddes, A. Hall, P. Isherwood, C. Littnan, B. Mooney, P. Nichols and volunteers from Scott Base for assistance in the field and laboratory. This study was supported by funds from an Australian Research Council Discovery Project grant (DP0342714), the University of Tasmania Institutional Research Grants Scheme and Sea World Research & Rescue Foundation, Inc. We thank Antarctica New Zealand for providing logistical support and the Australian Antarctic Division for providing some field clothing. S.T. Pochron and two anonymous reviewers provided constructive comments on the manuscript. Data were collected under permits from the University of Tasmania Animal Ethics Committee (A6790 and A6711), and the Department of Conservation of New Zealand (Per/22/2002/149 and Per/17/2003/188). This study was conducted in accordance with the current laws of Australia and New Zealand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jörg Ganzhorn.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wheatley, K.E., Bradshaw, C.J.A., Harcourt, R.G. et al. Feast or famine: evidence for mixed capital–income breeding strategies in Weddell seals. Oecologia 155, 11–20 (2008). https://doi.org/10.1007/s00442-007-0888-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0888-7