Abstract
Chromatic signals of offspring quality have been shown to play a role in parent–offspring communication in diurnal birds, but are assumed to be useless in dim light conditions because colour-based discrimination probably requires more light. A major ecological and evolutionary conundrum in this scenario is why the nestlings of some nocturnal owls display colourful beaks. Here, we test the hypothesis that yellow bill coloration of owlets of the nocturnal little owl Athene noctua may function as a chromatic signal revealing to parents aspects of quality of their offspring. In a first step, we examined physical variation in bill coloration and its covariation with owlet quality. Secondly, we studied parental provisioning in relation to an experimental manipulation of bill coloration of owlets. Bills of owlets showed higher within-nest variation in yellow–red chroma than in brightness. Plasma carotenoid concentration and nestling immunological status were not associated with chromatic or achromatic features of the bill. Interestingly, however, heavier owlets displayed more yellow bills than lighter ones. The effect of bill coloration on parental favouritism changed with brood size. Parents holding large broods preferentially fed owlets with enhanced over reduced yellow bill coloration, whereas those with small broods did not significantly bias feeding in relation to owlet bill coloration. Our results, based on integration of objective spectrophotometric assessment of colour and experimental procedures, confirm that parent little owls use bill coloration to reveal information on owlet body mass to adjust their feeding strategies, thus highlighting the importance of considering potential chromatic signals for a full comprehension of parent–offspring communication processes in nocturnal bird species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diurnal birds rely in the sense of vision for communicating with their offspring. Coloration of gapes is a key component of nestling begging displays in altricial birds (reviewed in Kilner 2006), and can provide parents with information on nestling level of satiation or health (e.g. Kilner 1997; Saino et al. 2000, 2003; Thorogood et al. 2008), or with a conspicuous target towards which to direct their feeds (Heeb et al. 2003; Avilés et al. 2008; Wiebe and Slagsvold 2009). It also influences parental feeding decisions (e.g. de Ayala et al. 2007; Ewen et al. 2008; Dugas 2009). Beyond coloration of gaping structures, chromatic characteristics of the skin may also function as quality signals of the offspring (Jourdie et al. 2004; Soler et al. 2007), and mediate parental favouristim (Bize et al. 2006; Avilés et al. 2011). Once nestlings became feathered, parents may also respond to changes in chromatic characteristics of plumage of their offspring (e.g. Tanner and Richner 2008; Galván et al. 2008; Griggio et al. 2009).
Among the factors influencing the efficacy of visual begging displays, light availability for reflection plays a key role (Ficken 1965; Kilner and Davies 1998; Heeb et al. 2003; Wiebe and Slagsvold 2009). Comparative evidence shows that nestlings of species nesting in poorly illuminated holes showed gaping designs maximizing achromatic-based discrimination (Kilner and Davies 1998; Avilés et al. 2008), suggesting that selection for nestling detectability in dim light may have favoured the evolution of particular achromatic features of gapes. Birds have two main classes of photoreceptors in their retinas, cones and rods, that mediate vision at different light levels (Kelber et al. 2003). In general, colour vision functions in bright lights, when the number of photons reaching at least two cone photoreceptors is large enough to create a contrasting signal given photoreceptor noise (Vorobyev et al. 2001). Avian colour vision, however, is lost at low light levels, and its functionality is replaced by achromatic discrimination based on the stimulation of single rod photoreceptors (Kelber et al. 2003). In agreement with a differential implication of cones and rods in visual discrimination at different light levels, anatomical studies of avian retina have shown that nocturnal species have a rod-dominated retina, whereas diurnal species have a cone-dominated one (Hart and Hunt 2007). Yet determining the light threshold for the functioning of avian colour vision, and its influence on the design of signals implicated in visual communication, remain major challenges in evolutionary ecology (Lind and Kelber 2009; Kelber and Lind 2010).
Nocturnal species must resolve the sensory challenge of feeding their offspring at night, when there might be insufficient light for chromatic-based discrimination (Martin 1990; Kelber et al. 2003). Therefore, nestlings of these species are expected to evolve non-visual begging signals and/or achromatic-based begging signals allowing visual discrimination at low light levels. Analogically, comparative evidence has shown that nestlings of hole-nesting species (i.e. fed under lower light levels) display brighter begging gapes than nestlings of open-nesting species (Avilés et al. 2008). In addition, plumage designs in nocturnal species are dominated by white plumage patches intermingled with high-contrasting dark backgrounds (Aragonés et al. 1999; Penteriani and Delgado 2009) producing achromatic (intensity related but colour blind) designs. For instance, nocturnal eagle owls Bubo bubo have evolved white plumage patches allowing them to communicate in dim light conditions (Penteriani and Delgado 2009). Male eagle owls displayed more frequently toward intruder male mounts with low-brightness white badges than toward male mounts with normal white badge in brightness (Penteriani et al. 2007a). Also, brightness-dependent physiological responses of owlets during the post-fledging dependence period may constitute evidence for achromatic-based parental preference in this species (Penteriani et al. 2007b). In addition, in the nocturnal scops owl Otus scops, sibling variation in ultraviolet colour of the cere of the bill signalled owlet body mass and was used by parents to decide their food allocation strategy (Parejo et al. 2010). Although the fact that parent scops owls responded to a manipulation of the UV reflectance of their offspring supports parental use of visual cues, it is unclear whether parental provisioning responses were due to changes in chromatic and/or achromatic features of the cere, as they were both affected by cere colour manipulation (see Parejo et al. 2010).
The little owl Athene noctua is a resident small cavity-nesting owl inhabiting a wide variety of semi-open habitats across its distributional range in Europe. It preys on small mammals, birds, reptiles and insects that are hunted from perches. Little owls hunt essentially during the crepuscule and night, showing a peak between dusk and midnight and then before resumption to dawn (Del Hoyo et al. 1999). However, the degree of nocturnal activity is variable between geographic regions and diurnal hunting activity has been frequently recorded in the south of Europe (Negro et al. 1990). Indeed, video-filming of nests in our Spanish population has even shown that food is frequently delivered to owlets during the day (J.M. Avilés and D. Parejo, unpublished data). The dark bill of little owl owlets adopts an adult-like colourful olive-yellow of unknown function appearance a few days before fledging (Cramp 1998; Fig. 1). Little owl owlets can climb up to the nest entrance when they are 3 weeks, and can even receive food deliveries from their parents outside the nests while branching for a few weeks before being fully fledged (Del Hoyo et al. 1999). Bill colour can change over a short time period when it relies on deposition of pigments (e.g. Faivre et al. 2003; Navarro et al. 2010; Karubian et al. 2011), and so might communicate information to parents (e.g. Dugas 2009; Thorogood et al. 2011). Indeed, we have recently shown in a sample of wild adult female little owls that yellowness of the bill was highly variable between individuals and positively correlated with owlet fitness prospects, as expected if chromatic components of bill coloration revealed aspect of female quality in a sexual selection scenario (Avilés and Parejo 2012).
The main aim of this study is to test the hypothesis that yellow bill coloration of little owl Athene noctua owlets may function as a chromatic stimulus revealing to parents aspects of quality of their offspring. In a first step, we examine physical variation in bill coloration and its covariation with owlet quality at fledging (i.e. body size, and immune responsiveness, estimated as phytohemagglutinin (PHA)-induced tissue swelling). We predicted that (1) if parents allocate resources within a nest on the base of bill color differences of their offspring, bill coloration between siblings should vary. In addition, we predicted (2) that bill coloration covaried with phenotypic traits of owlets. Previous studies have found that the yellow-orange beak of diurnal raptors may function as a carotenoid-based signal of adult quality (e.g. Bortolotti et al. 2000, 2003); therefore, aiming to preliminary investigate the mechanism behind bill coloration in little owls, we tested for a relationship between bill coloration and plasma carotenoid level. Specifically, we predicted (3) that yellowness covaried with plasma carotenoid level if carotenoids were the mechanism responsible of yellow bill coloration.
In a second stage, we studied parental provisioning behaviour at the end of the nestling period during nocturnal conditions in relation to owlet bill coloration. To this end, half of the owlets in each brood were treated with a paint that enhanced yellow bill coloration and their body mass gain was compared to that of siblings treated with a paint that dulled bill coloration. Interestingly, our bill colour manipulation affected chromatic but not achromatic aspects of bills (see “Materials and methods”), thus allowing the evaluation of the separate role of a likely chromatic signal of quality in parent-offspring communication in a nocturnal bird.
Materials and methods
Study population
The field study was carried out in a breeding population of little owls in the surroundings of Guadix (37º18′N, 3º11′W), southern-east Spain, in April–June during 3 years (2009–2011). The vegetation is sparse in the area, with cultivated cereals and some remains of holm-oak forests (see Avilés et al. 2008 for details). In this population, most little owls regularly breed in cork oak nest-boxes placed on trees (mainly holm oaks) or on artificial supports (i.e. electric pylons or walls) (see Parejo and Avilés 2011; Rodríguez et al. 2011 for details). Little owls are monogamous and lay clutches of two to six eggs [average (SD); 4.1(0.85) eggs; J.M. Avilés and D. Parejo, unpublished data] which are incubated solely by the female, while both parents actively care for the owlets during a nestling period of 30–35 days. Parents can store some food in the nest but they feed directly to their owlets until the end of the nestling period (J.M. Avilés and D. Parejo, unpublished data). All nesting attempts during the 2009–2011 breeding seasons were monitored, with occupation of nest-boxes, clutch size, date of clutch completion and hatching recorded.
Quality and colour measurements of owlets
In 2009, we measured yellow bill colour, owlet condition, and plasma carotenoid levels at day 22 after hatching of the first egg, and estimated immunocompetence at day 25 for all the owlets in the population. At that time, parents can assess bill coloration before feeding their offspring, because the owlets do not open their bills while begging as wide as do passerines.
Bill coloration of owlets from 320 to 700 nm was measured using a spectrometer [S2000 Ocean Optics equipment connected to a deuterium-halogen light (D2-W, mini) by a coaxial reflectance probe (QR-400-7-UV-vis) and the OOIBase32™ operating software; Ocean Optics, Dunedin, FL, USA)] (Fig. 1a). Reflectance was measured with a 45° angle probe completely touching the owlet to prevent any stray light from entering. Measurements were relative and referred to a standard white (WS-2) and to the dark (i.e. blocking the entering light by placing an opaque cap in the end of the reflectance probe), which we calibrated before the measurement of all brood mates in a nest. All measurements were taken within a portable hide with opaque wall set in the surroundings of the nests. Measurements were repeated three times and the three spectra obtained for each owlet were averaged and used in subsequent analyses, which is justified given that brightness and yellow chroma (see below) showed significantly high repeatability (repeatability > 95.4 %, F 57,116 > 63.81, P < 0.0001 in the two cases).
Despite the owlet bill being perceived by humans as yellow (Fig. 1a), reflectance spectra revealed that, besides a prominent peak around 550–625 nm (i.e. the yellow part of the spectrum), little owl bills had a second marked peak in the UV (350–400 nm) part of the spectrum (Fig. 1b). Therefore, we opted for summarizing reflectance spectra by using standard descriptors of color [i.e. brightness (i.e. summed reflectance between 320 and 700 nm)], yellow–red chroma (i.e. ratio in summed reflectance between 550 and 700 nm and total reflectance), ultraviolet chroma (i.e. ratio in summed reflectance between 320 and 400 nm and total reflectance) and hue (the wavelength at which the maximum peak of reflectance is reached). Ultraviolet chroma and hue were strongly correlated with yellow–red chroma so that bills with higher yellow–red chroma were invariably less saturated in the ultraviolet and had a hue displaced towards longer wavelengths (Table S1). Therefore, we disregarded using ultraviolet chroma and hue in further analyses given their obvious interdependence with yellow–red chroma.
After collection of colour data, we immediately took blood samples in heparinized capillary tubes that were stored refrigerated until centrifugation and collection of plasma. Plasma was frozen at −70 °C until analysis of carotenoid concentration. Owlets were also weighed with a Pesola spring balance to the nearest 0.5 g.
Three days later, all owlets were injected subcutaneously with phytohemagglutinin-P (PHA-P; Sigma Chemical) in the wing web to evaluate the in vivo T cell-mediated immune response following standardised protocols (e.g. Smits et al. 1999; Alonso-Alvarez and Tella 2001). Briefly, after measuring wing web thickness (with a Baxlo digital pressure-sensitive micrometer, model 3000, to the nearest 0.01 mm), we injected fledglings subcutaneously in the right wing web with 0.2 mg of PHA dissolved in 0.04 ml of physiological saline solution (Bausch and Lomb). The left wing web was injected with 0.04 ml of physiological saline solution. We measured the thickness of each wing web at the injection site before and 24 h after the injection and estimated T cell-mediated immune response as the change in thickness of the right wing web (PHA injection) minus the change in thickness of the left wing web. We repeated measurements of thickness of each wing web three times, which was highly repeatable (repeatability = 97.2 %, F 97,196 = 106.7, P < 0.0001) and, thus, the mean value was used in subsequent analyses.
Owlet perception
Avian visual perception has been modelled in complex ways, incorporating retinal sensitivities and ambient wavelengths in diurnal bird species (e.g. Vorobyev et al. 1998; Endler et al. 2005; Avilés and Soler 2009). Little owls, however, are active in dim twilight when visual models based on stimulation of single photoreceptor cones do not provide reliable estimates of chromatic performance (Vorobyev and Osorio 1998). Therefore, we relied on colour variables extracted from spectrophotometric data that have successfully been used to study the potential signalling of yellow bill coloration in diurnal (e.g. Peters et al. 2004; Navarro et al. 2010) and nocturnal birds (e.g. Parejo et al. 2010; Avilés and Parejo 2012).
Plasma carotenoid concentration
HPLC analysis performed on a randomly selected subsample of owlets indicated that the main carotenoid present in the plasma of little owls was lutein which co-eluted with relatively small quantities of zeaxanthin (J.M. Avilés and D. Parejo. unpublished data). Therefore, we measured total lutein concentration spectrophotometrically in all the samples (e.g. Tella et al. 1998; Bortolotti et al. 2000; Laaksonen et al. 2008). Plasma (40 μl) was mixed with pure acetone (240 μl) and centrifugated at 10,000 rpm during 10 min (Spectrafuge 24D; Labnet International, NJ, USA). We then determined the absorbance at 446 nm, the highest peak of the lutein spectrum in acetone, in the resulting supernatant by using a microplate reader (Anthos 2020; Austria). We confirmed linearity in a dilution series of five plasma samples [1:3–1:20; absorbance = 0.097 (lutein concentration) – 0.023, R 2 = 0.998] and estimated concentration (μg/mL) using the extinction coefficient of lutein in acetone at 446 nm (Laaksonen et al. 2008).
Experimental design
During the 2010 and 2011 breeding seasons, we studied parental food allocation in relation to owlet bill coloration by exaggerating a presumed carotenoid-rich bill (i.e. enhanced) of randomly chosen owlets within each brood and comparing their body mass gain with that of siblings in which brightness of the bill was slightly reduced (i.e. dulled) to compensate for the possible achromatic effect. To enhance bill coloration, we painted the bill with a nontoxic yellow Fabricolor felt-tipped art marker (TC-4000 no. 50, Yellow); to dull bill colour, we applied a gray Fabricolor marker (TC-4000 no.93, Grey). This method of colour modification has no harmful effects on birds (e.g. Velando et al. 2006) and their effects on bills lasted less than 24 h.
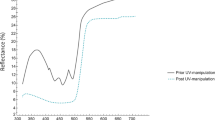
We estimated changes in bill reflectance before and after painting the bill on a subsample of 18 owlets at six nests of which 9 were painted to enhance and 9 to dull their yellow bill coloration. The yellow-enhancing treatment effectively enhanced yellow coloration as the yellow–red chroma of enhanced bills was significantly higher than that of bills before treatment application (t test for dependent samples, t 8 = 5.57, P = 0.0005; Fig. 2). However, the yellow-enhancing treatment did not significantly affect bill brightness (t test for dependent samples, t 8 = 2.18, P = 0.06). The dulling treatment affected both yellow–red chroma and brightness of bills as owlet bills had significantly lower yellow–red chroma (t test for dependent samples, t 8 = 2.48, P = 0.03) and brightness (t test for dependent samples, t 8 = 2.77, P = 0.024) after dulling (Fig. 2).
Manipulation of owlet bill coloration. a Photograph showing the appearance of bill-enhanced and bill-dulled siblings after treatment application. b Average reflectance spectra of natural (black line), yellow enhanced (yellow line) and yellow dulled (green line) owlet bills. The grey area delimits the natural range of reflectance spectra of bills in the population. Sample sizes are 18 owlets at six nests of which 9 were painted to enhance and 9 to dull their yellow bill coloration (colour figure online)
After the manipulation, colour spectra of enhanced and dulled bills where within the range of variation of bills in our population (Fig. 2). Yellow–red chroma of enhanced bills was on average 35 % higher than that of dulled bills (F 1,16 = 20.82, P = 0.0003). However, enhanced and dulled bills did not significantly differ in brightness (F 1,16 = 0.09, P = 0.76), suggesting that feeding parents should decide to feed between owlets differing in chromatic but not achromatic aspects of the bill in our experiment. Nonetheless, although the treatment did not influence brightness measured objectively, it cannot be ignored that it may have influenced perceived brightness under natural conditions.
At day 22, after hatching of the first egg, all owlets in a nest were ranked by weight. The heaviest nestling in the nest was assigned randomly to one of the two treatments; bill-enhanced or bill-reduced, whereas the remaining owlets were assigned alternately to each treatment following the ranking order (e.g. Parejo et al. 2010; Avilés et al. 2011). Nestlings were weighed with Pesola spring balances before and after a trial to accuracy of 0.25 g. Treatments were applied at dusk (i.e. 2000–2200 hours), and after 10 h we weighed the owlets again. Previous work has shown that nestling body mass gain is positively correlated with the amount of food provided by parents in birds (e.g. Heeb et al. 2003; Bize et al. 2006). A liner mixed model in which we controlled for the random effect of nest revealed that body mass of owlets did not significantly differ between bill-enhanced and bill-dulled owlets before the application of the treatment [mean (SD): 104.4 (19.0) g of bill-enhanced versus 103.9 (19.4) of bill-dulled owlets; treatment effect: F 1,50 = 0.06, P = 0.79; nest effect: F 23,50 = 7.68, P < 0.0001].
Statistical methods
Analyses were conducted using Statistica and SAS 9.2. Plasma carotenoid concentration was log transformed to fit normality. The level of T cell-mediated immune response, body mass of owlets, and variables describing bill coloration did not significantly differ from a normal distribution (Kolmogorov–Smirvov test for normality, P > 0.2).
Aiming to investigate the potential signalling role of bill coloration, we first estimated the percentage of total variation in this trait (i.e. brightness and yellow–red chroma) within nests rather than between nests from ANOVA means of squares using a variance method.
Linear Mixed Models (MIXED SAS procedure) were used to ascertain the potential informative content of bill coloration. In these models, variables describing bill coloration (i.e. brightness and yellow–red chroma) were used as the response variables with the various potential physiological correlates entered as explanatory variables so as to find the best combination of “information” that parents could be ascertaining from owlet bill coloration. Date and brood size at day of collection of colour measurements were entered as covariates in these models to control for possible environmental effects on owlet conspicuousness and quality (e.g. Bize et al. 2006; Avilés et al. 2011). In addition, the nest was entered as a random factor to control for non-independence of owlets from the same nest. Owlet body mass, immune response and carotenoid concentration were not significantly related in our sample of birds (Pearson correlations, r p < 0.12, P > 0.38 in all cases), which made collinearity unlikely.
We used a nested ANOVA design using the SAS PROC MIXED with REML to test the effect of our experimental manipulation of bill coloration on parental feeding allocation. In our bill colour manipulation experiment, we replicated the application of the two treatments (i.e. yellow-enhanced versus yellow-reduced) in 22 nests and body mass gain was measured for all the owlets of each nest. Therefore, in this analysis of variance, the treatment was included as a fixed factor, the nest nested within the treatment was considered a random factor and owlets were considered as residuals (see Quinn and Keough 2002). Date and brood size at the day of the experiment classed as small (i.e. two or three owlets) or large (i.e. four or five owlets) broods were entered as a covariate and a fixed factor, respectively, to control for possible environmental effects in parental provisioning. In addition, we entered owlet body mass at the beginning of the experiment as an additional covariate to control for owlet differences in size that may affect mass gain. The interactions between brood size and treatment and between owlet body mass at the beginning of the experiment and treatment were also included in the model because the effects of the experiment might differ for large and small broods (e.g. Avilés et al. 2011) and/or owlets initial mass (e.g. Parejo et al. 2010). Finally, we controlled for possible year effects in our experiment by entering study year as a random factor in the model.
Results
Variation in bill coloration
Owlet bill coloration in brightness varied more between than within nests (F 17,40 = 9.82, P < 0.001) and the percentage of total variation in bill brightness within nests was 9.23 %. Similarly, variation in yellow–red chroma of the bill was significantly larger between than within nests (F 17,40 = 1.97, P = 0.038), although the percentage of total variation in yellow–red chroma within nests reached 33.60 %, suggesting that a considerable fraction of bill yellow–red chromatic variation occurred between siblings.
Nestling quality and bill coloration
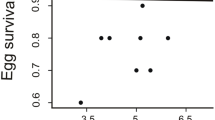
Neither plasma carotenoid concentration nor owlet PHA responses were significantly related with brightness or yellow–red chroma of the bill of owlets (Table 1). However, differences in body mass of owlets were associated with yellow–red chroma but not brightness of the bill (Table 1). Visualisation of the significant colour effects showed that heavier little owl owlets, both between and within nests, displayed more yellow bills than lighter owlets (Fig. 3).
Relationship between owlet body mass at day 22 of the brood and yellow–red chroma of the bill. a Relationships between average values per nest of mass of owlets and yellow–red chroma (n = 17 nests). The solid line is predicted by the linear regression of yellow–red chroma on owlet body mass. b Relationship between yellow–red chroma and body mass rank order assigned within each nest. Samples sizes on bars correspond to the number of nests in which a given rank order can be assigned
Bill coloration and parental provisioning
A total of 52 out of 67 owlets gained weight and all nests had at least 1 owlet that gained weight during the experiment. Owlets had an average mass gain (mean ± SE) of 4.55 ± 0.93 g per night, which suggests that parents were actively feeding nestlings during the experiment.
The effect of bill coloration on parental favouritism changed with brood size (interaction brood size treatment: F 1,22 = 5.75, P = 0.027; treatment effect: F 1,40 = 1.64, P = 0.20; brood size effect: F 1,22 = 1.02, P = 0.32) once we controlled for the random effect of nest nested within the treatment (Z = 1.79, P = 0.036), the effect of body mass of owlets at the beginning of the experiment (estimate ± SE = −0.16 ± 0.07, F 1,22 = 4.64, P = 0.04) and laying date (estimate ± SE = 0.03 ± 0.20, F 1,22 = 0.08, P = 0.77). Parents raising large broods preferentially fed enhanced over reduced bill-coloured owlets (Fig. 4), whereas those raising small broods did not significantly bias feeding in relation to owlet bill coloration (Fig. 4).
Average (±SE) body mass gain of little owl owlets after being treated with a marker enhancing and reducing the yellow coloration of the bill in relation to brood size (large broods and small broods include those with four and five owlets and with two and three owlets, respectively). Differences in body mass gain between siblings with enhanced and reduced coloration in small and large broods were tested with t test for dependent variables, results being displayed on average bars. Sample size was 22 tested broods (17 broods including 46 owlets with two or three owlets per nest and five broods including 21 owlets with four or five owlets per nest, respectively)
Discussion
The bill of little owl owlets adopts an adult-like yellow appearance to the human eye at the end of the nesting period. Bill reflectance measures have revealed that, besides yellowness, the bill of little owls strongly reflects in the UV part of the avian visible spectrum. In addition, we have found that a considerable portion of variation in bill yellowness occurred between siblings, and that it revealed information on owlet body mass. Finally, we have reported biases in adult food allocation toward siblings exhibiting chromatic features in their bills revealing high or low body mass.
Intriguingly, despite adults and owlets of some nocturnal owl species displaying colourful bare structures (e.g. beaks, ceres, or toes) (e.g. del Hoyo et al. 1999), the quality signalling function, if any, of these structures has been neglected, probably because the predominant belief was that visual communication based on chromatic discrimination was not possible under low light conditions. Only recently has the role of achromatic-based discrimination in visual communication of crespuscular and nocturnal owl species been investigated. For instance, eagle owls Bubo bubo display their white badge of throat during their territorial contents around sunset and sunrise (Penteriani and Delgado 2009). Also, in a parent–offspring communication scenario, achromatic signals may play a role as brightness-reduced eagle owlets showed poorer condition than control ones during the post-fledging dependence period, suggesting achromatic-based parental responses (Penteriani et al. 2007a). Here, we have shown that, besides achromatic signals, chromatic signals of quality emitted by bare structures may play a role in visual communication of a traditionally considered nocturnal species.
The use of colour vision by little owls is not surprising given current knowledge on Strigiidae visual performance and the width range of luminal condition in which little owls perform their activities. Anatomical evidence suggests that even strictly nocturnal owl species, such as the tawny owl Strix aluco, can achieve chromatic discrimination as they have three receptors types in their retina with peak sensitivities at 466, 556 and 530 nm (Bowmaker and Martin 1978). Indeed, laboratory experiments in which luminance levels (i.e. achromatic dimension) were controlled revealed that tawny owls had the capacity of discriminating between chromatic stimuli (Martin 1974). Therefore, it is likely that little owls also achieve colour vision. Furthermore, although little owls are essentially considered crepuscular and nocturnal regarding their hunting and vocal activities (Cramp 1998; Hardouin et al. 2008), they have been found hunting and feeding their offspring during the day in Spain (Negro et al. 1990; Cramp 1998), suggesting that important aspects of their life cycle can be completed in daylight under certain ecological conditions (Negro et al. 1990). Given that in birds color vision functions only in bright light (Kelber and Lind 2010), our findings of a quality signaling function of chromatic aspects of the bill in adult (Avilés and Parejo 2012) and young (this study) little owls may represent an overlooked but general pattern of signaling among nocturnal birds inhabiting a wide range of luminal conditions.
We found that heavier little owl offspring are yellower and that parental preference for owlet bill colour differed for pairs rearing large and small broods. Feeding pairs with large broods preferentially fed owlets displaying yellow-enhanced bills (i.e. colors associated with heavy nestlings), whereas an opposite non-significant parental preference was found in pairs with small broods. These findings may suggest that yellow bill coloration may have evolved as a signal of quality expressing the individual’s future reproductive value as an advertisement eliciting additional parental investment (see review in Mock et al. 2011). Several mutually non-exclusive alternative reasons, however, may explain this interactive effect of brood size and bill colour on parental preference. Firstly, perhaps older breeding little owls lay larger broods and show higher attentiveness than younger breeding little owls. Secondly, it is possible that the decline of environmental conditions through the season and their effects are more marked in large broods because their food needs are higher than in small broods and thus parents could aim to assure some success by feeding the best owlets (e.g. Bize et al. 2006). However, this possibility seems unlikely because we failed to detect seasonal changes in parental preferences (see “Results”).
The proximate mechanism behind the possible bill colour signalling function in little owl owlets remains elusive. Correlative (Leclaire et al. 2011; Dugas and Mcgraw 2011) and experimental (Ewen et al. 2008; Thorogood et al. 2008) evidence suggests that nestling bill coloration may function as a carotenoid-based signal of quality in nestlings of some species. Here, we failed to find a significant association between plasma carotenoid concentration and owlet bill coloration. It cannot be ignored, however, that carotenoid pigments present in the bill of owlets might not be present in the plasma (Mcgraw et al. 2004). Apart from pigments, integumentary bill coloration may be due to light-scattering on biological structures (i.e. structural coloration; Prum and Torres 2003; Jacot et al. 2010), or partly be mediated by deposition of cosmetic substances on the bill (Piault et al. 2008; Pérez-Rodríguez et al. 2011). Although disentangling the exact mechanism promoting the link between yellow bill coloration and owlet body mass deserves further research, our findings confirm the existence of such a link which is a prerequisite for the use of bill coloration as an indicator of individual quality in a parent–offspring communication scenario.
Summing up, bill chromatic variability and chromatic-dependent parental responses suggest that visual signalling based on chromatic stimulus may represent a widely overlooked element in nocturnal animal communication. These findings add to recent results suggesting that the chromatic variation in the bill of adult little owls may also play a role in sexual selection (Avilés and Parejo 2012). Given that colourful beaks are frequent in adults and owlets of several owls that extend their activities to some degree into daylight, or even in traditionally considered nocturnal species living in high latitudes where complete darkness at night is not fully achieved in summer time, our findings may represent an ignored but general pattern of signaling among birds inhabiting a wide range of luminal conditions that clearly deserves further attention.
References
Alonso-Alvarez C, Tella JL (2001) Effects of experimental food restriction and body-mass changes on the avian T-cell-mediated immune response. Can J Zool 79:101–105
Aragonés J, de Reyna LA, Recuerda P (1999) Visual communication and sexual selection ln a nocturnal bird species, Caprimulgus ruficollis, a balance between crypsis and conspicuousness. Wilson Bull 111:340–345
Avilés JM, Parejo D (2012) Covariation between bill coloration and fitness components in a nocturnal bird. J Avian Biol (in press) doi:10.1111/j.1600-048X.2012.05819.x
Avilés JM, Soler JJ (2009) Nestling coloration is adjusted to parent visual performance in altricial birds. J Evol Biol 22:376–386
Avilés JM, Pérez-Contreras T, Navarro C, Soler JJ (2008) Dark nests and conspicuousness in color patterns of nestlings of altricial birds. Am Nat 171:327–338
Avilés JM, Parejo D, Rodríguez J (2011) Parental favouritism strategies in the asynchronously hatching European Roller (Coracias garrulus). Behav Ecol Sociobiol 65:1549–1557
Bize P, Piault R, Moureau B, Heeb P (2006) A UV signal of offspring condition mediates context-dependent parental favouritism. Proc R Soc Lond B 273:2063–2068
Bortolotti GR, Tella JL, Forero MG, Dawson RD, Negro JJ (2000) Genetics, local environment and health as factors influencing plasma carotenoids in wild American kestrels (Falco sparverius). Proc R Soc Lond B 267:1433–1438
Bortolotti GR, Fernie KJ, Smits JE (2003) Carotenoid concentration and coloration of American Kestrels (Falco sparverius) disrupted by experimental exposure to PCBs. Funct Ecol 17:651–657
Bowmaker JK, Martin GR (1978) Visual Pigments and Color-Vision in A Nocturnal Bird, Strix aluco (Tawny Owl). Vis Res 18:1125–1130
Cramp S (1998) Cramp’s the complete birds of the Western palearctic. Optimedia, Oxford University Press, Oxford
de Ayala RM, Saino N, Møller AP, Anselmi C (2007) Mouth coloration of nestlings covaries with offspring quality and influences parental feeding behavior. Behav Ecol 18:526–534
Del Hoyo J, Elliott A, Sargatal J (1999) Handbook of the birds of the world, vol 5: Barn owls to hummingbirds. Lynx, Barcelona.
Dugas MB (2009) House sparrow, Passer domesticus, parents preferentially feed nestlings with mouth colours that appear carotenoid-rich. Anim Behav 78:767–772
Dugas MB, Mcgraw KJ (2011) Proximate correlates of carotenoid-based mouth coloration in nestling house sparrows. Condor 113:691–700
Endler JA, Westcott DA, Madden JR, Robson T (2005) Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution 59:1795–1818
Ewen JG, Thorogood R, Karadas F, Cassey P (2008) Condition dependence of nestling mouth colour and the effect of supplementing carotenoids on parental behaviour in the hihi (Notiomystis cincta). Oecologia 157:361–368
Faivre B, Gregorie A, Preault M, Cezilly F, Sorci G (2003) Immune activation rapidly mirrored in a secondary sexual trait. Science 300:103
Ficken MS (1965) Mouth color of nestling passerines and its use in taxonomy. Wilson Bull 77(71–75):1965
Galván I, Amo L, Sanz JJ (2008) Ultraviolet-blue reflectance of some nestling plumage patches mediates parental favouritism in great tits Parus major. J Avian Biol 39:277–282
Griggio M, Morosinotto C, Pilastro A (2009) Nestlings’ carotenoid feather ornament affects parental allocation strategy and reduces maternal survival. J Evol Biol 22:2077–2085
Hardouin LA, Robert D, Bretagnolle V (2008) A dusk chorus effect in a nocturnal bird: support for mate and rival assessment functions. Behav Ecol Sociobiol 62:1909–1918
Hart NS, Hunt DM (2007) Avian visual pigments: characteristics, spectral tuning, and evolution. Am Nat 169:S7–S26
Heeb P, Schwander T, Faoro S (2003) Nestling detectability affects parental feeding preferences in a cavity-nesting bird. Anim Behav 66:637–642
Jacot A, Romero-Diaz C, Tschirren B, Richner H, Fitze PS (2010) Dissecting carotenoid from structural components of carotenoid-based coloration: a field experiment with great tits (Parus major). Am Nat 176:55–62
Jourdie V, Moureau B, Bennett ATD, Heeb P (2004) Ultraviolet reflectance by the skin of nestlings. Nature 431:262
Karubian J, Lindsay WR, Schwabl H, Webster MS (2011) Bill coloration, a flexible signal in a tropical passerine bird, is regulated by social environment and androgens. Anim Behav 81:795–800
Kelber A, Lind O (2010) Limits of colour vision in dim light. Ophthalmic Physiol Opt 30:454–459
Kelber A, Vorobyev M, Osorio D (2003) Animal colour vision–behavioural tests and physiological concepts. Biol Rev 78:81–118
Kilner R (1997) Mouth colour is a reliable signal of need in begging canary nestlings. Proc R Soc Lond B 264:963–968
Kilner RM (2006) Function and evolution of color in young birds. In: Hill G, McGraw K (eds) Bird coloration: function and evolution, vol 2. Harvard University Press, Cambridge, pp 201–232
Kilner R, Davies NB (1998) Nestling mouth colour: ecological correlates of a begging signal. Anim Behav 56:705–712
Laaksonen T, Negro JJ, Lyytinen S, Valkama J, Ots I, Korpimaki E (2008) Effects of experimental brood size manipulation and gender on carotenoid levels of eurasian kestrels Falco tinnunculus. PLoS One 3:e2374
Leclaire S, White J, Arnoux E, Faivre B, Vetter N, Hatch SA, Danchin E (2011) Integument coloration signals reproductive success, heterozygosity, and antioxidant levels in chick-rearing black-legged kittiwakes. Naturwissenschaften 98:773–782
Lind O, Kelber A (2009) The intensity threshold of colour vision in two species of parrot. J Exp Biol 212:3693–3699
Martin GR (1974) Color-vision in tawny owl (Strix-Aluco). J Comp Physiol 86:133–141
Martin GR (1990) Birds by night. Poyser, London
Mcgraw KJ, Hill GE, Navara KJ, Parker RS (2004) Differential accumulation and pigmenting ability of dietary carotenoids in colorful finches. Physiol Biochem Zool 77:484–491
Mock DW, Dugas MB, Strickler SA (2011) Honest begging: expanding from signal of need. Behav Ecol 22:909–917
Navarro C, Perez-Contreras T, Avilés JM, Mcgraw KJ, Soler JJ (2010) Beak colour reflects circulating carotenoid and vitamin A levels in spotless starlings (Sturnus unicolor). Behav Ecol Sociobiol 64:1057–1067
Negro JJ, de la Riva MJ, Hiraldo F (1990) Daytime activity of little owls (Athene noctua) in southwestern Spain. J Raptor Res 24:72–74
Parejo D, Avilés JM (2011) Predation risk determines breeding territory choice in a Mediterranean cavity-nesting bird community. Oecologia 165:185–191
Parejo D, Avilés JM, Rodriguez J (2010) Visual cues and parental favouritism in a nocturnal bird. Biol Lett 6:171–173
Penteriani V, Delgado MD (2009) The dusk chorus from an owl perspective: eagle owls vocalize when their white throat badge contrasts most. PLoS One 4:e4960
Penteriani V, Delgado MD, Alonso-Alvarez C, Pina NV, Sergio F, Bartolommei P, Thompson LJ (2007a) The importance of visual cues for nocturnal species: Eagle owl fledglings signal with white mouth feathers. Ethology 113:934–943
Penteriani V, Delgado MD, Alonso-Alvarez C, Sergio F (2007b) The importance of visual cues for nocturnal species: eagle owls signal by badge brightness. Behav Ecol 18:143–147
Pérez-Rodríguez L, Mougeot F, Bortolotti GR (2011) The effects of preen oils and soiling on the UV-visible reflectance of carotenoid-pigmented feathers. Behav Ecol Sociobiol 65:1425–1435
Peters A, Denk AG, Delhey K, Kempenaers B (2004) Carotenoid-based bill colour as an indicator of immunocompetence and sperm performance in male mallards. J Evol Biol 17:1111–1120
Piault R, Gasparini J, Bize P, Paulet M, Mcgraw KJ, Roulin A (2008) Experimental support for the makeup hypothesis in nestling tawny owls (Strix aluco). Behav Ecol 19:703–709
Prum RO, Torres R (2003) Structural coloration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J Exp Biol 206:2409–2429
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rodríguez J, Avilés JM, Parejo D (2011) The value of nestboxes in the conservation of Eurasian Rollers Coracias garrulus in southern Spain. Ibis 153:735–745
Saino N, Ninni P, Calza S, Martinelli R, De Bernardi F, Møller AP (2000) Better red than dead: carotenoid-based mouth coloration reveals infection in barn swallow nestlings. Proc R Soc Lond B 267:57–61
Saino N, Ambrosini R, Martinelli R, Ninni P, Møller AP (2003) Gape coloration reliably reflects immunocompetence of barn swallow (Hirundo rustica) nestlings. Behav Ecol 14:16–22
Smits JE, Bortolotti GR, Tella JL (1999) Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct Ecol 13:567–572
Soler JJ, Avilés JM, Cuervo JJ, Pérez-Contreras T (2007) Is the relation between colour and immune response mediated by nutritional condition in spotless starling nestlings? Anim Behav 74:1139–1145
Tanner M, Richner H (2008) Ultraviolet reflectance of plumage for parent-offspring communication in the great tit (Parus major). Behav Ecol 19:369–373
Tella JL, Negro JJ, Rodríguez-Estrella R, Blanco G, Forero MG, Blázquez MC, Hiraldo F (1998) A comparison of spectrophotometry and color charts for evaluating total plasma carotenoids in wild birds. Physiol Zool 71:708–711
Thorogood R, Kilner RM, Karadas F, Ewen JG (2008) Spectral mouth colour of nestlings changes with carotenoid availability. Funct Ecol 22:1044–1051
Thorogood R, Ewen JG, Kilner RM (2011) Sense and sensitivity: responsiveness to offspring signals varies with the parents’ potential to breed again. Proc R Soc Lond B 278:2638–2645
Velando A, Beamonte-Barrientos R, Torres R (2006) Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149:535–542
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B 265:351–358
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Vorobyev M, Brandt R, Peitsch D, Laughlin SB, Menzel R (2001) Colour thresholds and receptor noise: behaviour and physiology compared. Vision Res 41:639–653
Wiebe KL, Slagsvold T (2009) Mouth coloration in nestling birds: increasing detection or signalling quality? Anim Behav 78:1413–1420
Acknowledgments
This research was funded by the Spanish Ministry of Education and Science/FEDER (CGL2008-00718) and the Spanish Ministry of Economy and Competitiveness/FEDER (CGL2011-27561) to J.M.A. and D.P. The authors thank Juan Rodríguez, Esther Campanario, Isaac Abdel and Fernando Goytre for their assistance in the field and in the laboratory and two anonymous referees for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Oliver Love.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avilés, J.M., Parejo, D. Colour also matters for nocturnal birds: owlet bill coloration advertises quality and influences parental feeding behaviour in little owls. Oecologia 173, 399–408 (2013). https://doi.org/10.1007/s00442-013-2625-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2625-8