Abstract
Carotenoid pigments are important for immunity and as antioxidants, and carotenoid-based colors are believed to provide honest signals of individual quality. Other colorless but more efficient antioxidants such as vitamins A and E may protect carotenoids from bleaching. Carotenoid-based colors have thus recently been suggested to reflect the concentration of such colorless antioxidants, but this has rarely been tested. Furthermore, although evidence is accruing for multiple genetic criteria for mate choice, carotenoid-based colors have rarely been shown to reflect both phenotypic and genetic quality. In this study, we investigated whether gape, tongue, eye-ring, and bill coloration of chick-rearing black-legged kittiwakes Rissa tridactyla reflected circulating levels of carotenoids and vitamins A and E. We further investigated whether integument coloration reflected phenotypic (body condition and fledging success) and genetic quality (heterozygosity). We found that the coloration of fleshy integuments was correlated with carotenoid and vitamin A levels and fledging success but only in males. Furthermore, the coloration of tongue and eye-ring was correlated with heterozygosity in both males and females. Integument colors might therefore be reliable signals of individual quality used by birds to adjust their parental care during the chick-rearing period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Red, orange, and yellow colorations are common in the feathers, skin, and bills of birds. In most species, such colors are produced by carotenoid pigments (review in McGraw 2006a). Animals cannot synthesize carotenoids de novo, so they have to acquire them in their diet. Carotenoid intake primarily depends on the quality and quantity of food, but it may also vary with individual efficiency in absorbing, modifying, and utilizing carotenoids (Olson and Owens 1998). In addition to providing coloration, carotenoids are antioxidants and are known to modulate the immune system (Blount et al. 2003; Chew 1993). This has lead to the assumption that there is a trade-off between allocating carotenoids to signals versus health-related functions. Recently, the antioxidant activity as the main role of carotenoids has been debated (Costantini and Møller 2008; Hartley and Kennedy 2004; Larcombe et al. 2010). Rather, it has been hypothesized that carotenoids being bleached by oxidative processes may reflect the concentration in major colorless antioxidants (e.g., vitamin C, E, or A) and therefore the healthy functioning of anti-oxidative systems (Hartley and Kennedy 2004). The common prediction of these hypotheses is that individuals in good health and with superior foraging ability can invest more carotenoids in sexual signals. Many studies have shown that birds with brighter carotenoid coloration have higher resistance to parasites (Baeta et al. 2008; Faivre et al. 2003; Horak et al. 2001), have higher reproductive success (Doutrelant et al. 2008; Préault et al. 2005), and survive longer (Horak et al. 2001). Carotenoid-based coloration is thus assumed to provide an honest signal of individual quality.
Although most studies on sexual selection focused on phenotypic quality (Andersson 1994), there is increasing evidence for multiple genetic criteria in mate choice (Mays and Hill 2004; Neff and Pitcher 2005). Females might choose males with “good genes,” for example males with specific alleles increasing fitness (Eizaguirre et al. 2009; Ekblom et al. 2004) or with high heterozygosity (Husseneder and Simms 2008; Roberts and Gosling 2003; Schwensow et al. 2008). In several species, heterozygosity is associated with individual quality (Acevedo-Whitehouse et al. 2003; Keller and Waller 2002; Roldan et al. 1998) and is conveyed to conspecifics through phenotypic traits, such as body scent, asymmetry, or song (Charpentier et al. 2010; Roldan et al. 1998; Van Oosterhout et al. 2003). Few studies have however shown a relation between carotenoid-based coloration and heterozygosity. In birds, only inbred zebra finches Taeniopygia guttata have been shown to be less colored than outbred zebra finches (Bolund et al. 2010).
Most studies linking individual quality and carotenoid coloration in birds have been performed in species showing carotenoid-based plumage coloration (review in Hill 2006). However, because feathers are non-living structures, their color is only indicative of a bird’s physiological status at the time of molt. In contrast, other parts of the body such as skin, caruncle, bill, cere, and tarsi may be brightly colored by carotenoid pigmentation (e.g., Blount et al. 2002; Faivre et al. 2003; Kristiansen et al. 2006; Velando et al. 2006) and may change color or shape rapidly (Faivre et al. 2003; Martínez-Padilla et al. 2007; Velando et al. 2006). Thus, they could provide accurate information about current individual physical condition. Although recent studies have focused on such traits, more research is required to understand the functional aspects of their use as honest signals (Hill 2006; Pérez-Rodríguez and Viñuela 2008). In particular, several gull species show conspicuous yellow to red gape color during the breeding season. Nestling birds in many passerines also often show rich gape colors that convey information such as hunger or health to parents (Kilner 1997; Saino et al. 2003). Although nestling gape color has been relatively well studied (de Ayala et al. 2007; Hunt et al. 2003; Saino et al. 2000, 2003; Soler and Aviles 2010; Thorogood et al. 2008), the signaling potential of gape color in adults is largely unexplored (but see Kristiansen et al. 2006).
The black-legged kittiwake Rissa tridactyla is one of the most common gull species in the Northern hemisphere. It feeds primarily on small schooling fishes (e.g., sand lance, capelin, Pacific herring) and secondarily on carotenoid-rich krill and copepods (Hatch et al. 1993a). It is a genetically monogamous bird with a slight sexual size dimorphism (Helfenstein et al. 2004a; Jodice et al. 2000) and quasi-similar parental behavior between sexes (Coulson and Johnson 1993; Leclaire et al. 2010; Roberts and Hatch 1993). Although kittiwakes are strictly monogamous over a breeding season (Helfenstein et al. 2004b), mate switching occurs in 19–47% of pairs each year (Coulson and Thomas 1983; Hatch et al. 1993b). In the chick-rearing period, cues that reliably indicate individual quality could thus be used for future pairing or adjustment of parental effort. Several studies have shown that differences between kittiwakes in survival and reproduction may be related to high differences in intrinsic individual quality (Cam and Monnat 2000; Coulson and Porter 1985). Furthermore, heterozygosity is positively correlated with chick growth and survival (Mulard et al. 2009), and it probably also affects adult fitness. However, secondary sexual traits indicating individual phenotypic or genetic quality have not been demonstrated as yet in this species. Both sexes show intense coloration during the breeding season, including the red eye-ring, orange gape, pink-orange tongue, and yellow bill (Fig. 1). As bill and eye-ring colors, gape and tongue colors might be important signaling cues as gape and tongue are often displayed to other individuals while birds are calling, in flight or at the nest.
Here, we test the signaling potential of gape, tongue, eye-ring, and bill colors of chick-rearing black-legged kittiwakes. Because both male and female kittiwakes provide parental care (Leclaire et al. 2010; Roberts and Hatch 1993) and because mate choice is thought to be reciprocal in this species, we predicted that integument coloration of chick-rearing kittiwakes should reflect carotenoid and vitamin levels, as well as individual phenotypic and genetic quality in both sexes. The bill is a keratinized structure, and the turnover of carotenoids within it may take place more slowly. Furthermore, while kittiwakes’ bill is bright yellow at the beginning of the breeding period, it is pale yellow and starts to be shed during the chick-rearing period (our personal observation). We therefore predicted that the bill color of chick-rearing kittiwakes should be less related to current antioxidant levels and individual quality than gape, tongue, or eye-ring color.
Material and methods
Study site
The study was conducted from late June to mid-August 2009 and 2010 on a population of black-legged kittiwakes nesting on an abandoned US Air Force radar tower on Middleton Island (59°26′ N, 146°20′ W), Gulf of Alaska. Artificial nest sites created on the upper walls can be observed from inside the tower through sliding one-way windows (Gill and Hatch 2002). This enabled us to capture and monitor the breeders and their chicks.
Field data collection
We caught 84 birds during the chick-rearing period (between 4 and 6 days after the hatching of the first chick; means, 5 ± 0 days). At capture, birds were weighed to the nearest 5 g with a spring scale and skull length (head + bill) was measured to the nearest 1 mm with a caliper. Forty six percent of birds were sexed based on molecular sexing, and 47% of birds were sexed based on copulation and courtship feeding behavior during the pre-laying period. Six percent of birds were sexed because their mate was sexed molecularly or behaviorally, and 1% of birds were sexed based on skull length (females’ skull length < 93 mm and males’ skull length > 96 mm; Jodice et al. 2000). All nest sites were checked twice daily to record events such as laying, hatching, fledging, or chick mortality. In 2009, we performed gape and tongue color measurements, to which bill and eye-ring color measurements were added in 2010.
Color measurement
Gape, tongue, and bill colors were measured with a reflectance spectrometer (Ocean Optics USB2000), a deuterium-halogen light source (DH2000, Top Sensor System), and a 200-μm fiber optic reflectance probe held at 45° to the integument surface. Reflectance was measured using SpectraSuite software (Ocean Optics, Inc.) and in relation to a dark and a white (Spectralon®, Labsphere) standard. The spectrometer was calibrated between each bird measurement. We measured gape color at the intersection between the upper and lower mandibles, tongue color at ca. 3 mm of the top, and bill color under the nostril on the upper mandible.
Since this study was set out to discuss the potential role of carotenoid-based coloration in kittiwakes, we focused on two physical measurements: yellow chroma ((R 700 nm − R 450 nm)/R 700 nm) and mean brightness (R 300–700 nm), measured from the reflectance spectra using the Avicol software (Gomez 2007). Reflectance spectra of kittiwake integument colors have peaks in both the ultraviolet (UV) and visible wavelengths (Fig. 2). Although UV coloration is most likely structural rather than pigment-based, there is some potential for carotenoids to contribute to the amount of UV signals. Carotenoids are subtractive pigments and increased carotenoids may obscure UV colors by acting as a filter (Mougeot et al. 2007; Shawkey and Hill 2005). As in other species for which reflectance spectra of bare parts have been studied (Mougeot et al. 2007; Thorogood et al. 2008), UV chroma (i.e., the proportion of the total reflectance falling in the UV range) of kittiwake gape, tongue, and bill co-varies negatively with yellow chroma (gape: r = −0.60, P < 0.0001; tongue: r = −0.25, P = 0.035; and bill: r = −0.50, P < 0.0001). However, we decided not to include the UV-chroma measurements in our analyses for the following reasons: (1) to limit the problem of multiple comparisons, (2) because we were interested in carotenoid-mediated color, and (3) because there is a covariance between UV chroma and yellow chroma.
Three measures of gape, tongue, and bill coloration were performed on 26 birds. They were highly repeatable (gape yellow chroma: intraclass correlation coefficient (ICC) = 79%, P = 0.0006; gape brightness: ICC = 79%, P = 0.0006; tongue yellow chroma: ICC = 74%, P = 0.0008; tongue brightness: ICC = 78%, P = 0.0006; bill yellow chroma: ICC = 83%, P = 0.0005; bill brightness: ICC = 49%, P = 0.0045).
The light emitted by the spectrometer is dangerous for the birds’ eyes. Eye-ring color was thus measured from digital photographs. Pictures were taken at a standard distance of ca. 40 cm using a digital camera with flash. For each photograph, a color swatch was placed next to the bird to standardize subsequent measurements (Montgomerie 2006). All pictures were analyzed using Adobe Photoshop 7.0. For each picture, the average components of red, green, and blue (RGB system) were recorded within the whole area of the eye-ring. RGB components were then converted into saturation and brightness values. Saturation represents color density (e.g., pink is less saturated than red), and brightness indicates whether a color is dark or light, independently of hue and saturation.
Antioxidant analyses: carotenoids and vitamins A and E
In 2009, a blood sample (∼500 μl) was collected from the brachial vein of 89 captured birds using heparinized syringes to determine carotenoid and vitamins A and E level in plasma. All blood samples were centrifuged immediately after collection, and plasma was stored at −20°C. Carotenoids and vitamins were analyzed following the protocol described in Surai and Speake (1998). Proteins were precipitated by adding 120 μl of absolute ethanol to 60 μl of plasma and vortexed for 1 min. Afterwards, 500 μl of hexane with 0.1% of buthylhydroxytoluène were added to the supernatant. The mixture was shaken for 1 min and centrifuged at 13,000 rpm/min at 4°C for 5 min. This was repeated twice to realize two extractions. The supernatant was evaporated to dryness under a nitrogen flow. Residual was re-suspended in 70 μl methanol/methyl tertiary butyl ether (MTBE) (50:50, v/v) and analyzed by high-performance liquid chromatography (HPLC, Waters™) using a reverse-phase HPLC column (Protonsil C30, 3 μm, 4.6 × 250 mm ID) with a mobile phase of methanol and MTBE. Peaks were identified by comparing their retention time and absorbent properties with reference carotenoids (astaxanthin, canthaxanthin, lutein, zeaxanthin, β-carotene, and β-cryptoxanthin; Extrasynthese, Genay) and reference vitamins (α-tocopherol and retinol; Sigma, St Louis). Quantity of pigments and vitamins (micrograms per milliliter of plasma) was calculated based on dilution curve constructed from known amounts of purified references. The estimated quantities were corrected by extraction output in reference to lycopene internal standard (100 ng/μl). We summed the concentrations of all pigments (i.e., lutein, iso-lutein, iso-asthaxanthin, zeaxanthin, β-cryptoxanthin, anhydrolutein, and β-carotene) to determine the total carotenoid level in plasma.
Heterozygosity analysis
At capture, blood was taken from the alar vein using a 1-ml syringe and a 25-gauge needle, and kept in a preservative solution (Longmire buffer; Longmire et al. 1988). Genomic DNA was extracted from each blood sample using the DNeasy® Blood and Tissue Kit (QIAGEN Group) and following the supplier’s guidelines. Samples were genotyped at 10 microsatellite loci (Mulard et al. 2009), distributed in two multiplexes. We amplified 1 μl extracted DNA in 10 μl reactions, using 0.75× QIAGEN® Multiplex PCR Master Mix (QIAGEN Group), and 0.05 to 0.45 μM of each primer. For the multiplex 1, the PCR thermal profile consisted of denaturation at 95°C for 15 min, followed by 35 cycles of 95°C denaturation for 30 s, 57°C annealing for 1 min and 30 s and 72°C elongation for 1 min, and a final elongation at 60°C for 30 min. For the multiplex 2, the thermal profile was the same except that the annealing was done at 60°C. One microliter of PCR product was then mixed with 8.7 μl of highly deionized formamide and 0.3 μl of GeneScan™ 600 LIZ® Size Standard (Applied Biosystems). The mixtures were denatured at 95°C for 5 min before separation with a 48-capillary 3730 DNA Analyzer (Applied Biosystems). Data analysis and genotyping were performed on GeneMapper® Software (Applied Biosystems).
Internal relatedness (described in Amos et al. 2001) was used as the estimate of heterozygosity and was calculated using the Nausicaa Software (Mulard et al. 2009). Internal relatedness was highly correlated with two other estimates of heterozygosity: heterozygosity and standardized heterozygosity (r² = 0.95; P < 0.0001).
Statistical analysis
Relationships between each color parameters (n = 8), fledging success, body condition, heterozygosity (internal relatedness), sex, and date were analyzed with General Linear Models or Generalized Linear Mixed Models. Fledging success, body condition, heterozygosity, date, and their interaction with sex were entered as fixed effects. Gape and tongue colors were measured in 2009 and 2010. Year and bird identity were therefore entered as random effects for gape and tongue color analyses. Body condition was estimated as the residuals of a linear regression between body mass and body size (i.e., skull length). Fledging success was estimated as the number of chicks that fledged. Bill, gape, and tongue brightness were log-transformed to meet normality assumption.
Carotenoid and vitamins A and E levels were not normally distributed. The link between antioxidant levels, and gape and tongue coloration was thus estimated using Pearson correlations. All statistical tests were performed with SAS (SAS Institute Inc 1999). Non-significant terms were backward dropped using a stepwise elimination procedure. All statistical tests are two-tailed type-3 tests with a significance level set to α = 0.05. Values are expressed as means ± SE throughout.
Results
Correlation between color parameters
In males, gape and tongue yellow chroma were significantly inter-correlated (r = 0.44, P = 0.01), and bill yellow chroma tended to be correlated with gape and tongue yellow chroma (r = 0.30, P = 0.08 and r = 0.33, P = 0.057). Gape, tongue, and bill brightness were all highly inter-correlated (all r > 0.55, all P < 0.001). Yellow chroma and brightness were not correlated (all P > 0.10). Eye-ring saturation and brightness were highly correlated (r = 0.48, P = 0.0044) but were not correlated with other integument parameters (all P > 0.05).
In females, gape, tongue, and bill yellow chroma were not inter-correlated (all P > 0.30). In contrast, gape, tongue, and bill brightness were all inter-correlated (all r > 0.40, all P < 0.01). Yellow chroma and brightness were not correlated, except in the case of bill color (r = −0.63, P < 0.0001). Eye-ring saturation and brightness were significantly correlated (r = 0.46, P = 0.0058) but were not correlated with other integument parameters (all P > 0.05).
Sexual dichromatism
Neither coloration of integument nor total carotenoid and vitamin levels were significantly different between males and females (coloration: MANOVA on all color parameters: F 8,55 = 0.97, P = 0.47 and all univariate tests P > 0.05, Fig. 2; antioxidant levels: see Table 1).
Color, fledging success, body condition, and heterozygosity
In males, body condition, fledging success, and heterozygosity were not correlated (all P > 0.15). In females, body condition and fledging success were correlated (r = 0.40, P = 0.013), but they were not correlated with heterozygosity (all P > 0.30). Body condition, fledging success, and heterozygosity were correlated with the date of capture neither in males nor in females (all P > 0.10).
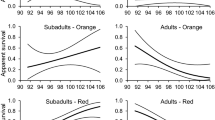
Gape and tongue yellow chroma depended upon the interaction between sex and fledging success (F 1,75.7 = 4.57, P = 0.036, Fig. 3a, and F 1,72 = 5.28, P = 0.025, Fig. 3b). Males with high gape yellow chroma had higher fledging success than males with low gape yellow chroma (F 1,30.4 = 4.91, P = 0.034, Fig. 3a), and males with high tongue yellow chroma tended to have higher fledging success than males with low tongue yellow chroma (F 1,36.5 = 3.22, P = 0.081, Fig. 3b). In contrast, gape and tongue yellow chroma did not depend upon fledging success in females (F 1,34.8 = 0.61, P = 0.44, Fig. 3a and F 1,33 = 1.85, P = 0.18, Fig. 3b). Surprisingly, homozygous birds had higher tongue yellow chroma than heterozygous birds (F 1,72 = 6.62, P = 0.012, Fig. 4a). Although the interaction between sex and heterozygosity was not significant (F 1,71 = 1.13, P = 0.29), when testing males and females separately, tongue yellow chroma depended upon heterozygosity only in females (females: F 1,33 = 6.38, P = 0.017; males: F 1,37 = 0.82, P = 0.37; Fig. 4a). Tongue yellow chroma increased with date in females but not in males (F 1,33 = 8.89, P = 0.0054 and F 1,37.9 = 0.00, P = 0.99; sex × date: F 1,72 = 4.31, P = 0.042). Tongue and gape brightness did not depend upon fledging success, body condition, or heterozygosity.
Tongue yellow chroma (a) and eye-ring saturation (b) in relation to internal relatedness in males (continuous line) and females (dashed line). Greater internal relatedness indicates decreased heterozygosity. Arrow in b shows an extreme value corresponding to a bird with large black patches in eye-rings. Tongue yellow chroma is expressed as the residual of a model with fledging success, sex, date, fledging success × sex, and date × sex as fixed effects, and year and individual ID as random effects
In males and females, bill yellow chroma decreased with date, whereas bill brightness increased with date (F 1,69 = 5.12, P = 0.027 and F 1,69 = 6.90, P = 0.011). Bill yellow chroma and bill brightness did not depend upon fledging success, body condition, or heterozygosity (all P > 0.05).
Eye-ring brightness depended upon the interaction between sex and fledging success. (F 1,63 = 11.77, P = 0.0011; Fig. 3c). Males with high eye-ring brightness had higher fledging success than males with low eye-ring brightness (F 1,31 = 13.86, P = 0.0008, Fig. 3c), whereas eye-ring brightness did not correlate with fledging success in females (F 1,32 = 1.63, P = 0.21; Fig. 3c). Eye-ring saturation was higher in heterozygous than homozygous individuals (F 1,61 = 5.25, P = 0.025; Fig. 4b). Eye-ring saturation decreased with date in males (F 1,29 = 7.55, P = 0.010), but it did not vary with date in females (F 1,31 = 0.15, P = 0.70; sex × date: F 1,61 = 5.42, P = 0.023).
Gape and tongue colors and antioxidant levels
Carotenoid pigments detected in kittiwake plasma were lutein, iso-lutein, zeaxanthin, anhydrolutein, iso-asthaxanthin, β-cryptoxanthin, and β-carotene (Table 1). Total carotenoid (i.e., the sum of all detected carotenoid pigments), vitamin A, and vitamin E levels were correlated together in males (all r > 0.53 and P < 0.0002) and females (all r > 0.36 and P < 0.02).
In males, gape and tongue yellow chroma were positively correlated with total carotenoid level (r = 0.37, P = 0.015, Fig. 5a, and r = 0.42, P = 0.0041, Fig. 6a) and vitamin A level (r = 0.44, P = 0.0029, Fig. 5b, and r = 0.40, P = 0.0071, Fig. 6b). Gape and tongue brightness were correlated with vitamin A level (r = −0.33, P = 0.026 and r = −0.37, P = 0.013), but only gape brightness was correlated with total carotenoid level (r = −0.29, P = 0.046 and r = −0.20, P = 0.20). Neither yellow chroma nor brightness of tongue and gape were correlated with vitamin E level (all P > 0.05).
Gape yellow chroma in relation to total carotenoid (a) and vitamin A (b) levels in males (continuous line) and females (dashed line). Carotenoid and vitamin A levels were log-transformed for the ease of representation. Statistical tests were however non-parametrics and performed on non-transformed values
Tongue yellow chroma in relation to total carotenoid (a) and vitamin A (b) levels in males (continuous line) and females (dashed line). Carotenoid and vitamin A levels were log-transformed for the ease of representation. Statistical tests were however non-parametrics and performed on non-transformed values
In females, gape brightness was correlated with vitamins A and E levels (r = −0.38, P = 0.012 and r = −0.38, P = 0.012) and with total carotenoid level (r = −0.33, P = 0.032). All other parameters were not significantly correlated with total carotenoid or vitamin levels (all P > 0.10).
Discussion
We investigated black-legged kittiwake integument coloration to determine whether it may signal antioxidant levels, as well as phenotypic and genetic quality during the chick-rearing period. The main findings of this study were that (1) gape and tongue yellow chroma were associated with carotenoid and vitamin A levels in males, (2) gape and tongue yellow chroma and eye-ring brightness reflected reproductive success in males, and (3) eye-ring saturation and tongue yellow chroma were correlated with heterozygosity in both males and females.
In males, circulating carotenoid and vitamin A levels were positively correlated with gape and tongue yellow chroma during the chick-rearing period (Figs. 5 and 6), while only vitamin A levels were correlated with gape and tongue brightness. Carotenoids and vitamin A may thus be involved in the expression of color signals in kittiwakes. Vitamin A plays a key role in various functions such as growth, reproduction, and vision, and its antioxidant and immune modulating properties are well recognized (Garbe et al. 1992; Stephensen et al. 2002; Surai 2002). Vitamin A is a better antioxidant than carotenoids, but it is colorless. Birds with a carotenoid-rich diet are expected to have high vitamin A levels, as vitamin A is formed from carotenoid precursors (β-carotene) in the intestinal mucosa. Furthermore, a high level of vitamins may prevent carotenoids from bleaching and thus carotenoid-based coloration from diminishing (Hartley and Kennedy 2004). It has thus been suggested that carotenoid-based signals do not advertise carotenoids as antioxidants but may advertise the healthy functioning of systems that prevent their oxidation (Hartley and Kennedy 2004). Our results provide evidence that in kittiwakes, carotenoid-based gape and tongue colors may be indicators of uncolored vitamin A levels. In spotless starlings Sturnus unicolor, beak color reflects circulating level of vitamin A during the mating period (Navarro et al. 2010). In yellow-legged gulls Larus michahellis, birds supplemented with vitamin E have larger red bill spots than non-supplemented birds (Pérez et al. 2008), and in male sticklebacks Gasterosteus aculeatus, males supplemented with vitamins E and C are more intensely colored and are preferred by females compared to controls (Pike et al. 2007). However, the causal link between vitamin A and carotenoid-based coloration remains to be demonstrated.
The carotenoid components of gape and tongue (i.e., yellow chroma), as well as eye-ring brightness, were related to male reproductive success (Fig. 3a–c). Although we lack correlation between antioxidant levels and eye-ring coloration, it is likely that eye-ring brightness also reflects carotenoid and vitamin A levels. Male coloration in the chick-rearing period may therefore be an honest signal of individual quality, indicating good health of the antioxidant system and reproductive success. Consequently, such coloration could play a role in the evaluation of mates for future pairing or the adjustment of parental effort by females (“differential allocation” hypothesis; Burley 1988; Sheldon 2000). These results are congruent with a previous study in kittiwakes, showing that males experimentally handicapped during the chick-rearing period had less bright integuments (measured from photos) than control males (our unpublished data). In several species, females have been shown to adjust clutch size, concentration of internal egg compounds, or food delivery according to male attractiveness (de Lope and Møller 1993; Gil et al. 1999; Helfenstein et al. 2008; Velando et al. 2006). In blue tits Parus caeruleus for instance, females mated to males with bright UV coloration feed theirs chicks at a higher rate (Limbourg et al. 2004), and in eastern bluebirds Sialia sialis, females mated to males with brighter plumage increase their investment in sons (Ligon and Hill 2010).
Contrary to male coloration, female coloration was not related to offspring fledging success, although female gape brightness was correlated with carotenoid and vitamins A and E levels. By evaluating gape color of females, males could estimate female health. Further studies are needed to determine in the extent to which gape coloration indicates female quality during the chick-rearing period.
Heterozygous kittiwakes were found to have higher eye-ring saturation (measured from pictures) (Fig. 4b) and lower tongue yellow chroma (Fig. 4a) than homozygous kittiwakes. In many species, heterozygosity is associated with individual quality (Acevedo-Whitehouse et al. 2003; Keller and Waller 2002; Roldan et al. 1998) and is conveyed to conspecifics through phenotypic traits, such as body scent, asymmetry, or song (Charpentier et al. 2010; Roldan et al. 1998; Van Oosterhout et al. 2003). In kittiwakes, heterozygosity is positively correlated with chick growth and survival (Mulard et al. 2009), and it probably also affects adult fitness. Kittiwakes may thus assess the genetic quality of conspecifics or mates through eye-ring and tongue color, and adjust their behavior accordingly. The negative correlation between tongue yellow chroma and heterozygosity (Fig. 4a) is intriguing because heterozygous individuals are expected to be high-quality birds and to have high carotenoid levels and high yellow chroma. However, the relationship between tongue yellow chroma and heterozygosity is mainly driven by females (Fig. 4a), in which tongue yellow chroma is not correlated with carotenoid levels (Fig. 6a). Furthermore, in females, tongue yellow chroma increased with date and tended to decrease with fledging success (Fig. 3b), potentially revealing a negative relationship between tongue yellow chroma and quality in females. Vascularized bare parts of many species are also colored by blood (McGraw 2006b), and blood flow and hemoglobin synthesis vary depending on bird physiological condition and metabolic rate. In kittiwakes, eye-ring color becomes less saturated and darker with the appearance of black patches as the breeding season progresses (our personal observation). Tongue and eye-ring color may thus depend on a combination of factors, e.g., the concentration of hemoglobin, the level of plasma carotenoid, the uptake of carotenoids from blood by integument cells, and the amount of melanin pigments. The color-based signal of heterozygosity in kittiwakes could therefore be determined by multiple factors rather than simply carotenoid levels. Very few studies have shown a relation between carotenoid-based coloration and heterozygosity. In zebra finch T. guttata and guppies Poecilia reticulata, inbred males are less colored than outbred individuals (Bolund et al. 2010; Sheridan and Pomiankowski 1997).
Contrary to fleshy integuments, the bill is a keratinized structure and the turnover of carotenoids deposited within it may take place more slowly. Therefore, bill coloration may not be linked to the current condition of the bird but may require more persistent stressful situations to change and thus reflect individual quality in the longer term. Bill color fades during the breeding season, from intense yellow at the beginning of the season to pale yellow and shedding at the end (our personal observations). Bill color may thus not reliably reflect individual quality during the chick-rearing period. This may explain why we did not observe any correlation between bill color and individual quality during the chick-rearing period.
To summarize, our main results indicate that the coloration of carotenoid-based fleshy integument in black-legged kittiwakes reveals information about phenotypic quality in males during the chick-rearing period. Experimental studies are now clearly required to determine whether coloration is used by females to adjust their parental effort.
References
Acevedo-Whitehouse K, Gulland F, Greig D, Amos W (2003) Disease susceptibility in California sea lions. Nature 422:35–35
Amos W, Worthington Wilmer J, Fullard K, Burg TM, Croxall JP, Bloch D, Coulson T (2001) The influence of parental relatedness on reproductive success. Proc R Soc London Series B Biol Sci 268:2021–2027
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Baeta R, Faivre B, Motreuil S, Gaillard M, Moreau J (2008) Carotenoid trade-off between parasitic resistance and sexual display: an experimental study in the blackbird (Turdus merula). Proc R Soc B 275:427–434
Blount JD, Surai PF, Nager RG, Houston DC, Moller AP, Trewby ML, Kennedy MW (2002) Carotenoids and egg quality in the lesser black-backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proc R Soc B 269:29–36
Blount JD, Metcalfe NB, Birkhead TR, Surai PF (2003) Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300:125–127
Bolund E, Martin K, Kempenaers B, Forstmeier W (2010) Inbreeding depression of sexually selected traits and attractiveness in the zebra finch. Anim Behav 79:947–955
Burley N (1988) The differential-allocation hypothesis—an experimental test. Am Nat 132:611–628
Cam E, Monnat JY (2000) Apparent inferiority of first-time breeders in the kittiwake: the role of heterogeneity among age classes. J Anim Ecol 69:380–394
Charpentier MJE, Crawford JC, Boulet M, Drea CM (2010) Message ‘scent’: lemurs detect the genetic relatedness and quality of conspecifics via olfactory cues. Anim Behav 80:101–108
Chew BP (1993) Role of carotenoids in the immune-response. J Dairy Sci 76:2804–2811
Costantini D, Møller AP (2008) Carotenoids are minor antioxidants for birds. Funct Ecol 22:367–370
Coulson JC, Johnson MP (1993) The attendance and absence of adult Kittiwakes Rissa tridactyla from the nest site during the chick stage. Ibis 135:372–378
Coulson JC, Porter JM (1985) Reproductive success of the kittiwake Rissa tridactyla—the roles of clutch size, chick growth-rates and parental quality. Ibis 127:450–466
Coulson JC, Thomas CS (1983) Mate choice in the kittiwake gull. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 361–373
de Ayala RM, Saino N, Moller AP, Anselmi C (2007) Mouth coloration of nestlings covaries with offspring quality and influences parental feeding behavior. Behav Ecol 18:526–534
de Lope F, Møller AP (1993) Female reproductive effort depends on the degree of ornementation of their mates. Evolution 47:1152–1160
Doutrelant C, Gregoire A, Grnac N, Gomez D, Lambrechts MM, Perret P (2008) Female coloration indicates female reproductive capacity in blue tits. J Evol Biol 21:226–233
Eizaguirre C, Yeates SE, Lenz TL, Kalbe M, Milinski M (2009) MHC-based mate choice combines good genes and maintenance of MHC polymorphism. Mol Ecol 18:3316–3329
Ekblom R, Saether SA, Grahn M, Fiske P, Kalas JA, Hoglund J (2004) Major histocompatibility complex variation and mate choice in a lekking bird, the great snipe (Gallinago media). Mol Ecol 13:3821–3828
Faivre B, Grégoire A, Préault M, Cézilly F, Sorci G (2003) Immune activation rapidly mirrored in a secondary sexual trait. Science 300:103–103
Garbe A, Buck J, Hammerling U (1992) Retinoids are important cofactors in T-cell activation. J Exp Med 176:109–117
Gil D, Graves J, Hazon N, Wells A (1999) Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128
Gill VA, Hatch SA (2002) Components of productivity in black-legged kittiwakes Rissa tridactyla: response to supplemental feeding. J Avian Biol 33:113–126
Gomez D (2007) AVICOL, a program to analyse spectrometric data. Available upon request from the author at dodogomez@yahoo.fr
Hartley RC, Kennedy MW (2004) Are carotenoids a red herring in sexual display? Trends Ecol Evol 19:353–354
Hatch SA, Byrd GV, Irons DB, Hunt GL Jr (1993a) Status and ecology of kittiwakes (Rissa tridactyla and R. brevirostris) in the North Pacific. In: Vermeer K, Briggs KT, Morgan KH, Siegel-Causey D (eds) The status, ecology, and conservation of marine birds of the North Pacific. Special publication, Canadian Wildlife Service, Ottawa
Hatch SA, Roberts BD, Fadely BS (1993b) Adult survival of black-legged kittiwakes Rissa tridactyla in a Pacific colony. Ibis 135:247–254
Helfenstein F, Danchin E, Wagner RH (2004a) Assortative mating and sexual size dimorphism in Black-legged Kittiwakes. Waterbirds 27:350–354
Helfenstein F, Tirard C, Danchin E, Wagner RH (2004b) Low frequency of extra-pair paternity and high frequency of adoption in black-legged kittiwakes. Condor 106:149–155
Helfenstein F, Losdat S, Saladin V, Richner H (2008) Females of carotenoid-supplemented males are more faithful and produce higher quality offspring. Behav Ecol 19:1165–1172
Hill GE (2006) Female mate choice for ornamental coloration. In: Hill GE, McGraw KJ (eds) Bird coloration. II. Function and evolution. Harvard University Press, London
Horak P, Ots I, Vellau H, Spottiswoode C, Moller AP (2001) Carotenoid-based plumage coloration reflects hemoparasite infection and local survival in breeding great tits. Oecologia 126:166–173
Hunt S, Kilner RM, Langmore NE, Bennett ATD (2003) Conspicuous, ultraviolet-rich mouth colours in begging chicks. Proc R Soc B 270:S25–S28
Husseneder C, Simms DM (2008) Size and heterozygosity influence partner selection in the Formosan subterranean termite. Behav Ecol 19:764–773
Jodice PGR, Lanctot RB, Gill VA, Roby DD, Hatch SA (2000) Sexing adult black-legged kittiwakes by DNA, behavior, and morphology. Waterbirds 23:405–415
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kilner R (1997) Mouth colour is a reliable signal of need in begging canary nestlings. Proc R Soc B 264:963–968
Kristiansen KO, Bustnes JO, Folstad I, Helberg M (2006) Carotenoid coloration in great black-backed gull Larus marinus reflects individual quality. J Avian Biol 37:6–12
Larcombe SD, Mullen W, Alexander L, Arnold KE (2010) Dietary antioxidants, lipid peroxidation and plumage colouration in nestling blue tits Cyanistes caeruleus. Naturwissenschaften 97:903–913
Leclaire S, Helfenstein F, Degeorges A, Wagner RH, Danchin E (2010) Family size and sex-specific parental effort in black-legged kittiwakes. Behaviour 147:1841–1862
Ligon RA, Hill GE (2010) Sex-biased parental investment is correlated with mate ornamentation in eastern bluebirds. Anim Behav 79:727–734
Limbourg T, Mateman AC, Andersson S, Lessers CM (2004) Female blue tits adjust parental effort to manipulated male UV attractiveness. Proc R Soc B 271:1903–1908
Longmire J, Lewis A, Brown N, Buckingham J, Clark L, Jones M, Meincke L, Meyne J, Ratliff R, Ray F, Wagner R, Moyzis R (1988) Isolation and molecular characterization of a highly polymorphic centromeric tandem repeat in the family Falconidae. Genomics 2:14–24
Martínez-Padilla J, Mougeot F, Pérez-Rodríguez L, Bortolotti GR (2007) Nematode parasites reduce carotenoid-based signalling in male red grouse. Biol Lett 3:161–164
Mays HL, Hill GE (2004) Choosing mates: good genes versus genes that are a good fit. Trends Ecol Evol 19:554–559
McGraw KJ (2006a) Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, London
McGraw KJ (2006b) Mechanics of uncommon colors: pterin, porphyrins and psittacofulvins. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, London, pp 354–398
Montgomerie R (2006) Analyzing colors. In: Hill GE, MCgraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge, pp 90–147
Mougeot F, Martinéz-Padilla J, Pérez-Rodríguez L, Bortolotti GR (2007) Carotenoid-based colouration and ultraviolet reflectance of the sexual ornaments of grouse. Behav Ecol Sociobiol 61:741–751
Mulard H, Danchin E, Talbot SL, Ramey AM, Hatch SA, White JF, Helfenstein F, Wagner RH (2009) Evidence that pairing with genetically similar mates is maladaptive in a monogamous bird. BMC Evol Biol 9:147
Navarro C, Pérez-Contreras T, Avilés JM, McGraw KJ, Soler JJ (2010) Beak colour reflects circulating carotenoid and vitamin A levels in spotless starlings (Sturnus unicolor). Behav Ecol Sociobiol 64:1057–1067
Neff BD, Pitcher TE (2005) Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol Ecol 14:19–38
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
Pérez C, Lores M, Velando A (2008) Availability of nonpigmentary antioxidant affects red coloration in gulls. Behav Ecol 19:967–973
Pérez-Rodríguez L, Viñuela J (2008) Carotenoid-based bill and eye ring coloration as honest signals of condition: an experimental test in the red-legged partridge (Alectoris rufa). Naturwissenschaften 95:821–830
Pike TW, Blount JD, Lindstrom J, Metcalfe NB (2007) Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biol Lett 3:353–356
Préault M, Chastel O, Cezilly F, Faivre B (2005) Male bill colour and age are associated with parental abilities and breeding performance in blackbirds. Behav Ecol Sociobiol 58:497–505
Roberts SC, Gosling LM (2003) Genetic similarity and quality interact in mate choice decisions by female mice. Nat Genet 35:103–106
Roberts BD, Hatch SA (1993) Behavioral ecology of black-legged kittiwakes during chick rearing in a failing colony. Condor 95:330–342
Roldan ERS, Cassinello J, Abaigar T, Gomendio M (1998) Inbreeding, fluctuating asymmetry, and ejaculate quality in an endangered ungulate. Proc R Soc B 265:243–248
Saino N, Ninni P, Calza S, Martinelli R, De Bernardi F, Moller AP (2000) Better red than dead: carotenoid-based mouth coloration reveals infection in barn swallow nestlings. Proc R Soc B 267:57–61
Saino N, Ambrosini R, Martinelli R, Ninni P, Moller AP (2003) Gape coloration reliably reflects immunocompetence of barn swallow (Hirundo rustica) nestlings. Behav Ecol 14:16–22
SAS Intitute Inc (1999) SAS user’s guide, version 8. Sas Institute Inc., Cary
Schwensow N, Fietz J, Dausmann K, Sommer S (2008) MHC-associated mating strategies and the importance of overall genetic diversity in an obligate pair-living primate. Evol Ecol 22:617–636
Shawkey MD, Hill GE (2005) Carotenoids need structural colours to shine. Biol Lett 1:121–124
Sheldon BC (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Sheridan L, Pomiankowski A (1997) Fluctuating asymmetry, spot asymmetry and inbreeding depression in the sexual coloration of male guppy fish. Heredity 79:515–523
Soler JJ, Aviles JM (2010) Sibling competition and conspicuousness of nestling gapes in altricial birds: a comparative study. PLoS ONE 5:e10509
Stephensen CB, Rasooly R, Jiang XW, Ceddia MA, Weaver CT, Chandraratna RAS, Bucy RP (2002) Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol 168:4495–4503
Surai PF (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham
Surai PF, Speake BK (1998) Distribution of carotenoids from the yolk to the tissues of the chick embryo. J Nutr Biochem 9:645–651
Thorogood R, Kilner RM, Karadas F, Ewen JG (2008) Spectral mouth colour of nestlings changes with carotenoid availability. Funct Ecol 22:1044–1051
Van Oosterhout C, Trigg RE, Carvalho GR, Magurran AE, Hauser L, Shaw PW (2003) Inbreeding depression and genetic load of sexually selected traits: how the guppy lost its spots. J Evol Biol 16:273–281
Velando A, Beamonte-Barrientos R, Torres R (2006) Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149:535–542
Acknowledgments
We are very grateful to F. Bailly, P. Blanchard, T. Merkling, C. DeFranceschi, and V. Frochot for their help in the field, and E. Lhuillier and C. Veyssière for their help in genotyping. We thank J. Cornuault for his help in using Avicol software and M. Giraudeau, F. Helfenstein, and C. Doutrelant for helpful discussion. Experiments were carried out in accordance with US laws and under permits from the US Fish and Wildlife Service and State of Alaska. This study was financed in part by the French Polar Institute Paul-Emile Victor (IPEV, program 1162). Any use of trade names is for descriptive purposes only and does not imply endorsement by the US government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Leclaire, S., White, J., Arnoux, E. et al. Integument coloration signals reproductive success, heterozygosity, and antioxidant levels in chick-rearing black-legged kittiwakes. Naturwissenschaften 98, 773 (2011). https://doi.org/10.1007/s00114-011-0827-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-011-0827-7