Abstract
In altricial birds, resource allocation during early developmental stages is the result of an interaction between parental feeding decisions and scramble competition between nestmates. Hatching asynchrony in birds leads to a pronounced age hierarchy among their offspring. Therefore, whenever parents exert control over resource allocation parents feeding asynchronous broods should simultaneously assess individual offspring internal condition and age. In this study, we first studied whether the highly ultraviolet (UV) reflective body skin of nestlings in the asynchronous European Roller (Coracias garrulus; roller hereafter) relates to nestling quality. In a second stage, we experimentally studied parental biases in food allocation towards senior and junior sibling rollers in relation to a manipulation of UV reflectance of the skin of their offspring. Heavier roller nestlings had less brilliant and less UV saturated skins than weaker nestlings. In our experiment, we found that parents with large broods preferentially fed nestlings presenting skin coloration revealing small body size (i.e. control nestlings) over nestlings presenting skin coloration revealing large body size (i.e. UV-blocked nestlings). Within the brood, we found that parental food allocation strategy depended on nestling age: parents preferentially fed senior nestlings signalling small body size, but did not show preference between control and UV-blocked junior nestlings. These results emphasise that parent rollers use UV cues of offspring quality while balancing the age of their offspring to adjust their feeding strategies, and suggest that parents may adopt finely tuned strategies of control over resource allocation in asynchronous broods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many animals, offspring depend entirely on resources provided by parents during early developmental stages (Clutton-Brock 1991). Parents are expected to allocate adaptively their limited resources among offspring so as to maximise parental fitness (Mock and Parker 1997), whereas begging offspring are expected to demand more food than parents are selected to provide, setting the scenario for a parent–offspring conflict over parental investment (Trivers 1974). Two main theoretical models have been proposed to explain the resolution of this conflict. The first model proposes that begging displays are the outcome of scramble competition among siblings, and rests on the assumption that offspring has full control over resource allocation (Macnair and Parker 1978; Parker and Macnair 1978; Macnair and Parker 1979; Parker and Macnair 1979; Parker et al. 2002). The alternative model dwells on the assumption that offspring reveal their need (i.e. the offspring’s marginal fitness gain from obtaining extra resources) to parents by begging towards them with costly signals (Godfray 1991; Godfray 1995; Mock and Parker 1997). In this scenario, parents would exert control over resource allocation within the brood and preferentially feed needy offspring on the basis of their begging displays.
Parents in many bird species start to incubate their eggs before clutch completion. This results in hatching asynchrony and leads to a pronounced age and hence size hierarchy among the nestlings in a brood (Magrath 1990). Empirical work has shown that variation in size rank caused by asynchronous hatching affects parental resource distribution within the brood (e.g. Lotem 1998; Cotton et al. 1999; Smiseth and Amundsen 2002). In general, senior nestlings actually receive more food than juniors even thought the former beg less intensively (Kilner 1995; Price and Ydenberg 1995; Lotem 1998; Cotton et al. 1999; Smiseth and Amundsen 2002). It remains unclear, however, whether this is because seniors outcompete junior siblings, or because parents have partial or full control over food allocation and actively preferred feeding senior offspring. So far, the only empirical test of parental control over resource allocation in asynchronous broods has focused on manipulating food availability in asynchronous bluethroat Luscinia svecica broods (Smiseth et al. 2003). Food limitation at the nests resulted in a stronger parental preference for senior over junior nestlings (Smiseth et al. 2003). However, food limitation also affected nestling behaviour, which hinders the interpretation of these findings in terms of parental and/or offspring control over food allocation.
Previous empirical work suggests that, beyond coloration of gaping structures, chromatic characteristics of the skin of nestlings may reveal quality of offspring and function as visual signals in altricial birds. Indeed, Jourdie et al. (2004) have shown that the body skin of nestlings of the European starling (Sturnus vulgaris) reflects substantially in the ultraviolet wavelength (see also Soler et al. (2007) for the spotless starling (Sturnus unicolor)), and that chicks in which this reflectance was artificially reduced gained less mass than controls. Also, Bize et al. (2006) showed that body skin reflectance for the hole-nesting Alpine swift (Apus melba; Apodiforme) and of the European starling showed a peak in the ultraviolet that correlated with nestling stature, and that parents used these signals of offspring condition to adjust resource allocation. Finally, the UV-reflecting cere (skin above the beak) reflects nestling body mass and mediates parental favouritism in the nocturnal scops owl (Otus scops; Parejo et al. 2010a). Here, we report an experiment on the European Roller (Coracias garrulus; roller hereafter), a secondary hole-nesting Coraciiform in which the two sexes incubate the eggs, brood and feed the offspring (Cramp 1998; Avilés 2006). Incubation begins before clutch completion, usually after the laying of the third egg, which results in patent size hierarchies within broods (Parejo et al. 2007). Previous work has shown that body skin of nestling rollers is also highly reflective in the ultraviolet waveband (Avilés et al. 2008), although it remains unstudied whether body skin colour may mediate parent–offspring in this asynchronous bird.
Here, we first studied whether skin reflectance of nestlings in the asynchronous roller (C. garrulus) varies according to nestling condition. In a second stage, we studied parental biases in food allocation towards senior and junior sibling rollers presenting skin coloration revealing high or low quality to their parents. To this end, half of the nestlings in a brood were treated with an UV-light block and spatially separated with a septum from control nestlings in their nests. Thus, our goal here was to elucidate whether parents use visual cues revealing nestling quality while assessing the age of their offspring to decide within-brood resource allocation.
Methods
Study population
The field study was carried out in the surroundings of Guadix (37°18′ N, 3°11′ W), southeastern Spain, in June–July 2009. Thirty-two roller pairs began reproduction (egg laying) in 2009 in nest boxes recently (2003–2005) installed in the area, although only 23 pairs hatched nestlings. The vegetation is sparse in the area, including cultivated cereals, some remains of holm oaks forests, groves of almond trees and olive trees, and other tree crops in irrigated areas surrounding villages (more details in Avilés et al. 2008). Average (SD) number of feeding trips per hour at day 8 from hatching of the first chick is 10.62 (6.88) (n = 29 nests), and the most frequent preys delivered to nestlings are Orthoptera and Araneae (Avilés 2006). Unpublished data collected in our population on 17 nests in which the two parents were ringed revealed that females and males feed the young at a similar rate (average (SD) number of feeding trips per hour: 4.94 (2.70) in females versus 5.23 (4.59) in males).
For each breeding pair, we recorded laying date, clutch size, date when the first and last egg hatched, and brood size at hatching. Average (SD) clutch size and brood size in our population during the year of the experiment were 4.4 (0.7) eggs and 4.0 (0.8) nestlings, respectively (n = 21 broods). Average hatching span per brood was 4.4 days (range 3–7 days, n = 20 broods in which hatching span could be assessed in a daily basis) and increased with clutch size (R p = 0.51, P = 0.02, n = 20), suggesting that, at least during the study year, clutch size was a reliable predictor of degree of hatching asynchrony.
Colour and body measurements of nestlings
We obtained reflectance data of the skin of roller nestlings on two body regions, namely the head and the neck, with an spectrometer [S2000 Ocean Optics equipment connected to a deuterium-halogen light (D2-W, mini) by a coaxial reflectance probe (QR-400-7-UV–vis) and the OOIBase32™ operating software (Ocean Optics, Inc. Dunedin, FL, USA)] the same day of the experiment (i.e. at day 6 after hatching of the first egg). Nestlings were weighed with a Pesola spring balance to the nearest 0.5 g, and reflectance was measured with a 45° angle probe completely touching the nestling to prevent any stray light from entering. Measurements were relative and referred to a standard white (WS-2) and to the dark (i.e. blocking the entering light by placing an opaque cap in the end of the reflectance probe), which we calibrated before the measurement of all brood mates in a nest. All measurements were taken within a portable hide with opaque wall set in the surrounding of the nests. Measurements were repeated three times at every body part, and mean values per nestling were calculated and used in the analyses.
Reflectance data were summarised by calculating standard colour descriptors (Montgomerie 2006): (1) total brightness was calculated as the summed reflectance in the interval 300–700 nm; (2) UV chroma as the summed reflectance in the interval 300–400 nm divided by total brightness; and (3) λUV peak as the wavelength at which the maximal value of reflectance is reached in the UV waveband (i.e. 300–400 nm). Colour measurements taken on the head and on the neck were correlated for the three colour descriptors (Pearson r coefficients = 0.28–53, P < 0.013 in the three cases, n = 77 nestlings). Furthermore, our results were qualitatively identical when using colour descriptors for the neck (results not shown). Hence, we used colour descriptors for the head as representative of entire body skin coloration for each nestling.
Experimental design
Body skin reflectance in roller nestlings has a marked peak in the UV part of the spectrum (Avilés et al. 2008; see also Fig. 1). In altricial birds, the skin of the head and the neck constitutes the most visible part of the nestling body together with the gape during their begging displays (Mock and Parker 1997). Thus, we assessed food allocation by parents in relation to UV reflectance of the body skin of their offspring, by applying a UV-light block treatment to the neck and head of randomly chosen nestlings within each brood and compared their body mass gain with siblings treated with a control treatment. The experiment was performed on 21 roller broods (i.e. 42 and 42 nestlings treated with the UV-light blocker and the control treatment, respectively). The UV-light block treatment was composed of an UV absorbing chemical (50/50 w/w blend of Parsol 1789 and MCX, Roche, Dubendorf, Switzerland) mixed with petroleum jelly (93.95% petroleum jelly, 6% Cetiol B and 0.05% BHT, Roche, Dubendorf, Switzerland). Control nestlings were treated with petroleum jelly only. Previous works have demonstrated that the UV-absorbing chemical product used to manipulate the UV reflectance of nestling skins does not induce harmful effects on bird skins (see also Bize et al. 2006; Penteriani et al. 2007; Parejo et al. 2010a; Wiebe and Slagsvold 2009).
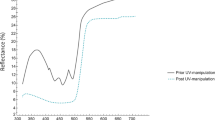
Average (±SD) reflectance spectra of the body skin of roller nestlings before and after they were treated with a petroleum jelly containing or not containing UV-light blocker (black before; dark grey control; light grey UV block). Sample sizes were 28 and 32 UV-reduced and control nestlings sampled at 17 broods, respectively
Indeed, the average weight of nestling treated with the UV-absorbing solution at fledging (i.e. 14 days after treatment application) did not differ from that of their nestmates treated with the control solution (mean (SD): 123.07 (21.70) g of control versus 130.46 (10.62) g of ultraviolet-reduced nestlings, paired t test: t = 1.72, P = 0.10, n = 16 nests). Also, previous studies did not find evidence that the application of petroleum jelly on the body skin would have negative consequences on birds (Bize et al. 2006; Parejo et al. 2010a), which seems to be the case for nestling rollers too, since the failure rate of nests after hatching in 2009 (i.e. when all nestlings were treated with petroleum jelly; 5 out of 22 nests) was similar to that reported in our population the previous year (5 out of 21 nests).
Nests were visited on a daily basis, and nestlings were individually marked with a marking pen at the tarsus the day they were hatched. At day 6, after hatching of the first egg, all nestlings in a nest were ranked by weight. The heaviest nestling in the nest was assigned randomly to one of the two treatments; control or UV-block, whereas the other nestlings were assigned alternately to each treatment following the ranking order. In order to exclude jostling by nestlings and thus that the treatments could be transferred among siblings, nestlings were separated by treatment with a cork septum. The septum was positioned perpendicularly to the entrance hole (e.g. Kilner 1995; Bize et al. 2006; Tanner et al. 2008; Parejo et al. 2010a) and nest treatment groups placed on either side of the septum at random. Thus, although the choice of using a septum to separate UV-reduced and control nestlings artificially increase parental control over food allocation (Tanner et al. 2008), nestlings were still free to scramble with each other for position in half of the nest box.
Nestlings were weighed with Pesola spring balances before and after a trial to an accuracy of 0.25 g. A linear mixed model in which we control for the random effect of nest revealed that body mass of nestlings did not differ between control and ultraviolet-reduced nestlings before the manipulation (mean (SE): 26.65 ± 2.58 g of control versus 25.84 ± 2.58 g of ultraviolet-reduced nestlings, Treatment effect: F 1,42 = 0.05, P = 0.82; Interaction Treatment*Nest: F 20,42 = 0.23, P = 0.99; Nest effect: Z = 4.58, P < 0.0001). A pilot study revealed that the UV-block effect persisted on head and neck of roller nestlings after 5 h. Treatments were applied in the morning, and within-brood trials lasted 2.2–4.7 h (median trial duration was 3.8 h). Variable duration of trials may result in uncontrolled biased in the dataset if only long trials were long enough to detect changes in body mass. Therefore, following Wiebe and Slagsvold (2009), we split the data into short (i.e. trial duration below the median) and long (i.e. trial duration above the median) trials and tested for the effect of trial duration on nestling mass gain. A linear mixed model in which we control for the random effect of nest showed that average mass gain of nestlings did not vary with duration of the trial (Duration effect: F 1,19 = 3.19, P = 0.09), and that this pattern was similar for control and UV-blocked nestlings (Interaction Treatment*Duration: F 1,19 = 2.34, P = 0.14; Nest effect: Z = 1.78, P = 0.03), suggesting a negligible effect of trial duration in our experimental setup.
Previous work has shown that nestling body mass is positively correlated with the amount of food provided by parents in synchronously hatching altricial birds (e.g. Heeb et al. 2003; Bize et al. 2006). In asynchronously hatched broods as in rollers, however, differences in body mass gain between senior and junior siblings may also reflect differences in developmental stage and food assimilation between seniors and juniors rather than amount of food ingested (Marchetti and Price 1989). Hence, here, we interpreted mass gain during the trial as a measure of parental favouritism only when contrasting variation in body mass gain between similar size siblings.
Effect of UV-block and control treatments on colour
We qualified changes in body skin reflectance before and after application of the UV blocker and the control treatments respectively in 28 and 32 nestlings from 17 randomly selected broods (Fig. 1). The control treatment did not significantly affect summed reflectance in the ultraviolet part of the spectrum (300–400 nm; (mean ± SD) 5,084.80 ± 1,204.58 before versus 4,647.09 ± 1,284.61 after; paired t test: t 32 = 1.53, P = 0.13; Fig. 1), and moderately diminished summed reflectance in the human visible part of the spectrum (400–700 nm; (mean ± SD) 9,098.21 ± 1,896.44 before versus 7,193.89 ± 1,659.60 after; paired t test: t 32 = 4.65, P = 0.0001; Fig. 1). The UV blocker drastically reduced reflectance in the ultraviolet part of the spectrum ((mean ± SD) 5034.41 ± 1320.05 before versus 639.66 ± 401.76 after; Paired t-test: t 28 = 19.89, P < 0.00001; Fig. 1) and reduced summed reflectance in the human visible part of the spectrum ((mean ± SD) 9222.34 ± 2452.72 before versus 7245.56 ± 1878.25 after; Paired t-test: t 28 = 3.99, P = 0.0001; Fig. 1). Therefore, the effects of the UV blocker and the control treatments above 400 nm were equivalent. We also compared reflectance of UV-blocked and control nestlings after treatment application with two linear mixed models in which we control for the random effect of nest. Reflectance in the visible part of the spectrum did not differ between control and UV-blocked nestlings (Treatment effect: F 1,36 = 0.03, P = 0.86; Nest effect: Z = 2.45, P = 0.007), whereas UV reflectance of nestlings treated with the UV blocker was significantly lower than that of control nestlings (Treatment effect: F 1,36 = 253.68, P < 0.0001; Nest effect: Z = 0.28, P = 0.38; Fig. 1).
Effect of UV-block and control treatments on nestling detectability within the nest
We ran physiological models (Vorobyev et al. 1998) with Avicol software version 3 (Gomez 2006), which account for nest box luminosity and bird sensitivity, to assess the effect of our control and UV-block treatment on perception of chromatic signal emitted by nestlings. Using this approach, we can assess whether the chromatic signal emitted by nestlings after being treated with the control and the UV-block treatment was under the threshold value for visual discrimination. In addition, this approach also allows us to assess whether the chromatic signal emitted by UV-blocked nestlings was within the natural range of signals emitted by nestlings to their parents in the luminal conditions prevailing in their nests. Nest box luminosity and average reflectance spectra of background in roller nests in our study area were extracted from Avilés et al. (2008). Evidence suggests that the European roller has a SWS1 opsin protein biased towards violet (Odeen and Hastad 2003). Thus, to model roller spectral sensitivity, we computed a model for a tetrachromatic vision with cone photoreceptor proportions of 1, 1.9, 2.2 and 2.1 and using spectral sensitivity data from the peafowl Pavo cristatus as representative of the violet-sensitive system (Hart 2002; Hastad et al. 2005; Avilés and Soler 2009). We obtained chromatic contrasts of nestling body skin against the nest background for all the sampled nestlings before and after applying the control and UV-block treatments. The units for chromatic matching estimated from our physiological model are JNDs (Just Noticeable Differences). The control treatment did not affect the intensity of the chromatic signal emitted by nestlings (14.79 ± 2.43 JND before versus 14.91 ± 2.29 JND after; paired t test: t 32 = 0.23, P = 0.82). Nestlings treated with the UV-block treatment still emitted a chromatic signal that exceeded largely the threshold value for nestling discrimination, although the UV-block treatment decreased the chromatic contrast between the nestlings and the nest background (14.14 ± 2.54 JND before versus 12.97 ± 2.01 JND after; paired t test: t 28 = 2.30, P = 0.02). Interestingly, although the application of the UV-block solution provoked a change in the spectral shape of the skin of nestlings (Fig. 1), nestlings treated with a UV-absorbed solution emitted an average chromatic signal within their nest that was within the natural range of variation of that emitted by the nestlings before the application of the treatment.
Statistical analyses
Analyses were performed using SAS 9.1. Nestling body mass and colour descriptors did not significantly differ from a normal distribution (Kolmogorov–Smirvov test for normality, P > 0.2). Within every nest, we established two distinct age-classes of offspring, senior and juniors, on the base of age in days from hatching. Seniors were the nestlings hatched in the first 24 h after the hatch of the first egg (typically two or three nestlings, see Parejo et al. 2010b), whereas the remaining nestlings were considered juniors. The weight of seniors the day of the experiment was twice that of juniors (average weight ± SD: 35.64 g ± 12.98 in seniors versus 16.45 g ± 7.48 in juniors, F 1,82 = 63.92, P < 0.0001), and seniors were in average 2.7 days older than juniors.
We studied the associations between colour descriptors with Pearson correlations. Linear mixed models (MIXED SAS procedure) were used to test for the relationships between nestling coloration (i.e. total brightness, UV hue and UV chroma) as independent variables and nestling body mass at day 6 as dependent variable. Date of experiment and brood size at the day of colour measurements were respectively entered as a covariate and a fixed factor to control for possible environmental effects on nestling colour and quality (e.g. Bize et al. 2006). In addition, the nest was entered as a random factor to control for non-independence of nestlings from the same nest.
To test for the effect of experimental reduction of ultraviolet reflectance on parental feeding allocation, we performed a linear mixed model (MIXED SAS procedure) with nestling body mass gain during the trial (corrected to a 3-h period) as dependent variable and experimental treatment (UV reduced versus control) as fixed effect. Nestling identity was nested within septum side as a random intercept and then septum side within nest as a random coefficient on treatment. Date and brood size were entered as a covariate and a fixed factor, respectively, to control for possible environmental effects. Because our aim was to test whether parental feeding strategies varied with nestling age, we also entered the interaction between nestling age (junior versus senior) and experimental treatment. Previous theoretical work has suggested that parental favouritism should increase with age differences between two offspring (Jeon 2008) and vary with season (Bize et al. 2006). Thus, in the knowledge that brood size was a reliable predictor of hatching span in our population (see above), we introduced the interaction between brood size and experimental treatment and date and experimental treatment to account for these possibilities.
Results
Ultraviolet reflectance of body skin and nestling quality
Reflectance of the skin showed a marked peak in the UV part of the spectrum (300–400 nm, Fig. 1). Nestlings with a lower UV hue (i.e. those having a peak in the ultraviolet at shorter wavelength) of the skin had greater UV chroma (R p = −0.35, P = 0.002, n = 77) and displayed less bright body skin (R p = 0.25, P = 0.027, n = 77). Brightness of the skin was not significantly associated with UV chroma (R p = −0.05, P = 0.63, n = 77).
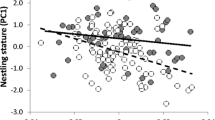
At day 6 of the brood, variation in body mass among nestlings was significantly associated with total brightness (Estimate ± SE = −0.0001 ± 0.0005, F 1,55 = 5.72, P = 0.02) and UV chroma of the skin (Estimate ± SE = −79.98 ± 41.51, F 1,55 = 3.71, P = 0.05) once we controlled for brood size (Estimate ± SE = 5.65 ± 2.46, F 1,55 = 5.27, P = 0.02) and laying date (Estimate ± SE = 0.75 ± 0.40, F 1,55 = 3.52, P = 0.07). UV hue was not significantly related to nestling body mass (Estimate ± SE = 0.65 ± 0.44, F 1,55 = 2.16, P = 0.15), and the random effect of nest was not significant (Z = 0.67, P = 0.25). Visualisation of the significant effects revealed that heavier roller nestlings had lower brightness (Fig. 2a) and less UV-saturated skins (Fig. 2b) than lighter nestlings.
Relationships between body skin coloration at day 6 of the brood and nestling quality. a Relationship between brightness and corrected body mass (i.e. residuals of the regression of body mass on brood size and UV chroma at day 6). b Relationships between UV chroma and corrected body mass (i.e. residuals of the regression of body mass on brood size and brightness at day 6)
Parental preferences in relation to body skin coloration
Sixty-three out of 84 roller nestlings gained weight, and all nests had a minimum of two nestlings that gained weight during the experiment. Nestlings had an average mass gain (mean ± SE) of 1.76 ± 0.22 g, which suggests that parents were actively feeding nestlings during the experiment.
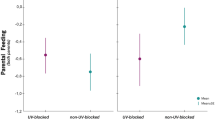
Among the nests, we found that parental favouritism towards UV-blocked and control nestlings changed with brood size (Table 1). Rollers with large broods preferentially fed control over UV-blocked nestlings, whereas rollers with small broods did not show a clear preference (Fig. 3). Within the brood, we found that rollers biased their food allocation depending on age differences between nestling (Table 1). Interestingly, we detected an evident parental favouritism towards control over UV-blocked senior nestlings, whereas feeding rollers did not show preference between control and UV-blocked junior nestlings (Fig. 4).
Average (±SE) body mass gain (g h−3) of roller siblings that were treated with a petroleum jelly containing or not containing ultraviolet-light blocker (UV-reduced and control nestlings, respectively) in relation to brood size. Results of post-hoc Scheffe tests are displayed on average bars. Sample size was 21 tested broods (5, 10 and 6 broods with 3, 4 and 5 nestlings, respectively)
Average (±SE) body mass gain (g h−3) of roller siblings that were treated with a petroleum jelly containing or not containing ultraviolet-light blocker (UV-reduced and control nestlings, respectively) in relation to nestling age (senior versus junior). Results of post-hoc Scheffe tests are displayed on average bars. Sample size was 21 tested broods
Discussion
We have shown that the body skin of nestlings of the roller strongly reflects in the UV. Furthermore, we found that individual variability in the intensity of UV skin coloration correlated with nestling condition. In addition, we reported that biases in food allocation towards nestlings exhibiting visual cue intensities revealing high or low nestling quality changed with age differences between nestlings and brood size. To our knowledge, these findings constitute the first empirical evidence that parents of asynchronous broods may adopt finely tuned strategies of control over resource allocation within the brood based on signalling (i.e. visual colour cues revealing nestling quality) and non-signalling cues, such as age.
There is ample evidence that parental feeding decisions are influenced by gape coloration that can reflect nestling needs or provide a conspicuous target towards which parents can direct their feeds (e.g. Kilner and Davies 1998; Kilner et al. 1999; Saino et al. 2000; Saino et al. 2003; Heeb et al. 2003; de Ayala et al. 2007; Avilés et al. 2008; Dugas 2009; Wiebe and Slagsvold 2009). Chromatic characteristics of the feathers of nestlings can also mediate parental favouritism (Tanner and Richner 2008; Galván et al. 2008; Griggio et al. 2009; see however Tschirren et al. 2005). The pattern of UV reflectance of nestling rollers closely resembled that reported for nestlings of other passerine and non-passerine altricial birds (Hunt et al. 2003; Avilés et al. 2008). Also, previous studies have reported that intensity of UV reflectance by the skin of nestlings of Alpine swifts, European starlings (Bize et al. 2006) was positively correlated with different aspects of nestling quality, and mediated parental favouritism. Here, we have found that heavy roller nestlings had lower brightness and less UV-saturated skins at day 6. Earlier studies have reported either positive (Bize et al. 2006) or negative (Parejo et al. 2010a) relationships between skin reflectance and nestling individual quality, which would suggest that the associations between body skin coloration and nestling age/mass can be a species-specific attribute. The exact mechanism leading to the relationship between nestling size and UV reflectance of the skin is yet unknown. Ultraviolet colours of avian skin are produced by coherent scattering by arrays of collagen fibres in the dermis (Prum and Torres 2003). Therefore, in adult birds, variation in UV skin reflectance could result from differences in the organisation of parallel collagen fibres in the dermis (Prum and Torres 2003). It follows that subtle changes in the hue of the skin can arise by derma shrinkage and derma growth affecting the collagen array size. Thus, perhaps, in nestling rollers, intensity of UV reflectance of the skin reflects to what extent collagen fibres were properly organised. Alternatively, UV reflectance of the skin may vary with the age and/or sex of nestlings. The organisation of collagen fibres of the dermis is likely to change throughout nestling development (Prum and Torres 2003), and a slight sexual size and colour dimorphism have been reported for adult rollers (Silva et al. 2008). Therefore, parents might show colour preferences revealing different condition (i.e. collagen fibres organisation in similar size siblings), as well as age and/or sex when feeding nestlings.
The effect of nestling colour manipulation on parental preference changed with brood size in rollers. Feeding pairs with large broods fed preferentially nestlings displaying skin reflectance intensities revealing low quality (i.e. control nestlings), whereas no clear parental preference was found in nests with lower brood sizes. Brood size was a reliable predictor of degree of hatching asynchrony in our roller population (see Methods). Thus, large broods result in a more evident pattern of size hierarchy within the brood than small broods. Our results, thus, provide support for the prediction that the degree of parental favouritism should increase as age differences between two offspring increase that emerges from theoretical models on age-structured broods (Jeon 2008). However, we did not manipulate degree of hatching asynchrony in this study, and thus, it cannot be excluded that older breeding rollers laid larger broods and showed higher attentiveness than young breeding rollers. Another possibility is that the detected decline of environmental conditions through the season and their effects were more marked on large broods because their food needs were higher than in small broods (e.g. Bize et al. 2006). However, this possibility seems unlikely because we failed to detect seasonal changes in parental preferences (see Table 1).
Within-brood parental responses to nestling colour cues revealing individual quality were affected by nestling age in rollers. The honest signalling model for the resolution of parent–offspring conflict predicts that because young in poorer condition benefits more by receiving extra food, parents should preferentially feed to needy offspring (Godfray 1995). This prediction implicitly assumes that all siblings in a brood have similar reproductive value. In highly asynchronous broods as in rollers, however, seniors may have higher reproductive value than juniors since the instantaneous rate of junior mortality trends to decrease with increasing age (Clutton-Brock 1991). Theoretical models have predicted that whenever resources are limited and large within-brood age differences existed, parents should preferentially invest in older over younger siblings in a brood (Jeon 2008). Interestingly, we found that parent rollers showed an evident feeding preference towards senior nestlings displaying skin intensities revealing low quality, whereas they disregarded skin colour information on nestling quality for juniors. Our results point out that the value of the information on nestling quality provided by skin coloration may change with age in rollers.
It is worth stressing that the UV manipulation performed in this study may result in biased allocation patterns due to parents being presented with chicks that were out of the natural range of variation in skin UV reflectance. The experimental approach used here (i.e. UV block manipulation) was similar to that used in previous studies on the function of UV reflectance in parent–offspring and inter-sexual communication (e.g. Bennett et al. 1996; Sheldon et al. 1999; Jourdie et al. 2004; Bize et al. 2006; Galván et al. 2008; Dugas 2009; Parejo et al. 2010a). This is probably due to the practical difficulty of using painting techniques to find a colourant that would modify UV signals within the natural range of variation (e.g. Dugas 2009). This possibility, however, seems unlikely because parents showed a trend to feed more junior nestlings treated with the UV-reduced treatment (Fig. 4). Moreover, using a visual model approach, we have shown that the chromatic signal emitted by nestlings after the application of the UV-block treatment was within the range of variation of chromatic signals emitted by the same nestling before the application of that treatment (see “Methods” section), which suggests that parents did not perceive UV-blocked nestlings as an artefact.
In conclusion, our study reveals that food distribution within broods of altricial birds can be at least partially influenced by parental preferences for visual colour cues revealing nestling quality and by nestling age. These findings provide a plausible explanation for an honest signalling resolution of parent–offspring conflict over parental care in asynchronous broods based on parental integration of information on nestling size (i.e. age) with information on nestling condition to feed preferentially the nestlings with the highest reproductive value.
References
Avilés JM (2006) La carraca. Enciclopedia virtual de los vertebrados españoles In: Carrascal LM, Salvador A (ed) Museo Nacional de Ciencias Naturales. http://www.vertebradosibericos.org
Avilés JM, Soler JJ (2009) Nestling colouration is adjusted to parent visual performance in altricial birds. J Evol Biol 22:376–386
Avilés JM, Perez-Contreras T, Navarro C, Soler JJ (2008) Dark nests and conspicuousness in color patterns of nestlings of altricial birds. Am Nat 171:327–338
Bennett ATD, Cuthill IC, Partridge JC, Maier EJ (1996) Ultraviolet vision and mate choice in zebra finches. Nature 380:433–435
Bize P, Piault R, Moureau B, Heeb P (2006) A UV signal of offspring condition mediates context-dependent parental favouritism. Proc R Soc Lond B 273:2063–2068
Clutton-Brock T (1991) The evolution of parental care. Princeton University Press, Princeton, New Jersey
Cotton PA, Wright J, Kacelnik A (1999) Chick begging strategies in relation to brood hierarchies and hatching asynchrony. Am Nat 153:412–420
Cramp S (1998) Cramp’s the complete birds of the Western Palearctic. Oxford University Press, Oxford, Optimedia
de Ayala RM, Saino N, Møller AP, Anselmi C (2007) Mouth coloration of nestlings covaries with offspring quality and influences parental feeding behavior. Behav Ecol 18:526–534
Dugas MB (2009) House sparrow, Passer domesticus, parents preferentially feed nestlings with mouth colours that appear carotenoid-rich. Anim Behav 78:767–772
Galvan I, Amo L, Sanz JJ (2008) Ultraviolet-blue reflectance of some nestling plumage patches mediates parental favouritism in great tits Parus major. J Avian Biol 39:277–282
Godfray HCJ (1991) Signaling of need by offspring to their parents. Nature 352:328–330
Godfray HCJ (1995) Evolutionary-theory of parent-offspring conflict. Nature 376:133–138
Gomez D (2006) AVICOL. A program to analyse spectrometric data., Vol. 1
Griggio M, Morosinotto C, Pilastro A (2009) Nestlings’ carotenoid feather ornament affects parental allocation strategy and reduces maternal survival. J Evol Biol 22:2077–2085
Hart NS (2002) Vision in the peafowl (Aves: Pavo cristatus). J Exp Biol 205:3925–3935
Hastad O, Victorsson J, Odeen A (2005) Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc Nat Acad Sci 102:6391–6394
Heeb P, Schwander T, Faoro S (2003) Nestling detectability affects parental feeding preferences in a cavity-nesting bird. Anim Behav 66:637–642
Hunt S, Kilner RM, Langmore NE, Bennett ATD (2003) Conspicuous, ultraviolet-rich mouth colours in begging chicks. Proc R Soc Lond B 270:S25–S28
Jeon J (2008) Evolution of parental favoritism among different-aged offspring. Behav Ecol 19:344–352
Jourdie V, Moureau B, Bennett ATD, Heeb P (2004) Ultraviolet reflectance by the skin of nestlings. Nature 431:262
Kilner R (1995) When do canary parents respond to nestling signals of need? Proc R Soc Lond B 260:343–348
Kilner R, Davies NB (1998) Nestling mouth colour: ecological correlates of a begging signal. Anim Behav 56:705–712
Kilner R, Noble DG, Davies NB (1999) Signals of need in parent-offspring communication and their exploitation by the common cuckoo. Nature 397:667–672
Lotem A (1998) Higher levels of begging behavior by small nestlings: a case of a negatively correlated handicap. Isr J Zool 44:29–45
Macnair MR, Parker GA (1978) Models of parent-offspring conflict. 2. Promiscuity. Anim Behav 26:111–122
Macnair MR, Parker GA (1979) Models of parent-offspring conflict. 3. Intra-brood conflict. Anim Behav 27:1202–1209
Magrath RD (1990) Hatching asynchrony in altricial birds. Biol Rev 65:587–622
Marchetti K, Price T (1989) Differences in the foraging of juvenile and adult birds—the importance of developmental constraints. Biol Rev 64:51–70
Mock DW, Parker GA (1997) The evolution of sibling rivalry. Oxford University Press, Oxford
Montgomerie R (2006) Analyzing colors. Bird coloration, vol. 1: mechanism and measurements. Harvard University Press, Harvard, pp 90–147
Odeen A, Hastad O (2003) Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol 20:855–861
Parejo D, Silva N, Avilés JM (2007) Within-brood size differences affect innate and acquired immunity in roller Coracias garrulus nestlings. J Avian Biol 38:717–725
Parejo D, Avilés JM, Rodriguez J (2010a) Visual cues and parental favouritism in a nocturnal bird. Biol Lett 6:171–173
Parejo D, Silva N, Avilés JM, Danchin E (2010b) Developmental plasticity varied with sex and position in hatching hierarchy in nestlings of the asynchronous European roller, Coracias garrulus. Biol J Linn Soc 99:500–511
Parker GA, Macnair MR (1978) Models of parent-offspring conflict. 1. Monogamy. Anim Behav 26:97–110
Parker GA, Macnair MR (1979) Models of parent-offspring conflict. 4. Suppression—evolutionary retaliation by the parent. Anim Behav 27:1210–1235
Parker GA, Royle NJ, Hartley IR (2002) Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol Lett 5:206–215
Penteriani V, Delgado MD, Alonso-Alvarez C, Pina NV, Sergio F, Bartolommei P, Thompson LJ (2007) The importance of visual cues for nocturnal species: eagle owl fledglings signal with white mouth feathers. Ethology 113:934–943
Price K, Ydenberg R (1995) Begging and provisioning in broods of asynchronously-hatched yellow-headed blackbird nestlings. Behav Ecol Sociobiol 37:201–208
Prum RO, Torres R (2003) Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J Exp Biol 206:2409–2429
Saino N, Ninni P, Calza S, Martinelli R, De Bernardi F, Møller AP (2000) Better red than dead: carotenoid-based mouth coloration reveals infection in barn swallow nestlings. Proc R Soc Lond B 267:57–61
Saino N, Ambrosini R, Martinelli R, Ninni P, Møller AP (2003) Gape coloration reliably reflects immunocompetence of barn swallow (Hirundo rustica) nestlings. Behav Ecol 14:16–22
Sheldon BC, Andersson S, Griffith S, Örnborg J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Silva N, Avilés JM, Danchin E, Parejo D (2008) Informative content of multiple plumage-coloured traits in female and male European Rollers. Behav Ecol Sociobiol 62:1969–1979
Smiseth PT, Amundsen T (2002) Senior and junior nestlings in asynchronous bluethroat broods differ in their effectiveness of begging. Evol Ecol Res 4:1177–1189
Smiseth PT, Bu RJ, Eikenaes AK, Amundsen T (2003) Food limitation in asynchronous bluethroat broods: effects on food distribution, nestling begging, and parental provisioning rules. Behav Ecol 14:793–801
Soler JJ, Avilés JM, Cuervo JJ, Pérez-Contreras T (2007) Is the relation between colour and immune response mediated by nutritional condition in spotless starling nestlings? Anim Behav 74:1139–1145
Tanner M, Richner H (2008) Ultraviolet reflectance of plumage for parent-offspring communication in the great tit (Parus major). Behav Ecol 19:369–373
Tanner M, Kolliker M, Richner H (2008) Differential food allocation by male and female great tit, Parus major, parents: are parents or offspring in control? Anim Behav 75:1563–1569
Trivers RL (1974) Parent-offspring conflict. Am Zool 14:249–264
Tschirren B, Fitze PS, Richner H (2005) Carotenoid-based nestling colouration and parental favouritism in the great tit. Oecologia 143:477–482
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Wiebe KL, Slagsvold T (2009) Mouth coloration in nestling birds: increasing detection or signalling quality? Anim Behav 78:413–1420
Acknowledgements
This research was funded by the Spanish Ministry of Education and Science/FEDER (CGL2008-00718) to JMA and DP and PIE 200930I029 to JMA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Pruett-Jones
Rights and permissions
About this article
Cite this article
Avilés, J.M., Parejo, D. & Rodríguez, J. Parental favouritism strategies in the asynchronously hatching European Roller (Coracias garrulus). Behav Ecol Sociobiol 65, 1549–1557 (2011). https://doi.org/10.1007/s00265-011-1164-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1164-8