Abstract
Recent meta-analyses and simulation studies have suggested that the relationship between soil resource heterogeneity and plant diversity (heterogeneity–diversity relationship; HDR) may be negative when heterogeneity occurs at small spatial scales. To explore different mechanisms that can explain a negative HDR, we conducted a mesocosm experiment combining a gradient of soil nutrient availability (low, medium, high) and scale of heterogeneity (homogeneous, large-scale heterogeneous, small-scale heterogeneous). The two heterogeneous treatments were created using chessboard combinations of low and high fertility patches, and had the same overall fertility as the homogeneous medium treatment. Soil patches were designed to be relatively larger (156 cm2) and smaller (39 cm2) than plant root extent. We found plant diversity was significantly lower in the small-scale heterogeneous treatment compared to the homogeneous treatment of the same fertility. Additionally, low fertility patches in the small-scale heterogeneous treatment had lower diversity than patches of the same size in the low fertility treatment. Shoot and root biomass were larger in the small-scale heterogeneous treatment than in the homogeneous treatment of the same fertility. Further, we found that soil resource heterogeneity may reduce diversity indirectly by increasing shoot biomass, thereby enhancing asymmetric competition for light resources. When soil resource heterogeneity occurs at small spatial scales it can lower plant diversity by increasing asymmetric competition belowground, since plants with large root systems can forage among patches and exploit soil resources. Additionally, small-scale soil heterogeneity may lower diversity indirectly, through increasing light competition, when nutrient uptake by competitive species increases shoot biomass production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil resource heterogeneity characterizes most plant communities (Fitter 1982; Jackson and Caldwell 1993) and is widely considered to promote species coexistence and diversity through increasing niche availability and thus reducing interspecific competition (Grime 1974; Harrison et al. 2010). The heterogeneity-diversity relationship (HDR) is one of the principal concepts in ecology, and until recently was assumed to be positive (Tilman 1982; Silvertown 2004). However, there is little empirical evidence for a positive HDR in experimental studies (Lundholm 2009), and this is especially true at small spatial scales (i.e., small grain size or soil patches), where non-significant or even negative HDRs appear to be more common (Tamme et al. 2010). Many studies have reported that individual plant species respond differently to heterogeneity of soil resources (Day et al. 2003; Maestre et al. 2007; Rajaniemi 2007), but little is known about how this impacts on plant communities. The few experimental studies that have examined the effects of small-scale soil resource heterogeneity on diversity have found positive, neutral or negative results (e.g., Wijesinghe et al. 2005; Reynolds et al. 2007; Wacker et al. 2008; Eilts et al. 2011). These conflicting results are likely due to the interactive effects of plant and grain size, and resource heterogeneity (Eilts et al. 2011). Tamme et al. (2010) took into account the relative sizes of plants and patches of heterogeneity, and proposed several new concepts to explain negative and neutral HDRs, which require further examination.
The mechanisms by which heterogeneity can lower plant diversity depend partly on the size of the soil patches and species root-foraging abilities (Farley and Fitter 1999; Hutchings et al. 2003; Hodge 2004). When the scale of soil resource patchiness is less than the size of individual plants, a negative HDR can occur when some species perform better in heterogeneous conditions. Hence, plants with larger and more plastic root systems have an advantage in exploiting resource-rich patches and can outcompete other species (Levins 1979; Grime 1994; Wilson 2000; Hutchings et al. 2003; Rajaniemi 2007). Tamme et al. (2010) proposed the term heterogeneity as a separate niche axis to describe this situation where certain species are advantaged by heterogeneity per se. Belowground competition is usually considered to be size symmetric, but heterogeneity can result in asymmetric competition when plants with good foraging abilities access and deplete resource-rich patches, suppressing other species through root competition (Hutchings et al. 2000; Fransen and de Kroon 2001; Schenk 2006). The effect of soil resource heterogeneity on diversity can also be indirect by increasing above-ground productivity of those species better able to access patchily distributed resources, thereby increasing asymmetric light competition (Wilson and Tilman 1993; Stevens and Carson 2002; Hautier et al. 2009; Lamb et al. 2009). Hence, heterogeneity as a separate niche axis explains a negative HDR.
When the size of the patches is similar to, or exceeds, the size of the root system of individual plants, greater heterogeneity may increase isolation among similar patches, reducing the ability of species to forage among suitable patches. Due to small-scale habitat ‘fragmentation’, plant individuals located in unfavorable patches face an increased risk of mortality unless they are able to occupy suitable patches through seed or vegetative dispersal (microfragmentation, sensu Tamme et al. 2010; Laanisto et al. 2013). Microfragmentation is more likely to occur when species are more specialized and adapted to a narrower range of environmental conditions, since they are more susceptible to being ‘isolated’ than other more generalist species (Laanisto et al. 2013).
We conducted a controlled greenhouse experiment to determine the nature of the heterogeneity–diversity relationship, and to test several explanations for the HDR, including the classic niche partitioning theory and some more recent concepts. We established mesocosm communities of common temperate grassland species which were grown in five treatments combining three levels of fertility (low, medium, high) and two levels of soil resource patchiness (large, small). The heterogeneity treatments had the same overall fertility (medium), and were constructed using chessboard combinations of low and high fertility patches. Large patches were designed to be approximately the same size or larger than root extent of common grassland plants (12.5 × 12.5 cm), while small patches were smaller than plant root extent (6.25 × 6.25 cm). Comparable patch sizes have been used in similar experiments (e.g., Wijesinghe et al. 2005), and are a reasonable choice according to the size of common grassland species, and their ability to exploit soil resources (Casper et al. 2003), particularly under greenhouse conditions.
Our main objective was to discover if the relationship between small-scale resource heterogeneity and plant diversity is positive, negative or neutral, and to determine the underlying mechanisms. We used shoot and root biomass as a proxy of overall community performance. Specifically, we considered the following hypotheses:
-
1.
According to the traditional positive HDR, diversity increases with heterogeneity. Hence, we expect higher diversity in the large-scale heterogeneous treatment than in the homogeneous treatments due to the increase in niche availability. We expect that the heterogeneous treatment should have greater biomass than the homogeneous treatment of the same fertility since niche complementarity favors community performance.
-
2.
According to heterogeneity as a separate niche axis concept, when soil patches are approximately smaller in size than plant root extent, heterogeneity may decrease diversity by altering competitive hierarchies since plants with large root systems are better able to exploit resource-rich patches, thereby outcompeting others. In this case, we expect that the small-scale heterogeneous treatment will have lower diversity, and higher productivity (shoot and root biomass) than the homogeneous treatment of the same overall fertility (medium). Additionally, low fertility patches of the small-scale heterogeneous treatment should have lower diversity and higher productivity than patches of the same size in the low fertility treatment. We do not expect differences in diversity and productivity between the high fertility patches of the same size in the small-scale heterogeneous and the high fertility treatment.
-
3.
According to the microfragmentation concept, when soil patches are larger than plant roots, diversity is reduced (i.e., a negative HDR). Hence, we expect lower diversity in the large-scale heterogeneous treatment than in the homogeneous treatments. Heterogeneity reduces habitat patch size and increases isolation among suitable patches, thus reducing plant performance. Therefore, we expect that low and high fertility patches in the large-scale heterogeneous treatment should have lower diversity and productivity than patches of the same size in the low and high fertility treatments, respectively, due to the reduction in overall habitat size and increasing isolation (i.e., surrounding patches can have a different fertility level). In addition, reduced plant performance in heterogeneous conditions will result in lower shoot and root biomass in the large-scale heterogeneous treatment than in the homogeneous treatment of the same fertility.
A summary of the three hypotheses and the expected outcomes are shown in Table 1.
Materials and methods
Experimental design
We conducted a mesocosm experiment in a greenhouse at the University of Tartu, Estonia (58°23′N, 26°43′E) from February to June 2011. The experiment consisted of five treatments (each replicated ten times) with three levels of fertility (low, medium and high), and two scales of resource heterogeneity (large and small patches; Fig. 1).
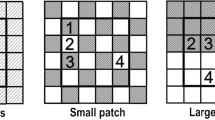
a Diagrammatic representation of the fertility and heterogeneity treatments used in the experiment and b photograph of plants in the small-scale heterogeneous (HetS) treatment 3 weeks after sowing. The boxes were 25 × 25 cm × 20 cm in depth and were subdivided into 16 quadrats of 6.25 × 6.25 cm. Low homogeneous low fertility, Med homogeneous medium fertility, High homogeneous high fertility, HetL heterogeneous large-scale
We used 50 galvanized steel square boxes (25 × 25 cm × 20 cm height), with six holes in the bottom (diameter = 0.5 cm) to ensure water drainage. The fertility gradient was created using three combinations of sand and compost (Biolan Must Muld®; 100 mg N l−1, 200 mg P l−1, 400 mg K l−1). The low fertility treatment (Low) consisted of a 4:1 mixture of sand and compost, the medium fertility treatment (Med) consisted of a 1:1 mixture of sand and compost, and the high fertility treatment (High) consisted of a 1:4 mixture of sand and compost. Each soil mixture was thoroughly homogenized in a cement mixer. For the heterogeneity treatments, we created chessboard combinations of the 4:1 and 1:4 sand:compost mixtures using two patch sizes. In the large-scale heterogeneity treatment (HetL) each four contiguous quadrats contained the same soil mixture (patch size of 12.5 × 12.5 cm). In the small-scale heterogeneity treatment (HetS) every quadrat was filled with a different soil mixture compared to its neighbor (patch size of 6.25 × 6.25 cm). Hence, treatments Med, HetL and HetS contained the same amount of nutrients per box, but varied in the scale of heterogeneity, and treatments Low, Med and High varied in the amounts of nutrients which were distributed homogeneously. The heterogeneous treatments HetL and HetS had the same total area of resource-poor and resource-rich patches but varied in their spatial arrangement (Fig. 1). A metallic partition was placed into each box before adding the sand and compost mixtures to separate each quadrat. Quadrats were filled to 5 cm depth with gravel in order to ensure water drainage, and were then filled with the sand and soil mixtures. The partition was removed after filling the boxes so all the quadrats were connected and root growth among soil patches was enabled.
We added a seed mixture of 15 northern European grassland species to each box on 15 February 2011. These species were selected because they differ in plant traits such as growth form, plant height and their preferences for soil fertility (Table 2). Seeds were obtained from a commercial seed supplier (B&T World Seeds, Paguignan, Aigues-Vives, France). We ensured a minimum of 32 seeds of each species per box (i.e., more than 1,600 seeds of each species were used in the experiment). To include microbial communities, we added 0.5 l of sieved natural grassland soil to each box. Soil was collected from a local grassland, air dried and sieved (mesh size = 0.1 mm) to remove seeds and vegetative plant parts. The natural soil was mixed with the seeds and spread on top of the soil and sand mixtures. An additional two boxes per treatment contained just natural soil, but no seeds, to determine potential contamination from the grassland soil. The boxes were covered with a transparent plastic sheet and watered every 2 days for 15 days after sowing in order to maximize plant germination. To ensure that subsequent differences in plant diversity among treatments were not caused by unequal seed germination, we counted the number of shoots of each species in four random quadrats per box 1 month after starting the experiment. For each box, we calculated the inverse of the Simpson’s dominance index (Gurevitch et al. 2002) using the number of shoots of each species as a measurement of abundance. We compared the index of diversity among the treatments and no significant differences were found (F 4,45 = 0.96; P = 0.437; Online Resource 1). After germination, the experiment ran for 90 days, giving a total duration of 105 days, which is comparable to a growing season in northern Europe. Growing conditions were full light (18 h light), air temperature 17 °C, relative air humidity 75 %, and photosynthetically active radiation 7.98 MJ m−2 day−1. Light conditions for each box were measured every 2 weeks after germination until 15 May, with a LI-190SA quantum sensor and LI-250A light meter (LI-COR Biosciences, USA). To obtain a measure of relative photosynthetic active radiation (below vegetation light/above vegetation light), we took four measurements below and one above the vegetation layer in each box. To minimize position effects in the greenhouse, boxes were randomized weekly.
Plants were harvested from 6 to 11 June 2011. For each quadrat (16 per box), we recorded species richness, the number of shoots per species, and harvested shoot biomass for all species together (clipped at the soil surface). We randomly selected two quadrats in each box to sample root biomass from the homogeneous treatments (Low, Med and High), while in the heterogeneous treatments (HetL and HetS) we choose one low fertility and one high fertility quadrat. To obtain the root samples, the entire block of soil and roots was removed from the box, and the 16 smaller quadrats of 6.25 × 6.25 cm × 20 cm were cut and separated from each other. Hence, the soil samples contained both the roots of species rooted in the quadrat and those foraging from neighboring quadrats. Soil samples were air dried, and roots were carefully separated from the soil. Shoot and root biomass were oven dried at 80 °C for 24 h and weighed (precision = 0.01 g).
Statistical analyses
We compared plant diversity, and shoot and root biomass at the end of the experiment using data from all the quadrats. The inverse of Simpson’s dominance index (Gurevitch et al. 2002) was calculated using the number of shoots of each species as a measurement of abundance, both at the box (25 × 25 cm) and quadrat scale (6.25 × 6.25 cm). At the box scale, we used generalized linear models (GLM) (Veneables and Ripley 2002) to test the effect of the treatments on species diversity, and shoot and root biomass, as well as root:shoot biomass ratio. We considered the following a priori comparisons according to our hypotheses: (1) HDR differs when comparing treatment HetL to treatments Low, Med and High (hypotheses 1 and 3; Table 1); (2) HDR differs when comparing treatment HetS to treatment Med (hypothesis 2; Table 1). These comparisons were also performed for shoot and root biomass, and root:shoot biomass ratio.
At the quadrat scale, we performed two analyses comparing diversity and shoot and root biomass in: (a) treatment Low with the low fertility patches of the two heterogeneous treatments (HetL Low and HetS Low, respectively); (b) treatment High with the high fertility patches of the heterogeneous treatments (HetL High and HetS High, respectively). Hence, we examine how the size of nutrient patches (625 cm2–156 cm2–39 cm2), and the average probability that a neighboring quadrat has the same nutrient content (100 %–67.5 %–41 %), influences shoot and root biomass, and species diversity (hypotheses 2 and 3; Table 1). Due to the nested structure of the experiment (i.e., quadrats in boxes), we used linear mixed-models accounting for box identity as a random factor (Zuur et al. 2009), except in the case of root biomass since in the heterogeneous treatments we only had one sample of each patch type (GLM was used). Prior to all the analyses, we determined if the variables had equal variances among groups and were normally distributed (Zuur et al. 2009). We used Gaussian models with identity link functions because all the variables were quantitative. To evaluate model fit, we examined the normality of the residuals, the relationship between residual and predicted values, and the relationship between the residuals and the predictor variables (Zuur et al. 2009). Due to the different number of quadrats among the treatments (i.e., HetL and HetS had half the number of Low and High quadrats than treatments Low and High), we included a fixed variance structure among groups to meet the assumptions of homogeneity of variances.
The glht function of the multcomp package (Hothorn et al. 2008) was used to study the a priori hypotheses at the box level, and the linear mixed-models were fitted with the nlme package (Pinheiro et al. 2011) of the R statistical language (R Development Core Team 2012).
We used structural equation modeling (SEM) to test if the influence of soil resource heterogeneity on diversity may be indirect by increasing asymmetric light competition (hypothesis 3; Table 1). SEM was used because it enables the study of direct as well as indirect (i.e., through the modification of a third factor) hypothesized relationships among the variables (Grace 2006). To construct our theoretical SEM we considered plant diversity, shoot biomass and a measurement of relative light (measured 21 days before harvesting on 15 May) of the three treatments of the heterogeneity gradient with the same overall fertility (i.e., Med, HetL and HetS). We hypothesized that heterogeneity can influence both species diversity and shoot biomass directly (i.e., competition for soil resources), but biomass can also indirectly influence diversity by increasing light competition. To reflect the combined influence of the treatments, we used a composite variable with two dummy indicators (since they account for the effect of the three treatments). To assess the overall fit of the model we used the χ2 statistic and its associated probability and the root mean square error of approximation (RMSEA) and its associated probability. Non-significant χ2 and RMSEA indexes (P > 0.05) indicate a reasonable fit of the model. The significance of each path specified in the model was based on the probability level (P < 0.05). SEM analysis was performed using the IBM SPSS Amos version 19 statistical software (Arbuckle 2010).
Results
At the box scale (25 × 25 cm), we found significant differences in species diversity, and root and shoot biomass and their ratio among treatments (Fig. 2; Table 3; Online Resource 2). Similarly, we found that plant diversity and biomass differed between the low fertility quadrats (6.25 × 6.25 cm) in treatments HetL and HetS and quadrats in the treatment Low (Fig. 3; Table 4). In the following sections, we examine the results according to the hypotheses considered in Table 1.
Treatment effects on a plant species diversity (inverse of Simpson’s dominance index), b shoot biomass and c root biomass at the box level (25 × 25 cm). Values are mean ± SE for Low, Med, HetL, HetS and High treatments. Dashed lines connect Low, High and two heterogeneous treatments (HetL and HetS); solid lines connect Low, Med and High treatments. Statistics are for treatment effects. For abbreviations, see Fig. 1
Treatment effects on a plant species diversity (inverse of Simpson’s dominance index) and b shoot and root biomass as a function of patch size and probability that a neighboring quadrat has the same nutrient level. White bars correspond to quadrats of the same type in the Low treatment and the low fertility patches of the large-scale (HetL-Low) and small-scale (HetS-Low) heterogeneous treatments. Black bars represent the high fertility quadrats of the High and HetL and HetS treatments. Bars are means; vertical lines are SEs. Results from the generalized linear and linear mixed models show differences among treatments. For abbreviations, see Fig. 1
Hypothesis 1: niche partitioning and a positive HDR
We did not find support for a positive HDR (Table 1) because plant diversity was not higher in treatment HetL than in any of the homogeneous treatments Low, Med and High (Fig. 2a; Table 3). Shoot and root biomass in the heterogeneous treatment HetL were higher than in treatment Low and lower than in treatment High (Fig. 2b, c; Table 3) following the fertility gradient. However, partially in accordance with niche partitioning theory (i.e., increased community performance due to niche complementarity) shoot and root biomass were higher in treatment HetL than in the homogeneous treatment of the same fertility (Med).
Hypothesis 2: heterogeneity as a separate niche axis and a negative HDR
We found support for heterogeneity as a separate niche axis (Table 1) because diversity in treatment HetS was lower than diversity in treatment Med (Fig. 2a; Table 3). In addition, shoot and root biomass were higher in treatment HetS than in treatment Med (Fig. 2b, c; Table 3). Heterogeneity as a separate niche axis was also supported by the analyses at the quadrat scale as we found: (1) low fertility quadrats of treatment HetS had lower diversity, and larger shoot and root biomass than the same quadrats in treatment Low (Fig. 3; Table 4); and (2) no differences were found in either diversity or biomass between the high fertility quadrats in treatment HetS and the same quadrats in treatment High. This suggests that when low fertility quadrats are surrounded by quadrats of higher fertility, as in treatment HetS, they have lower plant diversity than when low fertility quadrats are surrounded by similar quadrats (Table 1).
Further support for heterogeneity as a separate niche axis was found in our SEM, because soil resource heterogeneity had a negative influence on plant diversity by increasing asymmetric light competition (Fig. 4). The SEM results indicate that heterogeneity of soil fertility directly increased shoot biomass, but did not have a significant influence on diversity (Fig. 4). However, shoot biomass had a significant direct negative effect on relative light, and light positively influenced diversity. Therefore, there is an indirect negative influence of shoot biomass and heterogeneity on diversity (treatments HetL and HetS). The model accounted for 29 % of the variation in diversity, 37 % of the variation in shoot biomass, and 80 % of the variation in relative light. The values of the χ2 statistic and associated probability (χ2 = 2.11; P = 0.72) and the RMSEA (0.001; P = 0.74) indicated a good fit of the model. A complete summary of the results can be found in Online Resource 3. These results indicate that resource heterogeneity may lower plant diversity indirectly by increasing asymmetric light competition.
Structural equation model relating the heterogeneity gradient to shoot biomass, relative light and plant diversity. The analysis uses the Med and the HetL and HetS treatments. Values above the boxes indicate the error variance in each variable (i.e., fraction unexplained); values above single-headed arrows indicate the direct causal effect of one variable on another (raw and standardized coefficients separated by a slash). Positive effects are shown by a solid line, negative effects by a dashed line. Significant pathways (P < 0.05) are denoted by asterisks; doubled-headed arrows indicate non-studied correlations. For abbreviations, see Fig. 1
Hypothesis 3: microfragmentation and a negative HDR
The results obtained from the box-level (25 × 25 cm) analyses did not support the microfragmentation concept, since diversity in treatment HetL was not significantly different from that in the homogeneous treatments Low, Med and High (Fig. 2a; Table 3). Similarly, we found greater shoot and root biomass in treatment HetL than in treatment Med (Fig. 2b, c; Table 3). At the quadrat scale (6.25 × 6.25 cm), no differences in diversity were found between low fertility quadrats in treatment HetL and treatment Low, and between high fertility quadrats in treatment HetL and treatment High (Fig. 3a; Table 4). Hence, the reduction in patch size did not reduce diversity, which is inconsistent with the microfragmentation concept (Table 1). In addition, low fertility quadrats in treatment HetL had significantly greater shoot biomass that low fertility quadrats in treatment Low (Fig. 3b; Table 4), in contrast to the expectations of the microfragmentation concept.
Discussion
Recently published meta-analyses and computer simulations have sparked renewed interest in the relationship between heterogeneity and plant diversity (Lundholm 2009; Tamme et al. 2010; Allouche et al. 2012; Laanisto et al. 2013; Smith and Lundholm 2012). In accordance with our results, small-scale soil resource heterogeneity can lower plant diversity, and this is especially true when the size of soil patches is relatively smaller than plant root size. We found several pieces of evidence to support the hypothesis of heterogeneity as a separate niche axis (sensu Tamme et al. 2010), which suggests that some species are better adapted to heterogeneity per se, gaining a competitive advantage (Levins 1979; Wilson 2000). This is consistent with other studies examining species’ responses to heterogeneity (e.g., Baer et al. 2004; Wijesinghe et al. 2005; Eilts et al. 2011), and hence, this mechanism for a negative HDR may be more common than previously thought.
In heterogeneous environments, the performance of individual plants depends on their ability to deplete nutrients from resource-rich patches (Day et al. 2003; Maestre and Reynolds 2007; Wijesinghe et al. 2005; Rajaniemi 2011). Therefore, it is reasonable to expect that species with good foraging abilities may have a competitive advantage (Grime 1994; Wilson 2000; Hutchings et al. 2003), resulting in asymmetric competition for belowground resources (Hutchings et al. 2000; Fransen and de Kroon 2001). However, whether soil resource heterogeneity negatively influences diversity depends on the interactive effects of patch size and plant size (VivianSmith 1997; Collins and Wein 1998; Baer et al. 2004; Wijesinghe et al. 2005; Reynolds et al. 2007; Eilts et al. 2011). For example, Baer et al. (2004) found that adding N in patches of 2 m2 decreased species richness in restored prairies, and Eilts et al. (2011), found a negative influence of resource heterogeneity (patches of 625 cm2) on species richness. These two studies found evidence for heterogeneity as a separate niche axis concept (albeit they did not use this term) as soil resource heterogeneity enhanced the dominance of rhizomatous and clonal species, respectively, and decreased species richness. Similarly, Wijesinghe et al. (2005) conducted a manipulative experiment in which the soil patches were of a similar size to that used in our small-scale treatment (i.e., 39 cm2). Although they did not find a negative HDR, species composition was different in heterogeneous and homogeneous conditions suggesting that some species were better adapted to exploit patchy soils than others. In our study, we found that plant performance (measured as shoot and root biomass) was higher in heterogeneous than in homogeneous conditions, while plant diversity was lower. Moreover, low fertility quadrats had higher biomass and lower diversity if they were located adjacent to resource-rich patches (i.e., heterogeneous treatments) than if they were located adjacent to resource-poor patches (i.e., homogeneous low fertility treatment). Hence, some species may have a competitive advantage in exploiting resource-rich patches in heterogeneous conditions, resulting in asymmetric competition for soil resources and lowering plant diversity.
Soil resource patchiness can also decrease diversity indirectly by increasing shoot biomass and asymmetric light competition (Wilson 2000; Baer et al. 2004; Eilts et al. 2011). Lamb et al. (2009) demonstrated that soil fertility decreased diversity indirectly, since it enhanced shoot development of several species, and increased asymmetric light competition. Theoretically, above- and belowground productivity of heterogeneous systems can be similar to that observed under homogeneously fertile conditions since the potential for nutrient uptake from the nearby fertile patch may enhance shoot growth rates (Fransen and de Kroon 2001). In our study, this was supported by the greater shoot biomass found in the heterogeneous treatments compared to the homogeneous treatment of the same fertility. Moreover, the result of the SEM demonstrates that the increase in shoot biomass is strongly related to a reduction in relative light availability, and that this relationship is further intensified in the heterogeneous treatments. Finally, the results also demonstrate that higher relative light enhances plant diversity, supporting the hypotheses that the influence of soil resource heterogeneity on diversity may be indirect by increasing shoot biomass and asymmetric light competition.
We found greater shoot and root biomass in our large-scale heterogeneous treatment (patch size 156 cm2) than in the homogeneous treatment of the same fertility. This may indicate that plants located in the resource-poor patches can forage and exploit resource-rich patches. This was also confirmed by the greater shoot biomass found in the low fertility quadrats of the large-scale heterogeneous treatment when compared to the same size quadrats in the low fertility treatment. In addition, the results of SEM indicated that the negative effect of shoot biomass on species diversity, via reducing light availability, is of comparable intensity in the two heterogeneous treatments. Therefore, above- and belowground competition may be a feasible explanation for the negative HDR in both of the heterogeneous treatments, but the intensity of these interactions may be greater with smaller soil patches. These results indicate that either soil patches in the large-scale heterogeneous treatment were not large enough to avoid all plant species foraging, or that patch contrasts of the different fertilities were not strong enough, allowing root foraging on the edges between resource-rich and resource-poor patches (Hutchings et al. 2003; Schenk 2006). As a consequence, the influence of above- and belowground resource competition may override the potential influence of microfragmentation on plant diversity.
The negative influences of habitat microfragmentation on plant diversity depend not only on the interaction between plant root extent and soil patch size, but also on community species composition. In a recent modeling study Laanisto et al. (2013) found that plant specialist species (i.e., that have strong preferences for a particular soil type) were strongly influenced by small-scale habitat fragmentation whereas more generalist species were not. Specifically, they showed that communities containing only habitat-specialist species experienced a large decrease in diversity when the habitat was microfragmented, while plant diversity was unaffected when only habitat generalists were present. This study is the first to test the potential negative influence of habitat microfragmentation on either natural or experimentally assembled communities. Further studies, considering longer time spans than used in this experiment and focusing on plant populations, will be needed to disentangle the influence of microfragmentation on plant communities.
In conclusion, our experimental study demonstrates that small-scale heterogeneity of soil resources can lower species diversity, most likely due to some species’ adaptation to heterogeneity, increasing asymmetric competition for both above- and belowground resources. Using an experimental community allowed us to isolate the effect of soil heterogeneity from other confounding factors such as the size of the species pool (Dickson and Foster 2008; Zobel and Pärtel 2008), or variation in other resource levels (Fitter 1982). However, in natural ecosystems more pronounced soil variations occur at small spatial scales (Jackson and Caldwell 1993), environmental heterogeneity can involve more than just one factor (Reynolds et al. 1997; Farley and Fitter 1999) and species composition can display a wide variety of traits. Therefore, further studies using data from natural communities and along different environmental gradients are needed to determine the generality of the concepts presented here. This study highlights the importance of considering different scales of heterogeneity, because when heterogeneity occurs at small spatial scales (patches are equal to or smaller than plant size), it can have a negative influence on diversity by increasing asymmetric competition for above- and belowground resources (heterogeneity as a separate niche axis; Tamme et al. 2010).
References
Allouche O, Kalyuzhny M, Moreno-Rueda G, Pizarro M, Kadmon R (2012) Area-heterogeneity tradeoff and the diversity of ecological communities. Proc Natl Acad Sci USA 109:17495–17500. doi:10.1073/pnas.1208652109
Arbuckle JL (2010) IBM SPSS Amos 19.0 user’s guide. Amos Development
Baer SG, Blair JM, Collins SL, Knapp AK (2004) Plant community responses to resource availability and heterogeneity during restoration. Oecologia 139:617–629. doi:10.1007/s00442-004-1541-3
Casper B, Schenk H, Jackson R (2003) Defining a plant’s belowground zone of influence. Ecology 84:2313–2321
Collins B, Wein G (1998) Soil resource heterogeneity effects on early succession. Oikos 82:238–245
Day KJ, Hutchings MJ, John EA (2003) The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. J Ecol 91:541–553. doi:10.1046/j.1365-2745.2003.00799.x
Dickson TL, Foster BL (2008) The relative importance of the species pool, productivity and disturbance in regulating grassland plant species richness: a field experiment. J Ecol 96:937–946. doi:10.1111/j.1365-2745.2008.01420.x
Eilts JA, Mittelbach GG, Reynolds HL, Gross KL (2011) Resource heterogeneity, soil fertility, and species diversity: effects of clonal species on plant communities. Am Nat 177:574–588. doi:10.1086/659633
Ellenberg H (1988) Vegetation ecology of Central Europe. Cambridge University Press, Cambridge
Farley RA, Fitter AH (1999) The responses of seven co-occurring woodland herbaceous perennials to localized nutrient-rich patches. J Ecol 87:849–859. doi:10.1046/j.1365-2745.1999.00396.x
Fitter AH (1982) Influence of soil heterogeneity on the coexistence of grassland species. J Ecol 70:139–148
Fransen B, de Kroon H (2001) Long-term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. J Ecol 89:711–722. doi:10.1046/j.0022-0477.2001.00589.x
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, New York
Grime JP (1974) Vegetation classification by reference to strategies. Nature 250:26–31. doi:10.1038/250026a0
Grime JP (1994) The role of plasticity in exploiting environmental heterogeneity. In: Caldwell MM (ed) Exploitation of environmental heterogeneity by plants: ecophysiological processes above- and belowground. Academic Press, San Diego, pp 1–19
Gurevitch J, Scheiner SM, Fox GA (2002) The ecology of plants. Sinauer, Sunderland
Harrison S, Cornell H, Moore KA (2010) Spatial niches and coexistence: testing theory with tarweeds. Ecology 91:2141–2150
Hautier Y, Niklaus PA, Hector H (2009) Competition for light causes plant biodiversity loss after eutrophication. Science 324:636–638. doi:10.1126/science.1169640
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. doi:10.1111/j.1469-8137.2004.01015.x
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363. doi:10.1002/bimj.200810425
Hutchings MJ, Wijesinghe DK, John EA (2000) The effects of heterogeneous nutrient supply on plant performance: a survey of responses with special reference to clonal herbs. In: Hutchings MJ, John EA, Stewart AJA (eds) The ecological consequences of environmental heterogeneity. Cambridge University Press, Cambridge, pp 91–110
Hutchings MJ, John EA, Wijesinghe DK (2003) Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology 84:2322–2334
Jackson RB, Caldwell MM (1993) The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 74:612–614
Laanisto L, Tamme R, Hiiesalu I, Szava-Kovats R, Gazol A, Pärtel M (2013) Microfragmentation concept explains non-positive environmental heterogeneity-diversity relationships. Oecologia 171:217–226. doi:10.1007/s00442-012-2398-5
Lamb EG, Kembel SW, Cahill JF Jr (2009) Shoot, but not root, competition reduces community diversity in experimental mesocosms. J Ecol 97:155–163. doi:10.1111/j.1365-2745.2008.01454.x
Levins R (1979) Coexistence in a variable environment. Am Nat 114:765–783
Lundholm JT (2009) Plant species diversity and environmental heterogeneity: spatial scale and competing hypotheses. J Veg Sci 20:377–391. doi:10.1111/j.1654-1103.2009.05577.x
Maestre FT, Reynolds JF (2007) Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology 88:501–511
Maestre FT, Quero JL, Valladares F, Reynolds JF (2007) Individual vs. population plastic responses to elevated CO2, nutrient availability, and heterogeneity: a microcosm experiment with co-occurring species. Plant Soil 296:53–64. doi:10.1007/s11104-007-9289-2
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2011) nlme: linear and nonlinear mixed effects models. R package version 3.1-102
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rajaniemi TK (2007) Root foraging traits and competitive ability in heterogeneous soils. Oecologia 153:145–152. doi:10.1007/s00442-007-0706-2
Rajaniemi TK (2011) Competition for patchy soil resources reduces community evenness. Oecologia 165:169–174. doi:10.1007/s00442-010-1710-5
Reynolds HL, Hungate BA, Chapin FS, Dantonio CM (1997) Soil heterogeneity and plant competition in an annual grassland. Ecology 78:2076–2090
Reynolds HL, Mittelbach GG, Darcy-Hall TL, Houseman GR, Gross KL (2007) No effect of varying soil resource heterogeneity on plant species richness in a low fertility grassland. J Ecol 95:723–733. doi:10.1111/j.1365-2745.2007.01252.x
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739. doi:10.1111/j.1365-2745.2006.01124.x
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611. doi:10.1016/j.tree.2004.09.003
Smith TW, Lundholm JT (2012) Environmental geometry and heterogeneity–diversity relationships in spatially explicit simulated communities. J Veg Sci 23:737–744. doi:10.1111/j.1654-1103.2011.01380.x
Stevens MHH, Carson WP (2002) Resource quantity, not resource heterogeneity, maintains plant diversity. Ecol Lett 5:420–426. doi:10.1046/j.1461-0248.2002.00333.x
Tamme R, Hiiesalu I, Laanisto L, Szava-Kovats R, Pärtel M (2010) Environmental heterogeneity, species diversity and co-existence at different spatial scales. J Veg Sci 21:796–801. doi:10.1111/j.1654-1103.2010.01185.x
Tilman D (1982) Resource competition and community structure (Monographs in population biology—17). Princeton University Press, New Jersey
Veneables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
VivianSmith G (1997) Microtopographic heterogeneity and floristic diversity in experimental wetland communities. J Ecol 85:71–82
Wacker L, Baudois O, Eichenberger-Glinz S, Schmid B (2008) Environmental heterogeneity increases complementarity in experimental grassland communities. Basic Appl Ecol 9:467–474. doi:10.1016/j.baae.2007.08.003
Wijesinghe DK, John EA, Hutchings MJ (2005) Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. J Ecol 93:99–112. doi:10.1111/j.0022-0477.2004.00934.x
Wilson SD (2000) Heterogeneity, diversity and scale in plant communities. In: Hutchings MJ, John EA, Stewart AJA (eds) The ecological consequences of environmental heterogeneity. Cambridge University Press, Cambridge, pp 55–69
Wilson SD, Tilman D (1993) Plant competition and resource availability in response to disturbance and fertilization. Ecology 74:599–611
Zobel M, Pärtel M (2008) What determines the relationship between plant diversity and habitat productivity? Global Ecol Biogeogr 17:679–684. doi:10.1111/j.1466-8238.2008.00400.x
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank all of the students and technicians for their brave help in the greenhouse. This research was supported by the European Union through the European Regional Development Fund (Center of Excellence FIBIR), ERMOS programme Grant 14 (co-funded by Marie Curie Actions), MOBILITAS post-doctoral grant (MJD47), CSIC JAE DOC fellowship and Estonian Science Foundation (grant 8323). The experiments comply with the current laws of Estonia in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Schmid.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gazol, A., Tamme, R., Price, J.N. et al. A negative heterogeneity–diversity relationship found in experimental grassland communities. Oecologia 173, 545–555 (2013). https://doi.org/10.1007/s00442-013-2623-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-013-2623-x