Abstract
Life in saline environments represents a major physiological challenge for birds, particularly for passerines that lack nasal salt glands and hence are forced to live in environments that do not contain salty resources. Increased energy costs associated with increased salt intake, which in turn increases the production of reactive oxygen species, is likely a major selection pressure for why passerines are largely absent from brackish and marine environments. Here we measured basal metabolic rates (BMR) and oxidative status of free-ranging individuals of three species of Cinclodes, a group of passerine birds that inhabit marine and freshwater habitats in Chile. We used a combination of carbon, nitrogen, and hydrogen isotope data from metabolically active (blood) and inert (feathers) tissues to estimate seasonal changes in marine resource use and infer altitudinal migration. Contrary to our expectations, the consumption of marine resources did not result in higher BMR values and higher oxidative stress. Specifically, the marine specialist C. nigrofumosus had lower BMR than the other two species (C. fuscus and C. oustaleti), which seasonally switch between terrestrial and marine resources. C. fuscus had significantly higher total antioxidant capacity than the other two species (C. nigrofumosus and C. oustaleti) that consumed a relatively high proportion of marine resources. Nearly all studies examining the effects of salt consumption have focused on intraspecific acclimation via controlled experiments in the laboratory. The mixed results obtained from field- and lab-based studies reflect our poor understanding of the mechanistic link among hydric-salt balance, BMR, and oxidative stress in birds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Songbirds (Passeriformes) have diversified in all continents and occupy nearly all terrestrial ecosystems (Barker et al. 2004), but only a few species in this group inhabit marine and coastal environments (Wheelwright and Rising 1993; Martinez del Rio et al. 2009). A major physiological constraint limiting the geographic distribution of this diverse group of birds is the maintenance of osmotic homeostasis in environments where freshwater is scarce or unavailable (Johnston and Bildstein 1990; Casotti and Braun 2000; Sabat 2000; Bozinovic et al. 2011; Williams et al. 2012; Gutiérrez 2014). For passerines, marine and other saline environments are essentially dry environments as they cannot drink saline water, and most of the available prey (e.g., intertidal invertebrates, Larsen et al. 2014) are in osmotic equilibrium with environmental water. Indeed, feeding on salty marine foods and drinking seawater is especially challenging because these birds lack functional salt glands (Shoemaker 1972), and most of them have a limited ability to concentrate urine (Goldstein and Skadhauge 2000; Sabat and Martínez del Rio 2002; Sabat and Martinez del Rio 2006), which likely explains the paucity of obligate marine passerine species.
Some authors have suggested that the ingestion of salts and other osmotically active compounds represents an important energetic cost to seabirds and shorebirds, and may limit their distribution as well as colonization of new habitats (Nehls 1996; Gutiérrez et al. 2011). Although these osmoregulatory costs have been extensively studied in ectothermic animals (Tseng and Hwang 2008; Evans 2009), the topic has received little attention in endothermic vertebrates. For birds, a scarce amount of experimental evidence shows that consumption of saltwater for days or weeks increases basal (or resting) metabolic rate (BMR) in comparison to individuals that consume freshwater (Gutiérrez et al. 2011; Peña-Villalobos et al. 2013). This increase in BMR is assumed to be the cost of living in saline habitats, which is offset in seabirds and shorebirds by the osmotic work performed by the salt gland and kidneys.
Indirect evidence suggests that the costs of osmoregulation can be significant in birds and thus an important factor in prey selection and associated energy budgets in the field. For example, McNab (2009) concluded that seabirds have higher BMR than terrestrial species that inhabit forest or desert/grassland habitats. Furthermore, Gutierrez et al. (2012) found that BMR was significantly lower in shorebirds (Calidris alpina) inhabiting inland freshwater aquatic habitats in comparison to those that reside in coastal environments. Gutierrez et al. (2012) also found a similar pattern at the interspecific level among shorebirds after accounting for potential climatic effects. It is important to note that abiotic (e.g., temperature) and biotic (e.g., diet) factors known to influence BMR (Tieleman and Williams 2000; Tieleman et al. 2003; Cavieres and Sabat 2008; Swanson 2010; Maldonado et al. 2012; Naya et al. 2013) vary among habitats. Altogether, these studies suggest that saltwater consumption or consuming prey with high salt loads affects energy expenditure (BMR) in unpredictable ways, a topic that needs further research. In this context, obtaining an independent estimate of marine resource use is essential to identify the mechanisms that underlie physiological changes associated with salt intake in wild free-ranging populations.
On the other hand, an increase in metabolic activity may result in increased production of reactive oxygen species (ROS), a standard byproduct of the formation of ATP (Costantini and Verhulst 2009), whose accumulation ultimately leads to cell damage, disease, and even death (Dowling and Simmons 2009; Monaghan et al. 2009; van de Crommenacker et al. 2010; Selman et al. 2012). Increasing osmoregulatory function accelerates metabolic rates in several organisms including birds, but the precise effects of this acceleration on cellular oxidative status are unknown. Recently, Sabat et al. (2017) reported that when acclimated to drinking water of moderate salinity (200 mM NaCl), the rufous-collared sparrow (Zonotrichia capensis) exhibited changes in total oxidative status. Specifically, both antioxidant capacity and lipid peroxidation were higher in the liver of animals acclimated to saltwater than in those acclimated to freshwater. In addition, urine concentration and BMR increased in birds that drank salt water. Thus, it is important to note that there are intra- and interspecific differences in the resistance to environmentally mediated oxidative stress (Cohen et al. 2008; Beaulieu and Costantini 2014). Thus, an unresolved question is whether there are differences in the antioxidant capacity among phylogenetically related species if they differ in their seasonal use of marine resources, and more broadly what are the consequences of oxidative damage induced by salt consumption on physiological integrity.
Here we explore the presumed links between osmoregulation, BMR, and enzymatic mechanisms of oxidative stress in three species of Cinclodes, the only passerine genus that exhibits intra- and interspecific differences in marine resource use. By quantifying the physiological effects associated with the consumption of marine resources, we explored the proximate factors explaining the use of coastal habitats and dietary preferences in a widespread group of songbirds, which has implications for the question of why passerines as a group infrequently use saline resources. Furthermore, studying the effects of high salt loads experienced by Cinclodes will provide an understanding of how plastic animals can be in coping with diverse environmental stressors.
Materials and methods
Field collection
Birds were captured using mist nest and floor traps on the central coast of Chile in Algarrobo (33°22′09″S 71°40′05″W), El Quisco (33°24′S 71°42′W), and Los Molles (32°14′22″S 71°30′54″W) during austral winter (May–August). Blood samples were obtained with heparinized capillaries from the humeral vein of each bird immediately after capture. Blood samples were kept at 4 °C until centrifugation, which occurred within 12 h of capture. Blood was centrifuged at 10,000g for 10 min, and the plasma was separated and transported in liquid N2 to the laboratory and then stored at − 80 °C until analysis. In addition, a subsample of whole blood was collected for isotope analysis, which corresponded to the dietary resources assimilated during ~ 1–2 months prior to capture (Martinez del Rio et al. 2009), which occurred in the austral winter. Primary feathers (P9) were collected from each individual, which corresponds to the assimilated resources during the molting period that occurs during the austral summer in Cinclodes (Bertolero and Zavalaga 2003).
Basal metabolic rate
Basal metabolic rate (BMR) was measured via oxygen consumption using a continuous flow respirometry system (FoxBox, Sable Systems®, Nevada), as reported in Sabat et al. (2017). Briefly, birds were placed in metabolic chambers kept at a constant temperature (30 ± 0.5 °C) that received air free of water and CO2 removed via Drierite and CO2 absorbent at a flow of 750 mL/min; air flow was maintained at ± 1%. The O2 concentration in the chamber was recorded for 8 h per individual during the period of inactivity between 20:00 and 06:00. Because water vapor and CO2 were removed before breath samples entered the O2 analyzer, and the flow rates are measured downstream from the metabolic chamber, oxygen consumption was calculated according to equation (11.1) in Lighton (2008): VO2 = FR × 60 × (FiO2 − FeO2)/(1 − FiO2), where FR is the flow rate in mL min−1, and FiO2 and FeO2 are the fractional concentrations of inflow and outflow O2 in the metabolic chamber, respectively.
Oxidative stress
One limitation of using blood serum or plasma biochemical assays to characterize oxidative status in wild birds is the uncertainty that such measurements reflect the oxidative profile of the whole body. Although different tissues may show different biomarker responses to stressors in birds (e.g., Sabat et al. 2017), it is believed that plasma adequately reflects stressful situations, and hence the blood oxidative profile could be appropriate for field studies in birds and other vertebrate species (Costantini 2008; Costantini et al. 2012; Margaritelis et al. 2015). Finally, considering the short turnover time of blood plasma (Martinez del Rio et al. 2009), the use of this substrate for biochemistry assays allowed us to compare the oxidative status of birds in the winter months when the birds were captured. We used two common biomarkers of oxidative stress to assess the oxidative status in plasma samples: (1) the total antioxidant capacity (TAC) that is an indicator of the presence of molecular and enzymatic antioxidants in tissues, and (2) lipid peroxidation that is an indicator of oxidative damage. Total antioxidant capacity was measured by a colorimetric reaction between copper (I), which results of the reduction of copper (II) by antioxidants present in the sample, and a chromogenic reagent, generating a complex with a maximum absorbance at 490 nm (Cell Biolabs OxiSelect™ STA-360, San Diego, CA, USA). Lipid peroxidation was measured via formation of the malondialdehyde:thiobarbituric acid adduct at 532 nm (Cell Biolabs OxiSelect™ STA-330).
Stable isotope analysis
Whole blood was dried at 60 °C for 1 week and feathers were cleaned with a 2:1 chloroform:methanol solvent solution to remove surface contaminants. Approximately 0.5–0.6 mg of dried whole blood or feather tissue was weighed into tin capsules and carbon (δ13C) and nitrogen (δ15N) isotope values were measured on a Costech 4010 elemental analyzer coupled to a Thermo Scientific Delta V Plus isotope ratio mass spectrometer. Tissue hydrogen isotope (δ2H) values were measured with a Thermo Scientific thermal conversion elemental analyzer (TCEA) coupled to a Thermo Scientific Delta V Plus isotope ratio mass spectrometer. All isotope measurements were conducted at the University of New Mexico Center for Stable Isotopes (Albuquerque, NM). Stable isotope data are expressed as δ values using the equation δX = (RSample/RStandard) − 1, where X is any isotope system of interest (e.g., H, C, or N) and RSample and RStandard are the ratios of the heavy to light isotope (e.g., 2H/1H, 13C/12C, 15N/14N) for each sample and standard, respectively. The internationally accepted standards are Vienna Standard Mean Ocean Water (V-SMOW) for δ2H, Vienna Pee Dee Belemnite (V-PDB) for δ13C, and atmospheric nitrogen for δ15N; units are per mil (‰). Within-run analytical precision (SD) for δ2H measurements of organic materials was determined by analysis of internal reference materials described below and measured to be ≤ 3‰. Within-run precision (SD) for both δ13C and δ15N was estimated via analysis of internal reference materials and measured to be ≤ 0.2‰ for both isotope systems.
Approximately 8–15% of the organically bound hydrogen in proteins can potentially exchange with hydrogen in atmospheric water vapor at ambient temperature (Wassenaar and Hobson 2000; Bowen et al. 2005). To correct for exchangeable hydrogen in unknown samples, we used three keratins and two whole blood internal reference materials for which the δ2H composition of the non-exchangeable hydrogen fraction (δ2Hnon-ex) was determined via comparative equilibration experiments and statistical analyses identical to those described in Bowen et al. (2005). δ2Hnon-ex values of these reference materials ranged from − 55 to − 175‰ for the feather keratins and from − 80 to − 160‰ for whole blood, which brackets the δ2H values of the Cinclodes tissue samples we analyzed. Analyses of these four organic reference materials, two oil reference materials, and pure stearic acid were used to determine the within-run precision (standard deviation), which was determined to be ≤ 3‰ relative to V-SMOW.
Statistical analysis
We tested the effect of species on logarithmic transformed basal metabolic rate (log BMR) using ANCOVA with body mass (mb) as a covariate. A post hoc Fisher test was then performed to compare the least squares means (mass-corrected basal metabolic rate, BMRc) obtained from the ANCOVA analysis. We also tested the effect of species on total BMR and mass-specific metabolic rate (BMRm) by means of a one-way ANOVA. Significant differences in tissue isotope values and biomarkers of oxidative stress biomarkers in plasma were assessed with a Kruskal–Wallis test and a post hoc Wilcoxon test. Finally, we used linear regression to examine the relationship between plasma TAC (dependent variable) and whole blood δ13C values (independent variable).
Results
Stable isotope analysis
Both feather and whole blood δ13C, δ15N, and δ2H values were significantly different among species (Table 1, Fig. 1); see supporting Table S1 for statistical output of the Kruskal–Wallis tests. C. nigrofumosus had higher δ13C, δ15N, and δ2H values than C. fuscus in both whole blood and feathers indicative of winter and summer foraging, respectively. C. oustaleti had intermediate whole blood δ13C and δ15N values relative to C. nigrofumosus and C. fuscus; C. oustaleti and C. fuscus had similar whole blood δ2H values. Feather δ13C and δ15N values were similar for C. fuscus and C. oustaleti, and were much lower than for C. nigrofumosus. C. oustaleti had lower feather δ2H values than C. fuscus and C. nigrofumosus.
Basal metabolic rate
There is a significant effect of species on log BMR after controlling for body mass (ANCOVA F2,32 = 6.11; p = 0.006). Log BMR was significantly related to Log mb (F1,32 = 31.24; p < 0.0001) and there was no interaction between Log mb and species (F2,32 = 2.45; p = 0.11). A posteriori analysis revealed that the mass-corrected basal metabolic rate (BMRc) was higher in C. oustaleti than in C. nigrofumosus (p = 0.002) or in C. fuscus (p = 0.015). The log BMRc values of C. nigrofumosus and C. fuscus showed differences that are at the limit of significance (p = 0.045).
Oxidative stress
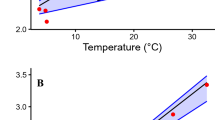
Plasma TAC differed among species (F2,15 = 6.67, p = 0.008; Fig. 2). C. fuscus had higher TAC concentrations than C. nigrofumosus and C. oustaleti. However, there were no significant differences in the levels of lipid peroxidation among species (F2, 11 = 0.33, p = 0.73). We found a negative relationship (r = − 0.64, F1,12 = 8.25, p = 0.014) between plasma TAC and δ13C value (Fig. 3), indicating that individuals with more terrestrial-based diets have higher levels of antioxidants.
Discussion
Seasonal ecological patterns via δ13C and δ15N analysis
Stable isotope analysis is ideal for characterizing marine versus terrestrial resource use (Schoeninger and DeNiro 1984; Hobson 1987) and latitudinal and/or altitudinal migration (Hobson 2008). This approach has been successfully applied to study the ecology (Martinez del Rio et al. 2009; Newsome et al. 2015), environmental physiology (Sabat and Martinez del Rio 2002, 2006; Sabat et al. 2006), and evolutionary biology (Rader et al. 2017) of Cinclodes. Since we collected birds in winter, metabolically active whole blood represents ecological information integrated over 1–2 months prior to capture (Martinez del Rio et al. 2009), while metabolically inert feathers represent ecological information from the previous summer when feathers are molted and regrown in this genus (Bertolero and Zavalaga 2003).
In agreement with previous work (Sabat and Martinez del Rio 2006; Martinez del Rio et al. 2009; Newsome et al. 2015), we found high blood and feather δ13C and δ15N values in C. nigrofumosus (Table 1 and Fig. 1), suggesting that this species is a resident marine specialist. In contrast, C. fuscus had relatively low blood and feather δ13C and δ15N values indicating that terrestrial resources dominate its diet throughout the year. Note that marine resource use by C. fuscus was higher in winter than summer (Fig. 1), but relatively low in comparison to the proportion of marine resources used by the other two species during the winter months. C. oustaleti had blood (winter) δ13C and δ15N values that were intermediate in comparison to the other two species, but feather (summer) isotope values were similar to those of C. fuscus. This pattern suggests that C. oustaleti is a mixed marine and terrestrial forager that based on associated δ2H data (see below), makes seasonal altitudinal movements to high elevations during summer.
Tissue δ2H patterns agreed with expectations based on seasonal shifts in diet gleaned from associated δ13C and δ15N data. C. nigrofumosus had the highest tissue δ2H values, which is likely the product of the ingestion of seawater that has a hydrogen isotope value of around 0‰. In contrast, C. fuscus had tissue δ2H values that are relatively low suggestive of the use of terrestrial resources fuelled by coastal precipitation, which in central Chile varies between − 20 and − 40‰ throughout the year (Bowen 2018). The greatest difference in whole blood versus feather δ2H was observed in C. oustaleti. Whole blood δ2H was similar to that of the marine forager C. nigrofumosus, but C. oustaleti feather δ2H values were significantly lower than the other species, indicating this species is an altitudinal migrant and spends the summer months foraging in freshwater aquatic environments at high altitude; a similar pattern was observed for this species by Newsome et al. (2015).
Metabolic adjustments related to marine resource use
Previous studies on free-ranging wild birds suggest that consumption of salty (food and water) resources imposes energetic costs, which are consistent with the elevated BMR observed in salt-acclimated animals in experimental studies (Peña-Villalobos et al. 2014; Sabat et al. 2017). Here, we examined individuals of three closely related species in the same season (winter) collected from the same localities, and used a robust indirect dietary proxy (stable isotope analysis) to characterize how marine resource use, and thus osmotic load (Sabat and Martínez del Rio 2002, 2006), varies significantly among species.
The relatively low BMR observed in the specialist marine forager C. nigrofumosus could result from phenotypic physiological adjustments that offset the osmoregulatory energy demands observed in birds subjected to acute osmotic stress (Gutiérrez et al. 2012; Sabat et al. 2017). For most birds, marine and other saline-rich environments (estuaries) are essentially dry habitats where freshwater is limiting because salt water is the only available source of drinking water and most marine invertebrate prey are in osmotic balance with environmental water. This seems to be the case for C. nigrofumosus (Sabat and Martínez del Rio 2005) that has a constant osmotic load in both the dry summer (735 mOsm/kg) and the more rainy winter (688 mOsm/kg) seasons. Thus, the water budget and specifically total evaporative water loss may also be a critical physiological variable constraining the consumption of salt-rich prey in Cinclodes. We hypothesize that the low BMR observed in C. nigrofumosus, which reduces endogenous heat production and water requirements (Dawson 1984; Bozinovic and Gallardo 2006; Maloney 2008), is a consequence of the consumption of marine resources with high osmotic loads (Sabat et al. 2004a). An interesting question for future research is to quantify the proportion of the total body water pool of Cinclodes species that use marine resources to various degrees (Rader et al. 2017) is composed of metabolic water versus pre-formed fresh or saline water sources.
Surprisingly, the highest BMR was observed in C. oustaleti, which may be associated with the high energetic and osmoregulatory costs of seasonally moving between coastal and high elevation Andean habitats (Table 2). C. oustaleti experiences seasonal changes in the osmotic load of its prey, which results in plastic modifications of its renal phenotype and function (Sabat et al. 2004b). Costs associated with such phenotypic plasticity (Pigliucci 2001) can be significant, and include changes organ mass, enzyme activities, and serum thyroid concentrations; such phenotypic changes have been observed in other bird species (Krebs 1950; Daan et al. 1990; Williams and Tieleman 2000; Vezina and Williams 2005; Swanson and Thomas 2007; Zheng et al. 2008; Maldonado et al. 2009; Sabat et al. 2017). Finally, tissue δ2H analysis revealed that C. oustaleti is an altitudinal migrant. Previous work has suggested that migratory species have higher BMR (Bushuev et al. 2018), however, the use of cold high-latitude breeding areas by migratory birds (Jetz et al. 2008) may also contribute to this pattern. In addition, some studies have reported that avian species from high elevation habitats have higher BMR compared with those inhabiting in low elevation areas (McNab 2009, 2013), but other studies did not find such a pattern (Londoño et al. 2015). We hypothesized that the relatively high basal metabolic rates found in C. oustaleti in comparison to low elevation resident species (e.g., C. nigrofumosus) could be explained by its elevational migratory behavior.
Oxidative status in free-ranging birds
One of the primary objectives of our study was to assess whether there is a functional association between oxidative status and the differential use of marine and freshwater resources among three species of closely related passerines collected from the same localities. We observed a negative association between plasma antioxidant biomarkers and marine resource use (Fig. 3), so species that primarily consumed marine resources (C. nigrofumosus and C. oustaleti) had a lower mean TAC compared with C. fuscus that mostly consumed terrestrial resources (Fig. 2). Thus, the absence of differences in plasma lipid peroxidation concentrations among species in conjunction with the observed pattern in TAC, suggests that the three species are subjected to different levels of ROS, which are compensated for by production of antioxidants as indicated by TAC.
These results differ from our expectations because our recent work showed a significant increase of enzymatic antioxidant defences in rufous-collared sparrows experimentally acclimatized to saline resources (Sabat et al. 2017). Thus, we expected to find higher values of TAC in plasma of C. nigrofumosus than in that of its congeners. This expectation was based on the assumption that the response to acute acclimatization (in the laboratory) should be similar, both in magnitude and in the direction of change, to acclimatization or evolutionary adaptation to natural environmental conditions associated with increased salt intake (i.e., marine resource use). C. nigrofumosus can specialize on marine resources because it possesses a unique physiology that includes large kidneys with a high proportion of medullary tissue, which enables it to concentrate urine (Sabat and Martínez del Rio 2002, 2006), an adaptation that is absent in all other species in the order Passeriformes. C. nigrofumosus also has lower relative water losses as measured by the ratio between total evaporative water (TEWL) and BMR (Sabat et al. 2004a), which enables it to simultaneously cope with high saline loads and avoid dehydration. In contrast, C. oustaleti has a high degree of phenotypic plasticity in renal function, paired with structural changes in the kidneys (Sabat et al. 2004b) that facilitate seasonal movement between (freshwater) terrestrial and marine resources (Martinez del Rio et al. 2009) without conferring significant costs to maintaining water balance. The greater capacity of C. nigrofumosus to avoid dehydration and the phenotypic plasticity observed in C. oustaleti may explain why these species have lower TAC in the face of increased salt loads in comparison to C. fuscus. For C. fuscus, a reduced capacity to deal with high osmotic loads suggests that consumption of a relatively low proportion of marine prey in the winter (Table 1) would be enough to produce significant salt stress, resulting in increased glucocorticoids concentrations that ultimately enhances ROS formation (Lin et al. 2004). Future experiments should include acute acclimation experiments at different osmotic load levels in different bird species to compare with results observed in free-ranging individuals.
In addition to salt intake, there are other potential explanations for the high levels of total antioxidants observed in C. fuscus relative to the other species. First, variation in tissue metabolic activity and its relationship with body mass could influence TAC. Mass-specific metabolic rates are negatively related to body mass, i.e., smaller animals have higher cellular metabolic activity than larger ones. The two smallest species in our study, C. fuscus and C. oustaleti, had similar mass-specific metabolic rates (Table 2) but significantly different TAC values (Fig. 2). Thus, the prediction that higher metabolic rates produce more ROS, which leads to an increase in antioxidant expression or to greater oxidative damage is not supported by our results. Even though some studies have found support for a functional relationship between metabolic rate and oxidative state (Williams et al. 2010), this pattern has been the subject of debate (Selman et al. 2012) and is not supported by some empirical datasets (Brzęk et al. 2014). Finally, we cannot discard other potential reasons for the observed differences in TAC among Cinclodes, such as variation in the concentrations of dietary vitamin E and carotenoids, which are ubiquitous (Bhosale and Bernstein 2007) antioxidants that reduce oxidative damage (Catoni et al. 2008).
Summary
Our study shows that some of the ecological traits unique to Cinclodes, such as persistent or seasonal marine resource use coupled with altitudinal migration, are correlated with organismal (e.g., BMR) and biochemical (e.g., TAC) physiological traits. We encourage future studies to include additional proxies of energy expenditure known to impact energy budgets in birds (e.g., metabolic scope or field metabolic rate), and additional indicators of oxidative status in a wider range of tissues or biological materials (urine, feces). The discrepancies between the patterns observed here and previous experimental data (Peña-Villalobos et al. 2014; Sabat et al. 2017) highlight substantial differences between intraspecific analyses that study the effects of acute acclimation in the laboratory versus interspecific comparative analyses of free-ranging species more indicative of evolutionary adaptation (Cruz-Neto and Bozinovic 2004). The disparity between these two levels of analysis may reflect our poor understanding of how the need to eliminate salts and conserve water influences oxidative status in birds.
References
Barker FK, Cibois A, Schikler P, Feinstein J, Cracraft J (2004) Phylogeny and diversification of the largest avian radiation. Proc Natl Acad Sci USA 101:11040–11045
Beaulieu M, Costantini D (2014) Biomarkers of oxidative status: missing tools in conservation physiology. Conserv Physiol. https://doi.org/10.1093/conphys/cou014
Bertolero A, Zavalaga C (2003) Observaciones sobre la biometría y la muda del churrete marisquero (Cinclodestaczanowskii) en Punta San Juan, costa sur de Perú. Ornitol Neotrop 14:469–475
Bhosale P, Bernstein PS (2007) Vertebrate and invertebrate carotenoid-binding proteins. Arch Biochem Biophys 458:121–127
Bowen GJ (2018) Waterisotopes Database. http://www.waterisotopes.org. Accessed 12 May 2018
Bowen JB, Chesson L, Nielson K, Cerling TE, Ehleringer JR (2005) Treatment methods for the determination of δ2H and δ18O of hair keratin by continuos-flow isotope-ratio mass spectrometry. Rapid Commun Mass Spectrom 19:2371–2378
Bozinovic F, Gallardo P (2006) The water economy of South American desert rodents: from integrative to molecular physiological ecology. Comp Biochem Physiol C Toxicol Pharmacol 142:163–172
Bozinovic F, Calosi P, Spicer JI (2011) Physiological correlates of geographic range in animals. Annu Rev Ecol Evol Syst 42:155–179
Brzęk P, Książek A, Ołdakowski Ł, Konarzewski M (2014) High basal metabolic rate does not elevate oxidative stress during reproduction in laboratory mice. J Exp Biol 217:1504–1509
Bushuev A, Tolstenkov O, Zubkova E, Solovyeva E, Kerimov A (2018) Basal metabolic rate in free-living tropical birds: the influence of phylogenetic, behavioral, and ecological factors. Curr Zool 64:33–43
Casotti G, Braun EJ (2000) Renal anatomy in sparrows from different environments. J Morphol 243:283–291
Catoni C, Schaefer HM, Peters A (2008) Fruit for health: the effect of flavonoids on humoral immune response and food selection in a frugivorous bird. Funct Ecol 22:649–654
Cavieres G, Sabat P (2008) Geographic variation in the response to thermal acclimation in rufous-collared sparrows: are physiological flexibility and environmental heterogeneity correlated? Funct Ecol 22:509–515
Cohen A, McGraw KJ, Wiersma P, Williams JB, Robinson WD, Robinson TR, Brawn JD, Ricklefs RE (2008) Interspecific associations between circulating antioxidant levels and life history variation in birds. Am Nat 172:178–193
Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–1251
Costantini D, Verhulst S (2009) Does high antioxidant capacity indicate low oxidative stress? Funct Ecol 23:506–509
Costantini D, Ferrari C, Pasquaretta C, Cavallone E, Carere C, von Hardenberg A, Réale D (2012) Interplay between plasma oxidative status, cortisol and coping styles in wild alpine marmots, Marmota marmota. J Exp Biol 215:374–383
Cruz-Neto AP, Bozinovic F (2004) The relationships between diet quality and basal metabolic rate in endotherms: insights from intraspecific analysis. Physiol Biochem Zool 77:877–889
Daan S, Masman D, Groenewold A (1990) Avian basal metabolic rates: their association with body composition and energy expenditure in nature. Am J Physiol 259:R333–R340
Dawson WR (1984) Physiological studies of desert birds: present and future considerations. J Arid Environ 7:133–155
Dowling DK, Simmons LW (2009) Reactive oxygen species as universal constraints in life history evolution. Proc R Soc B 276:1737–1745
Evans DH (2009) Osmotic and ionic regulation: cells and animals. CRC Press, Boca Raton
Goldstein DL, Skadhauge E (2000) Renal and extrarenal regulation of body fluid composition. In: Whittow GC (ed) Sturkie’s avian physiology. Academic, San Diego, pp 265–297
Gutiérrez JS (2014) Living in environments with contrasting salinities: a review of physiological and behavioural responses in waterbirds. Ardeola 61:233–256
Gutiérrez JS, Masero JA, Abad-Gómez JM, Villegas A, Sánchez-Guzmán JM (2011) Understanding the energetic costs of living in saline environments: effects of salinity on basal metabolic rate, body mass and daily energy consumption of a long-distance migratory shorebird. J Exp Biol 214:829–835
Gutiérrez JS, Abad-Gómez JM, Sánchez-Guzmán JM, Navedo JG, Masero JA (2012) Avian BMR in marine and non-marine habitats: a test using shorebirds. PLoS One 7:e42206
Hobson KA (1987) Use of stable-carbon isotope analysis to estimate marine and terrestrial protein content in gull diets. Can J Zool 65:1210–1213
Hobson KA (2008) Applying isotopic methods to tracking animal movements. In: Hobson KA, Wassenaar LI (eds) Tracking animal migration using stable isotopes. Academic, London, pp 45–78
Jetz W, Freckleton RP, McKechnie AE (2008) Environment, migratory tendency, phylogeny and basal metabolic rate in birds. PLoS One 3:e3261
Johnston JW, Bildstein KL (1990) Dietary salt as a physiological constraint on White Ibises breeding in an estuary. Physiol Zool 66:190–207
Krebs HA (1950) Body size and tissue respiration. Biochim Biophys Acta 4:249–269
Larsen EH, Deaton L, Onken H, O’Donnell M, Grosell M, Dantzler W, Weihrauch D (2014) Osmoregulation and excretion. In: Pollock DM (ed) Comprehensive physiology, vol 4. Wiley, New York, pp 405–473. https://doi.org/10.1002/cphy.c130004
Lighton JRB (2008) Measuring metabolic rates: a manual for scientists. Oxford University Press, Oxford
Lin H, Decuypere E, Buyse J (2004) Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus). 1. Chronic exposure. Comp Biochem Physiol B 139:737–744
Londoño GA, Chappell MA, Castañneda MR, Jankowski JE, Robinson SK (2015) Basal metabolism in tropical birds: latitude, altitude, and the “pace of life”. Funct Ecol 29:338–346
Maldonado K, Cavieres G, Veloso C, Canals M, Sabat P (2009) Physiological responses in rufous-collared sparrows to thermal acclimation and seasonal acclimatization. J Comp Physiol B 179:335–343
Maldonado K, Bozinovic F, Cavieres G, Fuentes C, Cortés A, Sabat P (2012) Phenotypic flexibility in basal metabolic rate is associated with rainfall variability among populations of rufous-collared sparrow. Zoology 115:128–133
Maloney SK (2008) Thermoregulation in ratites: a review. Aust J Exp Agr 48:1293–1301
Margaritelis NV, Veskoukis AS, Paschalis V, Vrabas IS, Dipla K, Zafeiridis A, Kyparos MG, Nikolaidis A (2015) Blood reflects tissue oxidative stress: a systematic review. Biomarkers 20:97–108
Martinez del Rio C, Sabat P, Anderson-Sprecher R, Gonzalez S (2009) Dietary and isotopic specialization: the isotopic niche of three Cinclodes ovenbirds. Oecologia 161:149–159
McNab BK (2009) Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol A 152:22–45
McNab BK (2013) The ecological energetics of birds in New Guinea. Bull Florida Mus Nat Hist 52:95–1599
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12:75–92
Naya DE, Spangenberg L, Naya H, Bozinovic F (2013) How does evolutionary variation in Basal metabolic rates arise? A statistical assessment and a mechanistic model. Evolution 67:1463–1476
Nehls G (1996) Low costs of salt turnover in common eiders Somateria mollissima. Ardea 84:23–30
Newsome SD, Sabat P, Wolf N, Rader JA, Martinez del Río C (2015) Multi-tissue d2H analysis reveals altitudinal migration and tissue-specific discrimination patterns in Cinclodes. Ecosphere 6:1–18
Peña-Villalobos I, Valdés-Ferranty F, Sabat P (2013) Osmoregulatory and metabolic costs of salt excretion in the rufous-collared sparrow Zonotrichia capensis. Comp Biochem A Physiol 164:314–318
Peña-Villalobos I, Nuñez-Villegas M, Bozinovic F, Sabat P (2014) Metabolic enzymes in seasonally acclimatized and cold acclimated Rufous-collared sparrow inhabiting a Chilean Mediterranean environment. Curr Zool 60:338–350
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. Johns Hopkins University Press, Baltimore
Rader JA, Newsome SD, Sabat P, Chesser RT, Dillon ME, Martínez del Rio C (2017) Isotopic niches support the resource breadth hypothesis. J Anim Ecol 86:405–413
Sabat P (2000) Birds in marine and saline environments: living in dry habitats. Rev Chil Hist Nat 73:401–410
Sabat P, Martinez del Rio C (2006) Osmoregulatory capacity and the ability to use marine food sources in two coastal songbirds (Cinclodes: Furnariidae) along a latitudinal gradient. Oecologia 148:250–257
Sabat P, Martínez del Rio C (2002) Inter- and intra-specific variation in the use of marine food resources by three Cinclodes (Furnariidae, Aves) species: carbon isotopes and osmoregulatory physiology. Zoology 105:247–256
Sabat P, Martínez del Rio C (2005) Seasonal changes in the use of marine food resources by Cinclodes nigrofumosus (Furnariidae, Aves): carbon isotopes and osmoregulatory physiology. Rev Chil Hist Nat 78:253–260
Sabat P, Nespolo R, Bozinovic F (2004a) Water economy of three Cinclodes (Furnariidae) species inhabiting marine and freshwater ecosystems. Rev Chil Hist Nat 77:219–225
Sabat P, Maldonado K, Rivera-Hutinel A, Farfan G (2004b) Coping with salt without salt-glands: osmoregulatory plasticity in three species of coastal songbirds (ovenbirds) of the genus Cinclodes (Passeriformes: Furnariidae). J Comp Physiol B 174:415–420
Sabat P, Maldonado K, Canals M, Martínez del Rio C (2006) Osmoregulation and adaptive radiation in the ovenbird genus Cinclodes (Passeriformes: Furnariidae). Funct Ecol 20:799–805
Sabat P, Narvaez C, Peña-Villalobos I, Contreras C, Maldonado K, Sanchez-Hernandez JC, Newsome SD, Nespolo R, Bozinovic F (2017) Coping with salt water habitats: metabolic and oxidative responses to salt intake in the rufous-collared sparrow. Front Physiol 8:654
Schoeninger MJ, DeNiro MJ (1984) Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 48:625–639
Selman C, Blount JD, Nusseym DH, Speakmanm JR (2012) Oxidative damage, ageing, and life-history evolution: where now? Trends Ecol Evol 27:570–577
Shoemaker V (1972) Osmoregulation and excretion in birds. In: Farner DS, King J, Parkes K (eds) Avian biology. Academic, New York, pp 527–574
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129
Swanson DL, Thomas NE (2007) The relationship of plasma indicators of lipid metabolism and muscle damage to overnight temperature in winter acclimatized small birds. Comp Biochem Physiol A 146:87–94
Tieleman BI, Williams JB (2000) The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol Biochem Zool 73:461–479
Tieleman BI, Williams JB, Buschur ME, Brown CR (2003) Phenotypic variation of larks along an aridity gradient: are desert birds more flexible? Ecology 84:1800–1815
Tseng YC, Hwang PP (2008) Some insights into energy metabolism for osmoregulation in fish. Comp Biochem Physiol C Toxicol Pharmacol 148:419–429
van de Crommenacker JN, Horrocks PC, Versteegh MA, Komdeur J, Tieleman BI, Matson KD (2010) Effects of immune supplementation and immune challenge on oxidative status and physiology in a model bird: implications for ecologists. J Exp Biol 213:3527–3535
Vezina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Wassenaar LI, Hobson KA (2000) Stable-carbon and hydrogen isotope ratios reveal breeding origins of red-winged blackbirds. Ecol Appl 10:911–916
Wheelwright NT, Rising JD (1993) Savannah Sparrow (Passerculus sandwichensis). In: Poole A, Stettenheim P, Gill F (eds) The birds of North America. American Ornithologists Union, Washington, pp 1–28
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among Hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Williams JB, Miller RA, Harper JM, Wiersma P (2010) Functional linkages for the pace of life, life-history, and environment in birds. Integr Comp Biol 50:855–868
Williams JB, Muñoz-Garcia A, Champagne A (2012) Climate change and cutaneous water loss of birds. J Exp Biol 215:1053–1060
Zheng WH, Li M, Liu JS, Shao SL (2008) Seasonal acclimatization of metabolism in Eurasian Tree Sparrows (Passer montanus). Comp Biochem Physiol A 151:519–525
Acknowledgements
This study was funded by Fondo Nacional de Desarrollo Cientı́fico y Tecnológico (1160115) and Fondo Basal (FB 0002-2014). We thank Andrés Sazo for field support, and Carolina Contreras, Cristobal Narvaez, Natalia Ramirez, and Karin Maldonado for technical support.
Author information
Authors and Affiliations
Contributions
PS, SDN, JCS-H, FB and RN conceived and designed the experiments. RT and PS conducted fieldwork. RT-M performed the experiments. RT-M, PS and SDN analyzed the data. RT-M, PS, SDN and JCS-H wrote the manuscript; other authors provided editorial advice.
Corresponding author
Additional information
Communicated by Blair Wolf.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tapia-Monsalve, R., Newsome, S.D., Sanchez-Hernandez, J.C. et al. Terrestrial birds in coastal environments: metabolic rate and oxidative status varies with the use of marine resources. Oecologia 188, 65–73 (2018). https://doi.org/10.1007/s00442-018-4181-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4181-8