Abstract
Woody encroachment into herbaceous ecosystems is emerging as an important ecological response to global change. A primary concern is alterations in C and N cycling and associated variations across a variety of ecosystems. We quantified seasonal variation in litterfall and litter N concentration in Morella cerifera shrub thickets to assess changes in litterfall and associated N input after shrub expansion on an Atlantic coast barrier island. We also used the natural abundance of 15N to estimate the proportion of litterfall N originating from symbiotic N fixation. Litterfall for shrub thickets ranged from 8,991 ± 247 to 3,810 ± 399 kg ha−1 year−1 and generally declined with increasing thicket age. Litterfall in three of the four thickets exceeded previous estimates of aboveground annual net primary production in adjacent grasslands by 300–400%. Leaf N concentration was also higher after shrub expansion and, coupled with low N resorption efficiency and high litterfall, resulted in a return of as much as 169 kg N ha−1 year−1 to the soil. We estimated that ∼70% of N returned to the soil was from symbiotic N fixation resulting in an ecosystem input of between 37 and 118 kg ha−1 year−1 of atmospheric N depending on site. Considering the extensive cover of shrub thickets on Virginia barrier islands, N fixation by shrubs is likely the largest single source of N to the system. The shift from grassland to shrub thicket on barrier islands results in a substantial increase in litterfall and foliar N concentration that will likely have a major impact on the size and cycling of ecosystem C and N pools. Increasing C and N availability in these nutrient-poor soils is likely to permanently reduce cover of native grasses and alter community structure by favoring species with greater N requirements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Woody plant encroachment in historically herbaceous ecosystems has been documented for a variety of ecosystems and is emerging as a key area in the study of global change (Archer et al. 1995; Wessman et al. 2004; Briggs et al. 2005; Sturm et al. 2005). The global nature of this phenomenon has led many to argue that expansion of woody plants is linked to global phenomena such as warming or atmospheric CO2 enrichment (Archer et al. 1995). While climate warming appears to be a key factor facilitating woody plant expansion in arctic and alpine systems (Sturm et al. 2005), Archer et al. (1995) makes a compelling case against the CO2 enrichment hypothesis and effectively argues that regional factors, such as changes in fire regime and grazing pressure are directly linked to woody encroachment. However, on barrier islands along the Virginia, USA coast, increases in atmospheric CO2 appear to be the only trend in global change that would favor woody expansion (Young et al. 2007). Virginia barrier islands lack the history of land management observed in arid and semi-arid systems discussed throughout Archer et al. (1995), yet have experienced rapid rates of woody encroachment in the last 60 years, even in the presence of a rising sea level (Young et al. 1995, 2007). While a further synthesis of existing data is necessary to better determine the role of CO2 enrichment on woody encroachment, the phenomenon does not follow traditional successional models of disturbance and recovery and could be viewed as a state transition induced by persistent global change (Briggs et al. 2005; Young et al. 2007).
While the extent of changes in woody abundance has been described for a variety of systems (Goslee et al. 2003; Briggs et al. 2005; Sturm et al. 2005; Young et al. 2007), quantifying impacts of shrub expansion on ecosystem properties is more difficult due to spatial and temporal complexity and the extended time-scale over which shifts in vegetation occur (Wessman et al. 2004). One of the few opportunities to study long-term consequences of shrub expansion is provided by accreting shorelines on barrier islands, which result in large variations in community age over relative small spatial scales (Hayden et al. 1991). Fluctuations in island size and shape are induced by natural changes in currents that affect erosion and deposition of sand and change shoreline position, often quite rapidly (Hayden et al. 1991). Subsequent colonization by dune-forming grasses is an expected outcome of this pattern but, from 1949 to 1989, Hog Island, a barrier island along the Virginia coast also experienced a 400% increase in shrub cover following expansion of the northern end of the island (Young et al. 1995). While an increase in shrub abundance in this system has generally been viewed in the context of primary succession, shrub expansion on Virginia’s barrier islands is not related directly to increases in upland area and shares many characteristics with the broader global trend of woody encroachment (Young et al. 2007).
One of the primary drivers of plant community composition and primary productivity on barrier islands is availability of nutrients, especially N (Art et al. 1974; Ehrenfeld 1990; Stalter and Odum 1993). As a consequence, the dominant woody species on many barrier islands of the southeastern United States is the N-fixing shrub Morella cerifera (Young 1992; Young et al. 1995). Commonly known as wax myrtle, M. cerifera is well adapted to low-nutrient coastal soils (Young 1992). A symbiotic association between members of Myricaceae (which includes the genera Morella and Myrica) and the actinomycete Frankia assures an adequate source of N (Morris et al. 1974; Vitousek and Walker 1989; Young et al. 1992). Furthermore, the evergreen leaf habit aides in nutrient conservation by allowing plants to retain and transport other foliar nutrients, including P, more efficiently (Monk 1966; Killingbeck 1996). These characteristics, along with high growth rates and bird-dispersed seeds, have enabled M. cerifera to form dense, nearly monospecific stands on islands that are otherwise dominated by herbaceous vegetation (Young et al. 1995; Kwit et al. 2004).
Changes in ecosystem function after shrub expansion, especially with regards to C and N cycling, are often quite substantial (Vitousek et al. 1987; McCarron et al. 2003; McCulley et al. 2004; Hughes et al. 2006). Shrubs and other woody vegetation in grasslands act to reduce soil erosion, subsidize nutrient inputs by intercepting atmospheric inputs and serve as a nutrient reservoir, especially in sandy and/or low-nutrient soils (García-Moya and McKell 1970; Vitousek et al. 1987; Joy and Young 2002). Furthermore, shrub expansion is often accompanied by substantial changes in annual net primary production (ANPP) and changes in tissue chemistry that affect both litter quality and quantity (McCarron et al. 2003; McCulley et al. 2004; Hughes et al. 2006). For example, Briggs et al. (2005) observed a consistent trend of increased leaf N concentration when shrubs replaced grasses, especially when expanding shrubs were N-fixers. Although N conservation is an important strategy for most plants in nutrient-poor soils, N-fixing species are often less proficient in resorption of N from senescing parts than other species and often contribute substantial N to the soil through litterfall (Killingbeck 1996; Sprent et al. 1978; Permar and Fischer 1983; Uliassi and Ruess 2002). Increased litter N concentration can be expected to increase rates of litter decomposition and increase N availability in soils thereby changing community dynamics (Melillo et al. 1982; Permar and Fischer 1983; Aber et al. 1990; Ulery et al. 1995; Berg et al. 1996). Previous estimates of soil N beneath M. cerifera shrub thickets and in soils without M. cerifera were 791 ± 195 and 321 ± 14 μg g−1, respectively (Young et al. 1992).
In addition to quantifying shifts in C and N cycling, it is also useful to determine principal sources of ecosystem N inputs. Previous studies have attempted to quantify atmospheric N2 fixation by stands of N-fixing plants by scaling up from C2H2 reduction assays that measure nitrogenase activity (Permar and Fischer 1983; Vitousek et al. 1987; Uliassi and Ruess 2002). However, spatial and temporal patterns of nitrogenase activity in root nodules are complex and highly variable and attempting to extrapolate assay results to annual N2 fixation in natural ecosystems is unreliable (Shearer and Kohl 1989; Halvorson et al. 1992; Sande and Young, 1992). Shearer and Kohl (1989) and Halvorson et al. (1992) suggested that measurements of N fixation using the natural abundance of 15N in plant tissues are more integrative and, therefore, more accurate.
To understand the impact of woody encroachment on C and N cycling, changes in litterfall and associated N inputs must be quantified and dominant sources of N determined. Our primary objectives were to quantify variations in litterfall and litter N concentration of four M. cerifera shrub thickets representing a chronosequence of shrub expansion. These data were compared to previously measured values of aboveground ANPP and foliar N of adjacent grasslands to determine how shifts in dominant growth form affect litterfall C and N inputs into the system. Furthermore, we examined seasonal trends in litterfall and litter N concentration to assess temporal variation of C and N return throughout the year. Finally, we used the natural abundance of δ15N to estimate the fraction of N in M. cerifera tissues that originated from actinomycete-induced N fixation.
Materials and methods
Study site
Fieldwork was conducted from April 2004 to November 2006 on the northern end of Hog Island (37°27′N, 75°40′W), a barrier island located ∼10 km east of the eastern shore of Virginia, USA. Hog Island is ∼1,200 ha, 10 km long and 2.5 km across at its widest point. The island is part of the Virginia Coast Reserve, managed by The Nature Conservancy, and is an NSF-funded Long-Term Ecological Research (LTER) site. The northern end of the island has been accreting ∼5 m year−1 for ∼140 years resulting in a chronosequence of progressively older soils as one moves west across the island from the ocean shoreline (Hayden et al. 1991; Shao et al. 1998). As the island has expanded, a series of dense thickets, dominated by the shrub M. cerifera, has developed with thicket age increasing with soil age. Thickets now cover ∼40% of the upland area on the island (Young et al. 2007). Four thickets in order of increasing age are the Colonizing thicket (8 years), the Young thicket (15 years), the Mid-Island thicket (25 years), and the Bay Side thicket (45 years). Adjacent grasslands are dominated by perennial grasses: Spartina patens and Ammophila breviligulata (Dilustro and Day 1997).
Experimental procedure
Ten sites in each thicket were randomly selected and a plastic litter trap, ∼0.30 m2 in area and 0.15 m deep, was placed at each site in April 2004. Litter was collected every 6 weeks from April 2004 to May 2005; however, the final sampling period was ∼12 weeks due to logistical difficulties associated with traveling to the island. Litter was dried at 70°C for 4 days, separated into leaf, woody and reproductive (i.e., fruits and flower parts) components, and weighed to the nearest 0.1 g. To analyze litter N concentration, ten leaves were selected from each thicket for each of three collection periods (May, September and January). Additionally, five samples each of woody and reproductive litter, taken throughout the year, were analyzed for each thicket. All samples were ground before analysis in a Wiley mill with a 40-mesh screen. N concentration was determined as a percentage of dry weight using the Pregl-Dumas pure-oxygen combustion method (Perkin-Elmer 2400 elemental analyzer; Perkin-Elmer, Wellesley, Mass.). Resorption efficiency of foliar N was determined for each thicket as a percentage of fresh leaf N concentration by comparing N per unit area of fresh leaves collected during September 2006 from sites adjacent to litter traps and leaf litter collected during November 2006. N content was converted to a weight per unit area basis using values of specific leaf area (leaf area per unit leaf weight) for fresh leaves and subsequent litter for each site (S. Brantley, unpublished data). Leaf area was determined as described in Young and Yavitt (1987).
Percent of N from fixation was estimated using the 15N natural abundance method as described by Shearer and Kohl (1989). In September 2006, fresh leaves were collected from non-N fixing species (hereafter referred to as “non-fixers”) growing within and immediately adjacent to shrub thickets. Non-fixers were selected based on location (particularly with respect to elevation) and rooting characteristics and included Baccharis halimifolia (also a shrub) and Rubus sp. Fresh leaves were also collected from each M. cerifera thicket, and from M. cerifera seedlings that rely primarily on N fixation (hereafter referred to as “fixers”) due to severe soil N limitation (Young et al. 1992). Fresh leaves were dried at 70°C for 4 days and ground in a Wiley mill with a 40-mesh screen. Isotopic composition of N was expressed as δ15N which represents the deviation from the ratio of 15N:14N for atmospheric N2. Fractional contribution of biological N fixation (F bfn) was estimated using the isotopic dilution expression:
where δ15Nsoil is the isotopic abundance in plants that rely primarily on soil N (“non-fixers”), δ15Nmix is the isotopic abundance in plants that use both soil and atmospheric fixation (M. cerifera thickets), and δ15Natm is the isotopic abundance of plants that rely primarily on symbiotic N fixation (“fixers”).
Statistical analysis
Leaf litterfall and leaf litter N concentration were analyzed using two-way ANOVA to test for interactions between site and season. Post hoc comparisons (Tukey) were performed as described in Zar (1999). Data for woody and reproductive litter N concentration, total litterfall, δ15N of fresh leaves, and δ15N of litter were analyzed by ANOVA and post hoc tests (Tukey). N concentration of fresh leaves and leaf litter were compared with Student’s t-tests and also to verify that there was a significant difference in δ15N between N fixers and non-fixers. Total content of biologically fixed N in leaves was estimated as the product of litter mass, litter N concentration, and the estimated fraction of fixed N. For all tests, P-values ≤ 0.05 were considered significant. Unless otherwise noted, all statistics were performed in SPSS 11.5.
Results
Total annual litterfall (i.e., leaves, woody, and reproductive litter) of M. cerifera varied over twofold among sites (F = 50.350, P < 0.001) with the Young thicket producing the most litter and the Bay Side thicket producing the least (Table 1). Leaf litterfall also varied significantly by site (F = 54.862, P < 0.001); however, there was no significant difference in leaf litterfall between the Young and Colonizing thickets. Higher total litterfall in the Young thicket was primarily due to a higher production of woody litter (Table 1). Reproductive litterfall did not vary among the three youngest thickets but was significantly lower in the Bay Side thicket (F = 6.135, P = 0.002).
Although leaf litterfall varied by season (F = 69.604, P < 0.001), there was a significant interaction (F = 8.221, P < 0.001) between site and season (Fig. 1). Litterfall increased significantly for all thickets from early May to late June which coincides with leaf flush at the start of the growing season. The Mid-Island thicket had the highest leaf litterfall of the four thickets during the late spring litterfall pulse and this was the highest rate observed during the study (34.3 ± 1.7 kg ha−1 day−1). Lowest leaf litterfall for all thickets was observed from late June to mid-August. Litterfall increased significantly beginning in mid-August and continued to increase to nearly the same rates observed during May for all sites except the Mid-Island thicket. The Mid-Island thicket experienced a small, though significant, increase in litterfall in late September but leaf litterfall in fall and winter did not approach spring levels.
Litter N concentration, averaged across all sites was 1.68 ± 0.04, 0.79 ± 0.04, and 1.49 ± 0.08% for leaf, woody and reproductive litter, respectively (Table 1). N concentration of woody (F = 1.811, P < 0.186) and reproductive (F = 0.846, P < 0.489) litter did not vary by site. In comparison, N concentration of leaf litter varied significantly (F = 20.837, P < 0.001) by site but did not vary by season (F = 3.251, P < 0.111) (Fig. 2). Estimated total N from litterfall (the sum of the product of litterfall and N concentration for all litter types) was highest for the Young thicket (169 kg ha−1 year−1) and lowest for the Bay Side thicket (53 kg ha−1 year−1) (Table 1). Averaged across all sites, 85% of total litter N was from leaf litter, 10% was from reproductive litter and 5% was from woody litter.
Mean N concentration of fresh M. cerifera leaves varied significantly among thickets during the growing season (F = 4.802, P = 0.022). Post hoc tests showed that only Mid-Island (1.75 ± 0.02%) and Bay Side (1.96 ± 0.02%) thickets differed significantly with neither of those thickets having significantly different values for leaf N than the Young (1.84 ± 0.06%) or Colonizing thickets (1.86 ± 0.05%). Overall resorption efficiency of M. cerifera for all thickets was 15%; however, this value also varied by site. The Colonizing thicket had the highest resorption efficiency with 26% and resorption declined with increasing soil age (15% in the Young thicket, 10% in the Mid-Island thicket, and 8% in the Bay Side thicket).
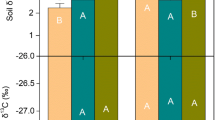
Overall, there was relatively little variation in δ15N among species and among sites (Fig. 3). No significant differences were detected in δ15N among M. cerifera thickets (F = 1.178, P = 0.362) so data were pooled across all sites before further analysis. Isotopic composition of the N-limited fixers was −1.2 ± 0.1 δ15N which compares very well to values observed in other studies for seedlings grown in an N-free medium (Hurd et al. 2005). The difference in δ15N between non-fixers and fixers was only 0.8. Although small, this difference was significant (t = 2.324, P = 0.036). Using the dilution expression described above with fixers and non-fixers, we estimated that ∼70% of foliar N concentration was from actinomycete-induced N fixation. When the fraction of fixed foliar N is factored with total litterfall N content, at least 37–118 kg ha−1 year−1 of N was fixed by M. cerifera thickets depending on age.
Natural abundance of 15N of foliar N for multiple species from Hog Island, represented as δ15N or the deviation from the atmospheric ratio of 15N:14N. Species represented include known N-fixers, shrub thickets that rely on a combination of soil N and symbiotic N fixation, and plants that lack N-fixing symbionts
Discussion
The influence of shrub thicket expansion on litterfall and associated N input in the barrier island ecosystem was substantial. High productivity of young stands of M. cerifera resulted in annual litterfall that exceeded litterfall reported for other shrub-dominated systems and temperate forests and compared with the lower end of values often cited for tropical forests (Gray and Schlesinger 1981; Barbour et al. 1999; Martinez-Yrizar et al. 1999; Norby et al. 2003). By comparison, aboveground annual net primary productivity in grasslands adjacent to shrub thickets ranged from 2,260 to 2,740 kg ha−1 year−1 (Dilustro and Day 1997). In our study, litterfall alone of shrub thickets was 1.4–4.0 times greater than grassland ANPP depending on site. N concentration of leaf litter from M. cerifera was also 1.6–4.6 times higher than N concentration of the two dominant grasses on the island (Dilustro and Day 1997). The coupling of high litterfall and high litter N concentration resulted in large quantities of N cycling through litterfall and explains the large differences in soil N between sites with and without M. cerifera previously observed by Young et al. (1992).
Our data are consistent with Uliassi and Ruess (2002), who concluded that the best predictor of ecosystem inputs of fixed N by Alnus tenuifolia was leaf area. The primary driver of N cycling in stands of M. cerifera was variation in litterfall. Although the Bay Side thicket occupies the oldest, most N-rich soils on the island (Young et al. 1992), this site consistently had the lowest litterfall of the four thickets while the two youngest thickets produced the most litter. Seasonal differences in litterfall were also observed across the chronosequence. Three of the four sites experienced two periods of increased leaf litterfall during the year: a brief spring pulse coinciding with the beginning of new leaf growth and a longer period of increased litterfall in autumn. However, the Mid-Island thicket did not show a large increase in litterfall during the autumn relative to other thickets, indicating that shrubs at this site retain more leaves throughout the winter. In spring, the Mid-Island thicket had the highest rate of litterfall even though the two younger sites had higher annual litterfall. The ability to retain more foliage through winter may be an important mechanism for nutrient conservation in two ways. First, retention of older foliage through spring leaf-out may facilitate more efficient translocation of nutrients, such as P (Monk 1966; Killingbeck 1996), that are required for N fixation but are extremely limiting in the sandy, barrier island soils (Art et al. 1974; Ehrenfeld 1990; Young 1992). Second, abscission of large quantities of N-rich leaf litter at the beginning of the growing season may reduce N loss from the stand and supplement the N supply during the growing season when nutrients are in highest demand. Most important for this coastal system, continuous litterfall and consistently high N concentrations result in a relatively constant input of organic matter and associated N to the soil rather than a single pulse characteristic for deciduous systems (e.g., Norby et al. 2003). This may be especially significant for N cycling in sandy soils typical of coastal systems where nutrient retention is minimal (Art et al. 1974).
Our results also suggest that a large fraction of foliar N in M. cerifera comes from actinomycete-induced fixation of atmospheric N2. We must qualify this statement based on the slight isotopic differences between fixers and non-fixers. Characteristics inherent to this system make it difficult to distinguish between N fixed by M. cerifera and N from other sources. For instance, atmospheric deposition is likely the main source of N for the system where M. cerifera is absent and the δ15N values of nitrates and NH4 + (dominant forms of N in atmospheric deposition) have been measured at −1.1 and −0.5, respectively (Russell et al. 1998); very similar to the −1.2 observed for N fixers in this study. According to Russell et al. (1998), while δ15N of dissolved organic N was +5, it accounted for only 13% of total N in wet deposition which, in any event, was relatively low. Furthermore, because soils are relatively young (∼5–140 years), enrichment of 15N often observed in better developed soils has yet to occur. Lack of variability in δ15N signatures from soils is likely the reason we were unable to detect differences among thickets across the chronosequence and as a result, we pooled our site data. Generally, such small differences in δ15N could be due to natural variations in N fractionation in the plants and would not be considered adequate for the model we used (Shearer and Kohl 1989); however, there were significant differences between the two end members of our model and our data followed the trend we expected.
We should also point out that our estimates are of input of fixed N through litterfall and do not reflect total N fixation because they do not account for fixed N that is incorporated into living stems or belowground structures. Nonetheless, our estimates for annual input of fixed N from litterfall are comparable to estimates of total N fixation for many other actinomycete–plant associations (Hibbs and Cromack 1990) and considerably higher than some estimates for other species within Myricaceae. For instance, Vitousek et al. (1987) estimated that Myrica faya contributed 18 kg ha−1 year−1 to volcanic soils and Sprent et al. (1978) estimated N loss through litterfall in Myrica gale was 30 kg ha−1 year−1 for wetlands in central Scotland. Other estimates are more comparable. Bond (1951) estimated Myrica gale fixation at 90 kg ha−1 year−1 based on laboratory studies and Permar and Fischer (1983) used in-field measurements to predict that stands of 100% Myrica cerifera could fix as much as 130 kg N ha−1 year−1. Both studies used C2H2 reduction assays to estimate potential N fixation and the latter value was based on an extrapolation from 8 to 100% cover for the sites studied. Laboratory studies of nitrogenase activity in M. cerifera seedlings using soils from Hog Island also show very high rates of N fixation (Sande and Young 1992), but extrapolation to island shrub thickets from seedlings grown in environmental chambers would be unrealistic.
Although incorporation of fixed N into other tissues (stems and roots) was not accounted for in our study, N content of leaves likely represents a majority of fixed N. While standing wood and belowground tissues represent the largest component of biomass in the system, relative N concentration of these tissues is substantially lower than photosynthetic tissues measured in our study (Conn and Day 1993; D, Young, unpublished data). Furthermore, Halvorson et al. (1992) concluded that N from atmospheric fixation in legumes was preferentially directed to photosynthetic tissues while roots contained elevated levels of 15N. Torrey (1978) also reported that N fixed in nodules is rapidly transported to the shoot and that fixed N is primarily returned to the soil through leaf litterfall.
Because shrub thickets now cover a large portion of the island, N fixation and subsequent litterfall in this species may be the single largest source of soil N for this system. Other sources of N for barrier islands include atmospheric deposition and fixation by free-living microbes (Ehrenfeld 1990). However, neither of these sources is likely to approach our estimation of annual N input by litterfall within shrub thickets (Sprent and Sprent 1990; Meyers et al. 2001). Atmospheric deposition for Hog Island Bay, the shallow lagoon that separates Hog Island from the mainland, has been estimated at ∼8 kg ha−1 year−1 (Meyers et al. 2001) which is less than 22% of our estimate for shrub litterfall input at the least productive site. Currently, no estimation for free-living microbial fixation exists for Hog Island or, to our knowledge, similar systems, and it is difficult to generalize based on current literature because of the wide variation in edaphic factors across the island (e.g., soil moisture, salinity, microbial diversity) (Stewart 1975; Sprent and Sprent 1990). Low P and organic matter content of the sandy soils is likely to limit N fixation by heterotrophic bacteria, and low incident light within shrub thickets and relatively low soil moisture content on dunes may limit N fixation by cyanobacteria (Stewart 1975; Sprent and Sprent 1990; Young et al. 1992; Brantley and Young 2007).
Previous studies on the consequences of shrub encroachment have shown that effects of shrub expansion on C and N cycling, including changes in C and N storage and soil respiration, vary widely depending on precipitation and/or edaphic characteristics including soil type and size of pre-existing C and N pools (Jackson et al. 2002; Hughes et al. 2006; Wheeler et al. 2007). McCulley et al. (2004) concluded that there was an increase in both soil respiration and ecosystem C and N storage after shrub expansion in subtropical savanna. However, McCarron et al. (2003) measured a significant decrease in soil respiration and no change in C or N storage in tallgrass prairie. Hughes et al. (2006) also measured no change in surface soil C and N pools, despite substantial changes in aboveground C and N. Jackson et al. (2002) concluded that mesic systems with large soil C pools could serve as a C source after replacement of grasses with woody vegetation because of increased soil respiration. Although further work is needed, ecosystem responses on barrier islands are likely to be greater than in systems with large pre-existing C and N pools because of young, sandy soils characteristic of the islands.
We show that dense thickets of M. cerifera on Hog Island produce a large quantity of N-rich litterfall that may rapidly increase C and N cycling. Increases in litter accumulation after thicket expansion, coupled with associated long-term increase in N inputs, will likely have irreversible effects on species composition by contributing to reduced cover and diversity of native grasses (Day et al. 2004). Even where shrubs have declined, thickets have been maintained by continued shrub recruitment (Brantley and Young 2007). In the absence of major disturbance, shrubs may be replaced by maritime forest species with higher N requirements (Ehrenfeld 1990). Perhaps more importantly, when ecosystem N limitation is mediated by expansion of N-fixing shrubs in nutrient-poor environments, associated increases in C sequestration may constitute an important terrestrial sink for atmospheric CO2 that must be accounted for in models of global C cycling (Houghton 2003; Woodbury et al. 2007). The dramatic shift in growth form we observed with barrier island shrub expansion further underscores the necessity for quantification of these changes on a global scale.
References
Aber JD, Melillo JM, McClaugherty CA (1990) Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–2208
Archer S, Schimel DS, Holland EA (1995) Mechanisms of shrubland expansion: land use, climate or CO2? Clim Change 29:91–99
Art HW, Bormann FH, Voigt GK, Woodwell GM (1974) Barrier island forest ecosystems: role of meteorological nutrient inputs. Science 184:60–62
Barbour MG, Burk JH, Pitts WD, Gilliam FS, Schwartz MW (1999) Terrestrial plant ecology, 3rd edn. Addison-Wesley, Longman, Menlo Park
Berg B, Ekbohm G, Johansson MB, McClaugherty C, Rutigliano F, Virzo de Santo A (1996) Maximum decomposition limits of forest litter types: a synthesis. Can J Bot 74:659–672
Bond G (1951) The fixation of nitrogen associated with root nodules of Myrica gale L., with special reference to its pH relation and ecological significance. Ann Bot 15:447–459
Brantley ST, Young DR (2007) Leaf-area index and light attenuation in rapidly expanding shrub thickets. Ecology 88:524–530
Briggs JM, Knapp AK, Blair JM, Heisler JL, Hoch GA, Lett MS, McCarron JK (2005) An ecosystem in transition: causes and consequences of the conversion of mesic grassland to shrubland. BioScience 55:243–254
Conn CE, Day FP (1993) Belowground biomass patterns on a coastal barrier island in Virginia. Bull Torrey Bot Club 120:121–127
Day FP, Conn C, Crawford E, Stevenson M (2004) Long-term effects of nitrogen fertilization on plant community structure on a coastal barrier island dune chronosequence. J Coast Res 20:722–730
Dilustro JJ, Day FP (1997) Aboveground biomass and net primary production along a barrier island dune chronosequence. Am Midl Nat 137:27–38
Ehrenfeld JG (1990) Dynamics and processes of barrier island vegetation. Rev Aquat Sci 2:437–480
García-Moya E, Mckell CM (1970) Contribution of shrubs to the nitrogen economy of a desert wash plant community. Ecology 51:81–88
Goslee SC, Havstad KM, Peters DC, Rango A, Schlesinger W (2003) High-resolution images reveal rate and pattern of shrub encroachment over six decades in New Mexico, USA. J Arid Environ 54:755–767
Gray JT, Schlesinger WH (1981) Biomass, production and litterfall in the coastal sage scrub of southern California. Am J Bot 68:24–33
Halvorson JJ, Franz EH, Smith JL, Black RA (1992) Nitrogenase activity, nitrogen fixation, and nitrogen inputs by lupines at Mount St. Helens. Ecology 73:87–98
Hayden BP, Dueser RD, Callahan JT, Shugart HH (1991) Long-term research at the Virginia Coast Reserve. BioScience 41:310–318
Hibbs DE, Cromack K (1990) Actinorhizal plants in Pacific Northwest forests. In: Schwintzer CR, Tjepkema JD (eds) The biology of Frankia and actinorhizal plants. Academic, San Diego, pp 343–358
Houghton RA (2003) Why are estimates of terrestrial carbon balance so different? Glob Change Biol 9:500–509
Hughes RF, Archer SR, Asner GP, Wessman CA, McMurty C, Nelson J, Ansley J (2006) Changes in aboveground primary production and carbon and nitrogen pools accompanying woody plant encroachment in a temperate savanna. Glob Change Biol 12:1733–1747
Hurd TM, Gökkaya K, Kiernan BD, Raynal DJ (2005) Nitrogen sources in Adirondack wetlands dominated by nitrogen-fixing shrubs. Wetlands 25:192–199
Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418:623–626
Joy DA, Young DR (2002) Promotion of mid-successional seedling recruitment and establishment by Juniperus virginiana in a coastal environment. Plant Ecol 160:125–135
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption proficiency. Ecology 77:1716–1727
Kwit C, Levey DJ, Greenberg CH, Pearson SF, McCarty JP, Sargent S (2004) Cold temperatures increase winter fruit removal rate of a bird-dispersed shrub. Oecologia 139:30–34
Martinez-Yrizar A, Nunez S, Miranda H, Burquez A (1999) Temporal and spatial variation of litterfall in Sonoran desert communities. Plant Ecol 145:37–48
McCarron JK, Knapp AK, Blair JM (2003) Soil C and N responses to woody plant expansion in a mesic grassland. Plant Soil 257:183–192
McCulley RL, Archer SR, Boutton TW, Harris FM, Zuberer DA (2004) Soil respiration and nutrient cycling in wooded communities developing in grassland. Ecology 85:2804–2817
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Meyers T, Sickles J, Dennis R, Russell K, Galloway J, Church T (2001) Atmospheric nitrogen deposition to coastal estuaries and their watersheds. In: Valigura RA, Alexander RB, Castro MS, Meyers TP, Paerl HW, Stacey PE, Turner RE (eds) Nitrogen loading in coastal water bodies: an atmospheric perspective. American Geophysical Union, Washington, DC, pp 53–76
Monk CD (1966) An ecological significance of evergreeness. Ecology 47:504–505
Morris M, Eveleigh DE, Riggs SC, Tiffney WN Jr (1974) Nitrogen fixation in the bayberry (Myrica pensylvanica) and its role in coastal succession. Am J Bot 61:867–870
Norby RJ, Sholtis JD, Gunderson CA, Jawdy SS (2003) Leaf dynamics of a deciduous forest canopy: no response to elevated CO2. Oecologia 136:574–584
Permar TA, Fischer RF (1983) Nitrogen fixation and accretion by wax myrtle (Myrica cerifera) in slash pine (Pinus ellotii) plantations. For Ecol Manage 5:39–46
Russell K, Galloway JN, Macko SA, Moody JL, Scudlark JR (1998) Sources of nitrogen in wet deposition to the Chesapeake Bay region—their chemistry and availability to phytoplankton. Atmos Environ 32:2453–2465
Sande E, Young DR (1992) Effect of sodium chloride on growth and nitrogenase activity in seedlings of Myrica cerifera L. New Phytol 120:345–350
Shao G, Young DR, Porter JH, Hayden BP (1998) An integration of remote sensing and GIS to examine the responses of shrub thicket distributions to shoreline changes on Virginia barrier islands. J Coast Res 14:299–307
Shearer G, Kohl DH (1989) Estimates of N2 fixation in ecosystems: the need for and basis of the15N natural abundance method. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotopes in ecological research. Springer, New York, pp 342–374
Sprent JI, Sprent P (1990) Nitrogen fixing organisms: pure and applied aspects. Chapman and Hall, New York
Sprent JI, Scott R, Perry KM (1978) The nitrogen economy of Myrica gale in the field. J Ecol 66:657–668
Stalter R, Odum WE (1993) Maritime communities. In: Martin WH, Boyce SG, Echternacht AC (eds) Biodiversity of the southeastern United States: lowland terrestrial communities. Wiley, Baltimore, pp 117–164
Stewart WDP (1975) Nitrogen fixation by free-living micro-organisms. Cambridge University Press, Cambridge
Sturm M, Schimel J, Michaelson G, Welker JM, Oberbauer SF, Liston GE, Fahnstock J, Romanovsky VE (2005) Winter biological processes could help convert Arctic tundra to shrubland. BioScience 55:17–26
Torrey JG (1978) Nitrogen fixation by actinomycete-nodulated angiosperms. BioScience 28:586–592
Ulery AL, Graham RC, Chadwick OA, Wood HB (1995) Decade-scale changes of soil carbon, nitrogen and exchangeable cations under chaparral and pine. Geoderma 65:121–134
Uliassi DD, Ruess RW (2002) Limitations to symbiotic nitrogen fixation in primary succession on the Tanana river floodplain. Ecology 83:88–103
Vitousek PM, Walker LR, Whiteaker LD, Mueller-Dombois D, Matson PA (1987) Biological invasion by Myrica faya alters ecosystem development in Hawaii, USA. Science 238:802–804
Vitousek PM, Walker LR (1989) Biological invasion by Myrica faya in Hawaii: plant demography, nitrogen fixation, and ecosystem effects. Ecol Monogr 59:247–265
Wessman CA, Archer S, Johnson LC, Asner GP (2004) Woodland expansion in US grasslands: assessing land-cover change and biogeochemical impacts. In: Guttman G, Janetos A, Skole D (eds) Land change science: observing, monitoring and understanding trajectories of change on the Earth’s surface. Kluwer, New York, pp 185–208
Wheeler CW, Archer SR, Asner GP, McMurtry CR (2007) Climate and edaphic controls on soil carbon–nitrogen responses to woody plant encroachment in desert grassland. Ecol Appl 17:1911–1928
Woodbury PB, Smith JE, Heath LS (2007) Carbon sequestration in the US forest sector from 1990 to 2010. For Ecol Manage 241:14–27
Young DR (1992) Photosynthetic characteristics and potential moisture stress for the actinorhizal shrub, Myrica cerifera (Myricaceae), on a Virginia barrier island. Am J Bot 79:2–7
Young DR, Yavitt JB (1987) Differences in leaf structure, chlorophyll, and nutrients for the understory tree Asimina triloba. Am J Bot 74:1487–1491
Young DR, Sande E, Peters GA (1992) Spatial relationships of Frankia and Myrica cerifera on a Virginia, USA Barrier Island. Symbiosis 12:209–220
Young DR, Shao G, Porter JH (1995) Spatial and temporal growth dynamics of barrier island shrub thickets. Am J Bot 82:628–645
Young DR, Porter JH, Bachmann CM, Shao G, Fusina RA, Bowles JH, Korwan D, Donato TF (2007) Cross-scale patterns in shrub thicket dynamics in the Virginia barrier complex. Ecosystems. doi:10.1007/s10021-007-9084-1
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River
Acknowledgements
This study was supported in part by NSF grant DEB-008031 to the University of Virginia for LTER-related work at the Virginia Coast Reserve. Paul Bukaveckas assisted with N concentration analyses. Colorado Plateau Stable Isotope Laboratory provided stable isotope analyses. The Virginia Coast Reserve LTER staff, especially Arthur Schwarzschild, assisted with island logistics. Data collection and experiments in this study comply with all laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Tim Seastedt.
Rights and permissions
About this article
Cite this article
Brantley, S.T., Young, D.R. Shifts in litterfall and dominant nitrogen sources after expansion of shrub thickets. Oecologia 155, 337–345 (2008). https://doi.org/10.1007/s00442-007-0916-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0916-7