Abstract
Leaf area index (LAI) and its seasonal dynamics are key determinants of terrestrial productivity and, therefore, of the response of ecosystems to a rising atmospheric CO2 concentration. Despite the central importance of LAI, there is very little evidence from which to assess how forest LAI will respond to increasing [CO2]. We assessed LAI and related leaf indices of a closed-canopy deciduous forest for 4 years in 25-m-diameter plots that were exposed to ambient or elevated CO2 (542 ppm) in a free-air CO2 enrichment (FACE) experiment. LAI of this Liquidambar styraciflua (sweetgum) stand was about 6 and was relatively constant year-to-year, including the 2 years prior to the onset of CO2 treatment. LAI throughout the 1999–2002 growing seasons was assessed through a combination of data on photosynthetically active radiation (PAR) transmittance, mass of litter collected in traps, and leaf mass per unit area (LMA). There was no effect of [CO2] on any expression of leaf area, including peak LAI, average LAI, or leaf area duration. Canopy mass and LMA, however, were significantly increased by CO2 enrichment. The hypothesized connection between light compensation point (LCP) and LAI was rejected because LCP was reduced by [CO2] enrichment only in leaves under full sun, but not in shaded leaves. Data on PAR interception also permitted calculation of absorbed PAR (APAR) and light use efficiency (LUE), which are key parameters connecting satellite assessments of terrestrial productivity with ecosystem models of future productivity. There was no effect of [CO2] on APAR, and the observed increase in net primary productivity in elevated [CO2] was ascribed to an increase in LUE, which ranged from 1.4 to 2.4 g MJ−1. The current evidence seems convincing that LAI of non-expanding forest stands will not be different in a future CO2-enriched atmosphere and that increases in LUE and productivity in elevated [CO2] are driven primarily by functional responses rather than by structural changes. Ecosystem or regional models that incorporate feedbacks on resource use through LAI should not assume that LAI will increase with CO2 enrichment of the atmosphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaf area is a primary determinant of plant productivity, whether assessed at the scale of the individual plant, a forest stand or grassland, or large regions. Most models of plant and terrestrial productivity include a dynamic determination of leaf area index (LAI, leaf area relative to ground area) (Woodward et al. 1995; Neilson and Drapeck 1998; Gower et al. 1999). Leaf area also is an important consideration in assessments of how plants and ecosystems will respond to the increasing concentration of CO2 [CO2] in the atmosphere. Leaves are the point of contact between plants and atmospheric CO2, and increased leaf area can enhance the opportunity for carbon uptake, albeit at the cost of a greater demand for water. Increased sugar availability in elevated [CO2] can stimulate leaf cell division and expansion (Ferris et al. 2001). Hence, it is often assumed that LAI will be higher in a CO2-enriched atmosphere, thereby counteracting lower foliar N concentration or the water-saving effect of stomatal closure in high [CO2] (Hymus et al. 2002). Both increases and decreases in peak LAI of various crop plants have been reported in response to elevated [CO2] in free-air CO2 enrichment (FACE) experiments (Kimball et al. 2002), but Drake et al. (1997) concluded that at canopy closure LAI of field-grown crops generally is not affected by [CO2]. In a model herbaceous community CO2 stimulated initial canopy development and cumulative LAI integrated over time, but not peak LAI (Hartz-Rubin and DeLucia 2001). LAI of some native grasslands also has been unaffected by elevated [CO2] (Niklaus et al. 1998).
Similar stand-level data do not exist for forest systems because of the difficulty in exposing forest stands to elevated [CO2] and the much longer time before a stand reaches canopy closure. There is ample evidence that the leaf area of individual tree seedlings and saplings increases in response to elevated [CO2] (Pritchard et al. 1999). Most of these reports, however, can be explained simply as part of a coordinated whole-plant response to increased [CO2]; that is, bigger plants have more leaf area. The stimulation by elevated [CO2] of carbon assimilation per unit leaf area is confounded with increases in total leaf area (Norby et al. 1995). Hence, data from young, exponentially growing tree seedlings and saplings do not inform us about how the leaf area of a continuous forest canopy will respond to elevated [CO2]. Leaf area of a closed-canopy forest is constrained by environmental resources (water, nutrients, or ultimately light) and cannot be expected to respond the same as an expanding canopy. This has been demonstrated in densely planted model ecosystems in which LAI reached a quasi-steady-state at the end of the experiment. Final LAI of dense thickets of beech and Norway spruce were not affected by CO2 enrichment (Egli et al. 1998), nor was there any effect on LAI in a model tropical forest community (Körner and Arnone 1992).
Although direct evidence is limited, mechanisms leading to changes in LAI (increases or decreases) in forests can be suggested. For example, if the light compensation point (LCP) in elevated [CO2] is lower, as indicated in some experiments (Long and Drake 1991; Kubiske and Pregitzer 1996; Drake et al.1997; Hättenschwiler 2001), leaves might maintain a positive carbon balance deeper in the canopy, sustaining a higher LAI (Long 1991; Hirose et al. 1997). On the other hand, optimization of water or nutrient requirements relative to carbon availability might suggest that forest stands will maintain a lower LAI in a CO2-enriched atmosphere in what might be considered a compensatory response (Norby et al. 1992). Without direct measurements of LAI in CO2-enriched forest stands, these potential mechanisms will remain speculative.
A primary rationale to undertake a FACE experiment in a deciduous forest was that the closed canopy would constrain growth responses. We have hypothesized, however, that the effect of [CO2] on growth per unit leaf area (canopy productivity index, CPI, Norby 1996) should be independent of LAI and therefore persist after LAI has reached a maximum, regardless of whether [CO2] alters maximum LAI. That is, the functional responses to [CO2] (increased carbon assimilation per unit leaf area) would support increased growth even without a concomitant structural response (LAI) (Norby et al. 1999). Here we report on the LAI of a closed-canopy sweetgum (Liquidambar styraciflua) forest stand before and during 5 years exposure to elevated [CO2]. Detailed assessment of LAI beginning in the second year of CO2 exposure required several different data streams, including light interception, litter mass, and leaf mass per unit area (LMA). The light interception data also permitted calculation of absorbed photosynthetically active radiation (APAR) and light-use efficiency (LUE), which are critical parameters for large-scale assessment of terrestrial productivity and are important links between terrestrial productivity models, remote sensing, and ground-based measurements in manipulative experiments.
Materials and methods
Site description
The experimental site is a planted sweetgum (Liquidambar styraciflua L.) monoculture, which was established in 1988 on the Oak Ridge National Environmental Research Park in Roane County, Tennessee (35°54′N, 84°20′W). The trees were planted at a spacing of 2.3×1.2 m, and based on measurements of basal area increment, they have been in a linear growth phase since 1993 (Norby et al. 2001). Five 25-m-diameter plots were laid out in 1996, and FACE apparatus (Hendrey et al. 1999) was assembled in four of them. Tree stand measurements within the inner 20-m-diameter part of the plots began in 1997, 1 year prior to the onset of treatment. There were approximately 94 trees per measurement plot with a total basal area of 32 cm2 m−2 and average height of 14 m (Norby et al. 2001). Across all plots, 42% of the trees were classified as dominant in 2000, 27% were co-dominant, 16% were intermediate, and 15% were suppressed. The understory comprises annual grasses, woody vines, and a few small tree seedlings; it is a minor component of stand productivity (Norby et al. 2002).
Exposure to elevated [CO2] commenced in two plots in April, 1998, and has continued during the growing season (April–November) since then. The average daytime [CO2] during the 1998–2002 growing seasons was 542 ppm in the two CO2-enriched plots, including periods when the exposure system was not functioning, and 391 ppm in ambient plots. The standard deviation of 1-min averages in the CO2-enriched plots was 60 ppm. The site and experimental design was fully described by Norby et al. (2001); environmental monitoring at the site was described by Wullschleger and Norby (2001). Hourly records of [CO2] and meteorological variables are given in Riggs et al. (2002a, 2002b).
Litter mass
Leaves were collected in litter traps as they fell, primarily during September and October. Litter traps were first placed in the stand as the plots were being laid out in 1996. Initially five 0.17-m2 laundry baskets were deployed in each plot. In 1998 these were replaced with seven 0.19-m2 baskets constructed from fiberglass screen suspended on a PVC frame. Leaf litter (woody litter was excluded) was collected from the baskets, usually within 1 week of when it fell, oven-dried (70°C), and weighed. Litter collections continued until all leaves of the canopy had fallen, which usually was determined by a storm in early November. The total leaf mass collected during the year, divided by the area of the litter baskets, provided an estimate of annual leaf litter mass production per square meter, which was an important component of the assessment of net primary productivity (NPP) of the plots (Norby et al. 2002).
Leaf mass per unit area
Conversion of litter mass to leaf area requires a canopy-averaged value for LMA, which is difficult given the three-fold variation in LMA with canopy depth. We collected fresh leaf litter from the forest floor and measured its area (LI-3100 area meter, LI-COR, Lincoln, Neb., USA) and dry mass, but these data were unreliable and prone to bias because of the difficulty in measuring the area of senescent leaves that often are desiccated and shriveled. A more reliable estimate came from green leaves collected from the canopy in August prior to senescence. Four leaves were collected at each meter of canopy depth, with access provided by a hydraulic lift, and their LMA was calculated from measurements of area and dry mass. A canopy-averaged LMA was calculated by weighting the LMA of each 1-m layer by the proportion of total leaf area in that layer, which was determined from previously cut trees (Norby et al. 2001). Litter LMA was 7% less than green leaf LMA because of loss of dry matter during senescence, determined through subsampling (Norby et al. 2001; Sholtis 2002). All measurements of leaf or litter mass and area included petioles.
PAR absorption
The progression of LAI in the spring was determined indirectly from the measurements of photosynthetically active radiation (PAR), following the terminology and relationships given in Russell et al. (1989). PAR sensors (LI-190SB, LI-COR, Lincoln, Neb., USA) were mounted above the canopy (22 m) and near the center of each plot at 2 m above the ground. Beginning in September 1998, PAR22 and PAR2 were recorded every 1 min, and the data were saved as 1-h averages. From these data we calculated fractional transmission of PAR (canopy transmittance, τ) as PAR2/PAR22. An estimate of the relationship between LAI (L) and radiation transmission is given by:
where k is an attenuation coefficient, provided that sun angle above the horizon is >20°, spatial distribution of leaves is random, and leaf angle distribution is spherical (Russell et al. 1989). The contribution of branches to canopy transmittance was subtracted from the daily total τ, where τbranch was set to the average of the 10 days prior to the beginning of leaf-out. The beginning of leaf-out was set as the date on which τ began to decrease (without retreat), which corresponded with visual observation of canopy activity. The value of k was determined from τ and L on the day when leaf production was complete, which was determined by visual inspection of buds in the upper canopy. L on that day was assumed to equal total leaf area production minus the small amount of leaf area that had already abscised. Values of k for the different plots and different years ranged from 0.28 to 0.43. Daily values of LAI were then calculated as:
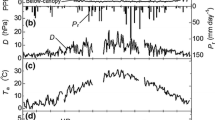
This approach was not adequate for estimating LAI throughout the year. Midsummer values of τ were noisy and did not correspond to observable increases in leaf abscission. Also, transmittance did not always return to the same baseline, τbranch, probably because summer storms created variable gaps in the canopy. Hence, transmittance was used to estimate LAI only during the period of leaf production (i.e., until early to mid-July). An asymmetric sigmoid curve was fit to the baseline-adjusted values of ln(τ/−k) such that the asymptote of the relationship was equal to the total leaf area production as determined from the litter basket collections (Fig. 1).
The calculation of leaf area index (LAI) through the growing season made use of data on photosynthetically active radiation (PAR) transmittance and leaf area collected in litter traps, shown here for plot 1 in 2001. The open circles and tick marks are PAR transmittance data, plotted as (ln τ–ln τbranch)/−k, where τ is fractional transmittance (daily PAR measured below the canopy divided by daily PAR above the canopy), corrected for transmittance prior to leaf-out (τbranch), and k is the extinction coefficient set to a value (0.41 in this example) such that total leaf area production matches that determined from litter traps. Transmittance data from leaf-out to bud-set (open circles) were used to generate a curve (dotted line) representing leaf area production. Solid circles represent abscised leaf area, and the dashed line is the cumulative progression of leaf loss until the end of the growing season when all leaves have fallen. The solid line is LAI, or the difference between production and loss. Note that the calculation of LAI was independent of the PAR data in the second half of the growing season (tick marks)
Total absorbed PAR (APAR, Q a) was determined as:
where Q is incident PAR (PAR22) summed over the growing season, p is the fraction of incident PAR reflected by the canopy, and Q τ (=τQ) is the total transmitted PAR (PAR2). Reflected PAR was determined to be 3% of incident PAR, based on measurements at the top of the canopy on both sunny and cloudy days. The fraction of PAR reflected by soil was negligible and not included in the calculation of APAR. APAR was converted from units of mol m–2 day–1 to MJ m–2 day–1 using the equivalence of 4.6 mol quanta per MJ of PAR (Russell et al. 1989). LUE was calculated as the dry matter: radiation quotient (εN, g MJ–1), by dividing annual NPP (g DM m–2 day–1) by APAR. NPP data for 1999 and 2000 were reported by Norby et al. (2002) and include stem and coarse root increment, leaf litter, and fine root production; NPP for 2001 and 2002 was calculated similarly.
Calculation of LAI
The litter basket collections provided the time course of leaf loss. A logistic curve was fit to the data, or in some cases separate relationships were used for the loss of leaves during summer and the rapid loss due to normal senescence in the autumn. The progression of LAI through the year was then calculated by subtracting the leaf loss curve from the leaf production curve (Fig. 1). Leaf area duration (LAD) was calculated as the sum of daily LAI values, or the area under the LAI versus time curve. The seasonal average LAI is the LAD divided by the number of days. The effective length of growing season (canopy duration) was calculated as the number of days for which LAI was at least 50% of the peak LAI. The effects of [CO2], year, and [CO2] × year interaction on LAI and related parameters were tested statistically by analysis of variance using type III sum of squares with plot as the experimental unit and [CO2] and year as fixed effects.
Optical assessment of LAI
During the 2000 growing season LAI also was assessed by analysis of hemispheric photos of the canopy (Fig. 2). The photos were taken with a Nikon Coolpix 950 digital camera with a fisheye lens. The images were saved as 1,600×1,200 pixel black and white TIFF images and analyzed using WinScanopy software (Regent Instruments, Quebec, Canada). The calculation of LAI was corrected for clumping using the negative binomial model of Neumann et al. (1989):
where g is the index of foliage dispersion, P 0 is the gap frequency, G is the leaf orientation function and µ is the cosine of the zenith angle. The values for g (2.42) and G (0.5) were originally calculated for an oak-hickory forest in Oak Ridge, Tennessee by Baldocchi et al. (1985).
Canopy density
Canopy density, or the amount of leaf area in the volume of space occupied by the canopy, was determined in August 2000. Canopy volume of 25 trees per plot was calculated from measurements of height to base of canopy, height to top of canopy, and maximum canopy width. Canopy heights were measured with a Vertex hypsometer (Haglöf, Sweden); canopy width was measured with the aid of a right-angle prism. Most of the volume was assumed to be a cylinder except for the conical tops of the taller trees that emerged above the average canopy top. The average volume per tree was multiplied by the number of trees per plot and divided by the 314 m2 area of the plot. Canopy density (m2 m–3) was then calculated as LAI divided by canopy volume per m2 plot area.
Light compensation points for CO2 assimilation
Light response curves were determined three times in 1999 and 2000, in fully expanded leaves near the tips of branches in the upper and lower canopy, on 4–6 leaves per plot. Upper canopy leaves that were exposed to full sunlight at least part of the day were selected from the top 2 m of the canopy. Lower canopy leaves were approximately 3–4 m down into the canopy and received approximately 20–50% of full PPFD during the day, as estimated with a 1-m line quantum sensor (LI-191SA, LI-COR, Lincoln, Neb., USA) after full canopy development.
Photosynthetic light response curves were measured with the LI-6400 steady state photosynthesis system (LI-COR, Lincoln, Neb., USA) using a 6-cm2 cuvette. Net CO2 assimilation of each leaf was measured after equilibration at nine irradiances, beginning at 2,000 µmol m−2 s−1 PAR. Illumination was provided by a red/blue LED source (LI 6400–02B, LI-COR, Lincoln, Neb., USA). Cuvette conditions (temperature, vapor pressure deficit, and [CO2]) were set to approximate prevailing mid-day atmospheres (Gunderson et al. 2002). CO2 concentrations were regulated using a CO2 mixer and injector system (LI-6400–01, LI-COR, Lincoln, Neb., USA) and cartridges of compressed CO2. Inlet concentrations were set to 365 ppm for measurements in the ambient [CO2] plots, and 565 ppm in the elevated [CO2] plots. Net photosynthesis for each leaf was described as a function of PPFD using the least-squares fit of a non-rectangular hyperbola (Prioul and Chartier 1977; Photosyn Assistant software, Dundee Scientific, Dundee, Scotland). Light compensation points (LCP) for each leaf were determined as the x-intercept of this curve (where assimilation =0).
Results
Leaf area dynamics
The seasonal pattern of canopy development was typical of sweetgum trees. Sweetgum shoot extension is sustained for a longer fraction of the growing season than for many temperate trees. There is a distinct pattern of leaf dimorphism in that some leaves develop from preformed initials in an overwintering terminal bud, and additional (neoformed) leaves develop from new primordia that are formed during a free-growth stage (Brown and Sommer 1992). In the Oak Ridge FACE experiment, leaf initiation began each year in mid-April, and 50% of peak LAI was reached in mid-May (Table 1). New leaves continued to be initiated and expand until early to mid-July, when bud set was observed (Sholtis 2002). Tree height increased by 0.5–1.0 m each year (Norby et al. 2001), but as the top of the canopy grew higher, lower branches were cast off, so the canopy depth remained about 6 m. The top of the canopy increased in roughness as a few trees in each plot emerged from the average top of the stand. A few trees died each year (less than three per plot); these always were highly suppressed trees that had been contributing little to stand LAI.
The canopy was highly clumped because the branches of individual trees did not overlap those of adjacent trees (Fig. 2). Green leaves fell from the canopy throughout the spring and summer, primarily during severe storms. Leaf senescence and abscission generally began in the lower canopy in September or early October (Table 1), progressed to the top of the canopy, and abscission was complete by early November. In years with low rainfall in August and September (1998 and 1999), 50% of the peak LAI had fallen by mid-September to the beginning of October, but leaves were retained longer in years with normal late-season precipitation. The pattern in 2002 was an exception, starting early in association with low September rainfall, but ending later in November than in previous years after heavy rainfall and warm weather in late September and October.
CO2 enrichment during 1999–2002 had a significant effect on the duration of the canopy (Table 1), but the effect varied year to year (CO2 × year significant at P<0.088). Canopy duration (the number of days with at least 50% of the peak LAI) was 6 days less in elevated [CO2] in 1999, 2 days less in 2001, and 15 days less in 2002, but 2 days longer in 2000. The difference in 1999 was due to later leafout in the spring in CO2-enriched trees, whereas the difference in 2002 occurred during the drought-related early senescence in the fall.
The pattern of LAI development was generally similar in all plots each year (Fig. 3). Discontinuities are apparent in the PAR transmittance data, especially on 28 July 1999 (day 209), corresponding with a major storm that created canopy gaps and clumps where none had been previously. There has been no trend through time in total annual leaf area production since 1996 when observations first began (not shown). Regressions of leaf area production versus year were not statistically significant for any individual plot or collectively across all plots. There also was no apparent pre-treatment bias that would need to be accounted for in analyzing subsequent responses to [CO2].
LAI of each plot in 1999–2002. Solid circles represent abscised leaf area, and crosses are PAR transmittance data. The analytical approach is described in Fig. 1. The graph from plot 1, year 2000 also includes LAI values calculated from hemispheric photos (solid triangles). Daily LAI data from each plot are archived at the Carbon Dioxide Information and Analysis Center, Oak Ridge, Tennessee (http://cdiac.ornl.gov/programs/FACE/ornldata/ornldata.html)
There was no effect of [CO2] on peak LAI, average LAI, or LAD in 1999–2002 (Fig. 4). Peak LAI, which occurred in mid-July varied from 5.2 to 6.4, and average LAI varied from 3.4 to 4.7. LAD, which perhaps best represents the total capacity of the canopy for carbon uptake, varied from 709 to 914 m2 day m-2. Averaged across the 4 years, the difference in LAD between ambient and elevated plots was 3%, ranging from an 8% increase with CO2 enrichment in 2000 to a 3% decrease in 2002; none of these small differences was statistically significant. Peak canopy mass, however, was significantly higher in elevated [CO2], reflecting the increase in LMA (Table 2). The limited replication in FACE experiments creates concern that biologically significant effects are undetected. However, given the replication and variance in this data set, a CO2 effect of less than 6% in the 4-year means of peak LAI, average LAI, or LAD would be statistically significant (P<0.05), and this difference is less than the year-to-year variation that was observed.
Leaf area parameters calculated from the data in Fig. 3. a LAI averaged over the growing season; b peak LAI; and c leaf area duration (LAD), or the integration of LAI over time. Open bars are the means of plots in ambient CO2 (n=3), and solid bars are means for elevated CO2 (n=2); error bars represent 1 SE. CO2 concentration and CO2 × year were not statistically significant sources of variation for any of the parameters (P>0.10); year was significant at P<0.004 for average LAI and P<0.064 for LAD
Hemispheric photos and canopy structure
Gap fraction in 2000 as calculated from hemispheric photos was 14% in both ambient and elevated [CO2] (Table 3). Canopy volume and canopy density in 2000 also were not affected by [CO2] (Table 3). LAI values calculated from hemispheric photos were approximately 2 throughout most of the growing season, with peak values of 3, when actual LAI was above 4 (Fig. 3, plot 1, year 2000). Similar discrepancies occurred in all five plots. Measurements made in 2002 with the LAI-2000 Plant Canopy Analyzer (LI-COR, Lincoln, Neb., USA), which uses similar assumptions and algorithms as the hemispheric photo approach, produced similar results (A. W. King, personal communication).
APAR and light-use efficiency
APAR did not differ between CO2 treatments, as might be expected given the similar LAD (Table 4). The fraction of PAR absorbed (f APAR) was 89%. Since NPP over the 4 years was 22% higher in elevated [CO2], the light-use efficiency εN also was 22% higher (range 16–28%).
Light compensation points
In upper canopy foliage, light compensation points were reduced an average of 21% by elevated [CO2] (Fig. 5). The influence of canopy position on LCP, however, was larger than the effect of CO2 treatment, such that leaves in the lower portions of the canopy had 50% lower LCP than upper canopy leaves in ambient [CO2] and 38% lower LCP than upper canopy leaves in elevated [CO2]. Acclimation to reduced light environments thus eclipsed the effects of elevated [CO2] on LCP, and for leaves in the mid- to lower canopy, no additional reduction in LCP was associated with [CO2].
Light compensation points in upper and lower canopy sweetgum leaves during late June, 1999 and 2000, and August, 2000. Open bars represent the means of plots in ambient [CO2] (n=3), and solid bars are means for elevated [CO2](n=2); error bars represent 1 SE. Four to six leaves contributed to each plot mean. Statistical significance of main and interactive effects: canopy position, P<0.001; CO2 concentration, P<0.062; date, P<0.001; canopy position × CO2, P<0.074; other interactive terms not significant
Discussion
The separation of plant productivity into functional and structural components provides a useful construct for analyzing responses to elevated [CO2] and for relating responses across a wide range of scales (Woodward et al. 1995; Norby 1996). LAI is the most important structural component in this regard, and determination of its response to elevated [CO2] in forest ecosystems has been a high priority research question requiring the employment of larger-scale research facilities. Here we provide evidence that LAI is not affected by CO2 enrichment in a deciduous forest. Despite year-to-year variation in LAI, and independent of the observed functional responses to elevated [CO2] (e.g., increased photosynthesis, Gunderson et al. 2002), no expression of leaf area, including average LAI, peak LAI, LAD, or canopy density, showed any response to [CO2] over the 4 years reported here. Leaf area was unaffected by [CO2] despite increased carbon allocation to leaves in elevated [CO2] (Norby et al. 2002), which resulted in increased foliage mass and mass per unit area. Increased LMA (decreased specific leaf area), which is a common response to elevated [CO2] (Yin 2002), could represent a structural change at the leaf level (morphological changes in mesophyll) or an accumulation of non-structural carbohydrates, with different implications for the regulation of assimilation (Peterson et al. 1999), but these alternatives cannot be evaluated in this study.
While we have confidence that our primary measurements of LAI are close to the true values, optical assessment of the canopy through analysis of hemispheric photos greatly underestimated LAI. Much of the error is probably associated with the highly clumped nature of the sweetgum canopy (Gower et al. 1999). Optical approaches (hemispheric photos and the LAI-2000 Plant Canopy Analyzer) have underestimated LAI in other studies, especially in forest stands with non-random leaf area distribution (Mussche et al. 2001). Caution is urged in the use of these methods in ongoing efforts to "ground truth" satellite assessments of LAI, especially in stands with high LAI and clumped canopies, without more direct measurements to verify their accuracy.
There are only a few other observations of forest LAI in elevated [CO2], but all of them indicate that there is no substantial sustained effect of [CO2]. LAI is more difficult to measure in the evergreen, needle-leaf Pinus taeda stand in the FACE experiment in North Carolina, and reports have been inconsistent regarding the effect of [CO2] (Lichter et al. 2000; Luo et al. 2001; DeLucia et al. 2002). If there have been any effects of [CO2] in that experiment, apparently they have been small. Elevated [CO2] increased LAI in a Florida scrub-oak ecosystem prior to canopy closure (Hymus et al. 2002), but there is no basis for projecting this response to a closed-canopy system. In the FACE experiment in central Italy in a developing stand of Populus genotypes, LAI was significantly higher in the elevated [CO2] plots during the first year of stand establishment, but there were no effects of [CO2] on LAI once canopy closure occurred during the second growing season (Gielen et al. 2001). The rapid growth of this stand has precluded longer-term monitoring of leaf area responses after canopy closure. In the much slower-growing Quercus ilex stands adjacent to CO2 springs in central Italy, there was no difference in LAI of tree stands that had been exposed to elevated [CO2] for decades and that of reference plots (Hättenschwiler et al. 1997). Coupled with observations of dense tree stands in model ecosystems (e.g., Egli et al. 1998), which also showed no effect of [CO2] on LAI, the current evidence seems convincing that LAI of non-expanding forest stands will not be different in a future CO2-enriched atmosphere. Nevertheless, the number of intact stands with a fully developed canopy that have been measured is very small, and it is premature to conclude that forest LAI will never increase with increasing [CO2].
One physiological observation that has previously been used to suggest that LAI might increase is the decrease in light compensation point of leaves in elevated [CO2] (Long 1991; Hirose et al. 1997). That theory was not supported in this experiment. Although upper canopy leaves in full sun did have a lower LCP in elevated [CO2], there was no change in the LCP of shaded leaves deeper in the canopy, and it is these leaves which would need to be retained longer for an increase in LAI by this scenario. DeLucia and Thomas (2000) similarly reported no effect of [CO2] on LCP of saplings of four hardwood species in deep shade, and Herrick and Thomas (1999) saw no effect of [CO2] on LCP of sub-canopy sweetgum trees, but Hättenschwiler (2001) reported decreases of 22–32% in five other deciduous species exposed to elevated [CO2] in deep shade.
Even if the magnitude of leaf production (e.g., peak LAI) is not altered by [CO2], the timing, or phenology, of leaf initiation and leaf senescence could be. The phenology of canopy development is an important determinant of productivity, and satellite surveillance (Myneni et al. 1997) has suggested that phenology in the north temperate zone has been changing in recent decades. Earlier leaf initiation is most likely to be associated with warmer spring temperatures. There have been some reports that [CO2] alters phenology of trees (Jach et al. 2001), but many more reports indicate no or inconsistent effects on phenology. Over the 4 years of this study, [CO2] had a statistically significant effect on the duration of the canopy, reducing it by as much as 15 days in 2002. However, the effect was very inconsistent (CO2 × year interaction marginally significant), and the largest effects (in 1999 and 2002) resulted from different causes. Most importantly, the reduced canopy duration in elevated [CO2] was not associated with differences in LAD—the integration of LAI over time—because phenological differences in LAI occurred when LAI was low. However, the later leaf-out in 1999 may have reduced total water use by the CO2-enriched plots (Wullschleger and Norby 2001).
Satellite surveillance also is used to estimate the fractional absorption of PAR (f APAR) from the spectral characteristics of canopy reflectance (Myneni et al. 2002), and f APAR is used to calculate APAR, a primary determinant and predictor of productivity when coupled with light-use efficiency (Haxeltine and Prentice 1996). We were able to measure APAR and NPP directly, thereby providing a direct test of how [CO2] will affect the relationship between NPP and APAR, expressed as the light-use efficiency, εN (Medlyn 1998). Since there was no effect of [CO2] on LAD, it is not surprising that APAR was similar in the different treatments, and differences in εN reflected differences in NPP. The value εN is analogous to the CPI derived to separate the CO2 response of productivity from the confounding influence of increasing leaf area in exponentially growing young trees (Norby 1996). A primary hypothesis of the FACE experiment was that the increase in CPI observed in young trees grown in elevated [CO2] (29% in 650 ppm; Norby et al. 1999) would persist after canopy closure in a forest stand; that is, that the fundamental functional response is independent of the structural response. For convenience, CPI was calculated from experimental data as annual aboveground dry matter increment per unit leaf area, and it would not be valid if there were important shifts in allocation (Medlyn et al. 2001). In this sweetgum experiment, there have been important shifts in allocation such that the increase in NPP in elevated [CO2] appears primarily in the fine root component and not in increased aboveground woody increment (Norby et al. 2002). Hence, CPI is not increased as proposed. Nevertheless, the more general concept that productivity (i.e., NPP) per unit functioning leaf area, as captured in εN, would increase in elevated [CO2] after canopy closure is supported.
Light-use efficiency measured in this sweetgum stand is comparable to other observations, and the increases in εN observed in elevated [CO2] also are generally consistent with other experimental and modeling results. Our values of εN, ranging from 1.4 to 2.4 g MJ−1, are within the range reported elsewhere: 0.2 to 3.6 g MJ−1 for a variety of vegetation types (Ruimy et al. 1994). A calculated mean value of εN for temperate deciduous forests developed in a global modeling effort (Ruimy et al. 1994) was 1.01 g MJ−1; our value would be expected to be higher in that it was based on PAR absorbed by leaves only and excluded PAR absorbed in the winter. In elevated [CO2] (+180 ppm, excluding early morning hours), εN in our sweetgum stand increased 22%. This was somewhat less than that reported by DeLucia et al. (2002) for a Pinus taeda stand of similar stature in a similarly designed FACE experiment. In that experiment ε increased from 0.49 to 0.62 g MJ−1 (27%) based on biomass increment (rather than NPP). The average increase in CPI of young trees in open-top chambers with +300 to +350 ppm CO2 was 26% (Norby et al. 1999), corresponding to a 14% increase with +180 ppm CO2, if we conservatively assume a linear response to CO2 enrichment. Model results with three species of trees in Australia indicated that ε could increase 20 to 35% with a 350 ppm increase in [CO2] (Medlyn 1996), corresponding to a 10–18% increase with +180 ppm. Hence, we can conclude that light-use efficiency increases in elevated [CO2], the response is fairly well constrained in different systems, and the increase is driven primarily by functional responses (photosynthesis and dry matter production) rather than by structural changes (LAI and PAR absorption), although a role for structural changes at the leaf level associated with increased LMA cannot be excluded.
The simple model relating NPP to APAR, which has been useful in large-scale analyses and remote sensing of terrestrial productivity, is an appropriate basis for linking results of manipulative experiments to ecosystem models of future productivity in a CO2-enriched atmosphere and to observations of current patterns of productivity (e.g., Haxeltine and Prentice 1996). Nevertheless, it must also be recognized that there are other sources of variation influencing NPP, as evidenced by the 30% plot-to-plot and year-to-year within-treatment variation in εN in this experiment, which emphasizes the lack of a clear understanding of the physiological basis of light-use efficiency (Medlyn 1998). Improvements in the estimation of εN are important to efforts to estimate NPP from satellite data (Ruimy et al. 1994). In particular, LUE strongly depends on the diffuse and direct composition of global incident PAR (Gu et al. 2002)
The evidence presented here and in a few other studies is that LAI in non-expanding forests does not increase in elevated [CO2]. Many analyses predicting responses to global change have assumed otherwise, and those conclusions should be reconsidered. For example, in the equilibrium vegetation distribution model MAPSS (Neilson and Drapek 1998), doubled [CO2] is assumed to create a 35% reduction in stomatal conductance. Through internal feedbacks the reduction in conductance (or increased water-use efficiency) leads to an increase in LAI such that there is no or little effect of [CO2] on evapotranspiration. Our experimental results do not support this scenario. Although elevated [CO2] did reduce stomatal conductance, this response was not associated with increased LAI. Evapotranspiration was unchanged, as suggested by MAPSS, but for reasons unrelated to adjustments in LAI (Wullschleger et al. 2002). Our experiment, however, does not address the possibility of regional-scale increases in LAI related to CO2 effects on plant distribution. The importance of LAI in regulating system-level feedbacks through resource use and allocation emphasizes the importance of knowing how LAI of regionally important ecosystems will respond to CO2 enrichment of the atmosphere.
References
Baldocchi DD, Hutchison BA, Matt DR, McMillen RT (1985) Canopy radiative transfer models for spherical and known leaf inclination angle distributions: a test in an oak-hickory forest. J Appl Ecol 22:539–555
Brown CL, Sommer HE (1992) Shoot growth and histogenesis of trees possessing diverse patterns of shoot development. Am J Bot 79:335–346
DeLucia EH, Thomas RB (2000) Photosynthetic responses to CO2 enrichment of four hardwood species in a forest understory. Oecologia 122:11–19
DeLucia EH, George K, Hamilton JG (2002) Radiation-use efficiency of a forest exposed to elevated concentrations of atmospheric carbon dioxide. Tree Physiol 22:1003–1010
Drake BG, Gonzàlez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48:609–639
Egli P, Maurer S, Gunthardt-Goerg MS, Körner C (1998) Effects of elevated CO2 and soil quality on leaf gas exchange and above-ground growth in beech-spruce model ecosystems. New Phytol 140:185–196
Ferris R, Sabatti M, Miglietta F, Mills RF, Taylor G (2001) Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant Cell Environ 24:305–315
Gielen B, Calfapietra C, Sabatti M, Ceulemans R (2001) Leaf area dynamics in a closed poplar plantation under free-air carbon dioxide enrichment. Tree Physiol 21:1245–1255
Gower ST, Kucharik CJ, Norman JM (1999) Direct and indirect estimation of leaf area index, f(APAR), and net primary production of terrestrial ecosystems. Remote Sensing Environ 70:29–51
Gu L, Baldocchi D, Verma, SB, Black TA, Vesala T, Falge EM, Dowty PR (2002) Advantages of diffuse radiation for terrestrial ecosystem productivity. J Geophys Res 107(No D6):10.1029/2001JD001242
Gunderson CA., Sholtis JD, Wullschleger SD, Tissue DT, Hanson PJ, Norby RJ (2002) Environmental and stomatal control of photosynthetic enhancement in the canopy of a sweetgum (Liquidambar styraciflua L.) plantation during three years of CO2 enrichment. Plant Cell Environ 25:379–393
Hartz-Rubin JS, DeLucia EH (2001) Canopy development of a model herbaceous community exposed to elevated atmospheric CO2 and soil nutrients. Physiol Plant 113:258–266
Hättenschwiler S (2001) Tree seedling growth in natural deep shade: functional traits related to interspecific variation in response to elevated CO2. Oecologia 129:31–42
Hättenschwiler S, Miglietta F, Raschi A, Körner Ch. (1997) Morphological adjustments of mature Quercus ilex trees to elevated CO2. Acta Oecol 18:361–365
Haxeltine A, Prentice IC (1996) A general model for the light-use efficiency of primary production. Funct Ecol 10:551–561
Hendrey GR, Ellsworth DS, Lewin KF, Nagy J (1999) A free-air enrichment system for exposing tall forest vegetation to elevated atmospheric CO2. Global Change Biol 5:293–309
Herrick JD, Thomas RB (1999) Effects of CO2 enrichment on the photosynthetic light response of sun and shade leaves of canopy sweetgum trees (Liquidambar styraciflua) in a forest ecosystem. Tree Physiol 19:779–786
Hirose T, Ackerly DD, Traw MB, Ramseier D, Bazzaz FA (1997) CO2 elevation, canopy photosynthesis, and optimal leaf area index. Ecology 78:2339–2350
Hymus GJ, Pontailler JY, Li J, Stiling P, Hinkle CR, Drake BG (2002) Seasonal variability in the effect of elevated CO2 on ecosystem leaf area index in a scrub-oak ecosystem. Global Change Biol 8:931–940
Jach ME, Ceulemans R, Murray MB (2001) Impacts of greenhouse gases on the phenology of forest trees. In Karnosky DF, Ceulemans R, Scarascia-Mugnozza G, Innes JL (eds) The impact of carbon dioxide and other greenhouse gases on forest ecosystems. CAB International, Wallingford, pp 193–235
Kimball BA, Kobayashi K, Bindi M (2002) Responses of agricultural crops to free-air CO2 enrichment. Adv Agron 77:293–368
Körner C, Arnone JA III (1992) Responses to elevated carbon dioxide in artificial tropical ecosystems. Science 257:1672–1675
Kubiske ME, Pregitzer KS (1996) Effects of elevated CO2 and light availability on the photosynthetic light response of trees of contrasting shade tolerance. Tree Physiol 16:351–358
Lichter J, Lavine M, Mace KA, Richter DD, Schlesinger WH (2000) Throughfall chemistry in a loblolly pine plantation under elevated atmospheric CO2 concentrations. Biogeochemistry 50:73–93
Long SP (1991) Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations—has its importance been underestimated? Plant Cell Environ 14:729–739
Long SP, Drake BG (1991) Effect of the long-term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3 sedge, Scirpus olneyi. Plant Physiol 96:221–226
Luo Y, Medlyn B, Hui D, Ellsworth D, Reynolds JF, Katul G (2001) Gross primary productivity in the Duke Forest: modeling synthesis of the free-air CO2 enrichment experiment and eddy-covariance measurements. Ecol Appl 11:239–252
Medlyn BE (1996) Interactive effects of atmospheric carbon dioxide and leaf nitrogen concentration on canopy light use efficiency: a modeling analysis. Tree Physiol 16:201–209
Medlyn BE (1998) Physiological basis of the light use efficiency model. Tree Physiol 18:167–176
Medlyn BE, Rey A, Barton CVM, Forstreuter M (2001) Above-ground growth response of forest trees to elevated atmospheric CO2 concentrations. In: Karnosky DF, Ceulemans R, Scarascia-Mugnozza G, Innes JL (eds) The impact of carbon dioxide and other greenhouse gases on forest ecosystems. CAB International, Wallingford, pp 127–146
Mussche S, Samson R, Nachtergale L, De Schrijver A, Lemeur R, Lust N (2001) A comparison of optical and direct methods for monitoring the seasonal dynamics of leaf area index in deciduous forests. Silva Fenn 35:373–384
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386:698–702
Myneni RB, Hoffman S, Knyazikhin Y, Privette JL, Glassy J, Tian Y, Wang Y, Song X, Zhang Y, Smith GR, Lotsch A, Friedl M, Morisette JT, Votava P, Nemani RR, Running SW (2002) Global products of vegetation leaf area and fraction absorbed PAR from year one of MODIS data. Remote Sensing Environ 83:214–231
Neilson RP, Drapek RJ (1998) Potentially complex biosphere responses to transient global warming. Global Change Biol 4:505–521
Neumann HH, Den Hartog G, Shaw RH (1989) Leaf area measurements based on hemispheric photographs and leaf-litter collection in a deciduous forest during autumn leaf-fall. Agric For Meteorol 45:325–345
Niklaus PA, Spinnler D, Körner C (1998) Soil moisture dynamics of calcareous grassland under elevated CO2. Oecologia 117:201–208
Norby RJ (1996) Forest canopy productivity index. Nature 381:564
Norby RJ, Gunderson CA, Wullschleger SD, O'Neill EG, McCracken MK (1992) Productivity and compensatory responses of yellow-poplar trees in elevated CO2. Nature 357:322–324
Norby RJ, Wullschleger SD, Gunderson CA, Nietch CT (1995) Increased growth efficiency of Quercus alba trees in a CO2-enriched atmosphere. New Phytol 131:91–97
Norby RJ, Wullschleger SD, Gunderson CA, Johnson DW, Ceulemans R (1999) Tree responses to rising CO2: implications for the future forest. Plant Cell Environ 22:683–714
Norby RJ, Todd DE, Fults J, Johnson DW (2001) Allometric determination of tree growth in a CO2-enriched sweetgum stand. New Phytol 150:477–487
Norby RJ, Hanson PJ, O'Neill EG, Tschaplinski TJ, Weltzin JF, Hansen RT, Cheng W, Wullschleger SD, Gunderson CA, Edwards NT, Johnson DW (2002). Net primary productivity of a CO2-enriched deciduous forest and the implications for carbon storage. Ecol Appl 12:1261–1266.
Peterson AG, Ball JT, Luo Y, Field CB, Curtis PS, Griffin KL, Gunderson CA, Norby RJ, Tissue DT, Forstreuter M, Rey A, Vogel CS, CMEAL participants (1999) Quantifying the response of photosynthesis to changes in leaf nitrogen content and leaf mass per area in plants grown under atmospheric CO2 enrichment. Plant Cell Environ 22:1109–1119
Prioul JL, Chartier P (1977) Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: a critical analysis of the methods used. Ann Bot 41:789–800
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Global Change Biol 5:807–837
Riggs JS, Tharp ML, Norby RJ (2002a) ORNL FACE CO2 Data (http://cdiac.ornl.gov/programs/FACE/ornldata/co2files.html). Carbon Dioxide Information Analysis Center, U.S. Department of Energy, Oak Ridge National Laboratory, Oak Ridge, Tennessee
Riggs JS, Tharp ML, Norby RJ (2002b) ORNL FACE Weather Data (http://cdiac.ornl.gov/programs/FACE/ornldata/weatherfiles.html). Carbon Dioxide Information Analysis Center, U.S. Department of Energy, Oak Ridge National Laboratory, Oak Ridge, Tennessee
Ruimy A, Saugier B, Dedieu G (1994) Methodology for the estimation of terrestrial net primary production from remotely sensed data. J Geophys Res 99(D3):5263–5283
Russell G, Jarvis PG, Monteith JL (1989) Absorption of radiation by canopies and stand growth. In Russell G, Marshall B, Jarvis PG (eds) Plant canopies: their growth, form and function. Cambridge University Press, Cambridge, pp 21–39
Sholtis JD (2002) Effects of elevated CO2 on sweetgum ecophysiology. PhD Dissertation, Texas Tech University, Lubbock, Texas
Woodward FI, Smith TM, Emanuel WR (1995) A global land primary productivity and phytogeography model. Global Biogeochem Cycles 9:471–490
Wullschleger SD, Norby RJ (2001) Sap velocity and canopy transpiration for a 12-year-old sweetgum stand exposed to free-air CO2 enrichment. New Phytol 150:489–498
Wullschleger SD, Gunderson CA, Hanson PJ, Wilson KB, Norby RJ (2002) Sensitivity of stomatal and canopy conductance to elevated CO2 concentration–interacting variables and perspectives of scale. New Phytol 153:485–496
Yin X (2002) Responses of leaf nitrogen concentration and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Global Change Biol 8:631–642
Acknowledgements
We appreciate the critical reviews of an earlier manuscript by Stan Wullschleger and Lianhong Gu. Research was sponsored by the U.S. Department of Energy, Office of Science, Biological and Environmental Research. Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract DE-AC05–00OR22725.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norby, R.J., Sholtis, J.D., Gunderson, C.A. et al. Leaf dynamics of a deciduous forest canopy: no response to elevated CO2 . Oecologia 136, 574–584 (2003). https://doi.org/10.1007/s00442-003-1296-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1296-2