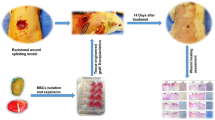
Abstract
We have investigated the wound-healing effects of mesenchymal stem cells (MSCs) in combination with human amniotic membrane (HAM) when grafted into full-thickness skin defects of rabbits. Five defects in each of four groups were respectively treated with HAM loaded with autologous MSCs (group A), HAM loaded with allologous MSCs (group B), HAM with injected autologous MSCs (group C), and HAM with injected allologous MSCs (group D). The size of the wounds was calculated for each group at 7, 12, and 15 days after grafting. The wounds were subsequently harvested at 25 days after grafting. Sections stained with hematoxylin and eosin were used to determine the quality of wound healing, as based on the characteristics and amount of granulated tissue in the epidermal and dermal layers. Groups A and B showed the most pronounced effect on wound closure, with statistically significant improvement in wound healing being seen on post-operative days 7, 12, and 15. Although a slight trend toward improved wound healing was seen in group A compared with group B, no statistically significant difference was found at any time point between the two groups. Histological examination of healed wounds from groups A and B showed a thin epidermis with mature differentiation and collagen bundle deposition plus recovered skin appendages in the dermal layer. In contrast, groups C and D showed thickened epidermis with immature epithelial cells and increased fibroblast proliferation with only partially recovered skin appendages in the dermal layer. Thus, the graft of HAM loaded with MSCs played an effective role during the healing of skin defects in rabbits, with no significant difference being observed in wound healing between autologous and allologous MSC transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coverage of skin defects remains a major concern when the defective area is widespread, severely contaminated by microorganisms, or poorly vascularized, as can be the case with irradiation defects, congenital skin disorders, or extensive burns. Numerous materials for skin coverage, including temporary substitutes, such as porcine xenografts, synthetic membranes, and allogeneic substitutes, or permanent skin substitutes, such as cultured epidermis and dermal substitutes, have been investigated (Sheridan and Tompkins 1990). Among these materials, artificial dermal substitutes are structurally optimized to be incorporated into the surrounding tissue and to allow cell invasion by fibroblasts and capillaries for subsequent dermal remodeling (Suzuki et al. 1990).
In a recent study, allologous mesenchymal stem cells (MSCs) or epidermal cells have been demonstrated playing an effective role in promoting wound healing when injected into the skin defect, either alone or in combination with human amniotic membrane (HAM; Kim et al. 2008). However, some questions remain unresolved regarding this technique, such as the way that the transplanted stem cells effect healing and whether autologous or allologous stem cells are the most effective for transplantation. The combined application of both cell sources (MSCs and HAM) is expected to have wide clinical use for the treatment of skin lacerations and warrants further analysis. The purpose of this study has been to investigate the wound-healing effects of HAM combined with MSCs grafted to full-thickness skin defects in rabbits.
Materials and methods
Animals
From 21 New Zealand white rabbits aged approximately 15 weeks at the beginning of the experiment and weighing 2–2.5 kg, one rabbit was used for the cultivation of allologous MSCs, and 20 rabbits were divided into four groups, each containing five animals. The protocol for the experiment was approved by the Institutional Animal Care and Use Committee of Dong-A University. Animals were handled according to the guidelines established for animal care at the center. Each rabbit had free access to both sterile water and standard rodent soft chow ad libitum.
Preparation of HAM
HAM was obtained after Caesarian deliveries. Briefly, the placenta was cleaned of blood clots with sterile Ringer’s solution containing antibiotics. The HAM was peeled from the chorion, rinsed in Ringer’s solution, and placed on nitrocellulose paper (Bio-Rad, Gainesville, Fl., USA) with the epithelium facing up. The tissue was then stored at −80°C in Dulbecco’s modified Eagle’s medium (Life Technologies, Gaithersburg, Md., USA) and glycerol at a ratio of 1:1 (vol/vol). Before use of the HAM, it was thawed at 37°C for 30 min, peeled from the nitrocellulose membrane, and placed on a glass slide with the epithelium facing up. The HAM was de-epithelialized via incubation in 0.25% trypsin-EDTA at 37°C for about 30 min followed by mechanical scraping and washing with phosphate-buffered saline.
Isolation and culture of MSCs
MSCs were obtained for autologous transplantation by puncturing the distal femur of 10 rabbits, and allologous MSCs were obtained from another rabbit (not included in this experiment) under sterile conditions. Approximately 2 ml bone marrow was collected in a sterile tube containing heparin. Red blood cells were then induced to settle by using 10% Pentaspan (Jeil Pharm, Seoul, Korea), and the plasma was removed from the upper layer of the sample. The marrow sample was centrifuged at 1,000 rpm for 20 min at room temperature in 1.077 g/ml Ficoll-Hypaque (Amersham Biosciences, Sweden) with a density gradient. The enriched cells were collected from the interphase, resuspended in basal medium and stimulatory supplements (Stem Cell Technologies, Vancouver, BC, Canada) in the presence of 10% fetal bovine serum (Stem Cell Technologies) and antibiotics (100 IU/ml penicillin G and 100 μg/ml streptomycin), and then transferred to tissue culture flasks (25T flask) at densities of about 1 × 106 cell/ml (Fig. 1). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air in an incubator (HERAcell, Heraeus, Germany). The medium was changed after 48 h and then every 2–3 days. Primary cultures were usually maintained for 7–10 days, during which time the non-adherent hematopoietic cell fraction was depleted. After the cells had grown to near confluence, they were passaged 2–3 times by digestion with 0.25% trypsin and 0.02% EDTA (GIBCO, Grand Island, N.Y., USA).
Transfection and loading of MSCs onto surface of HAM
Cells for transfection were pre-conditioned by using fully supplemented medium for 4 h at 37°C in an atmosphere of 5% CO2. The phenotype and purity of MSCs were assessed by flow cytometry with CD45, CD73, CD105, and CD166. We transfected one cytokine gene (stem cell factor) into ex-vivo-expanded MSC and confirmed transfection, after cells were loaded onto the HAM, by fluorescence microscopy with green fluorescent protein (GFP). Flow cytometry analyses demonstrated that MSCs expressed CD73, CD105, and CD166 in all samples, but were negative for CD45. The purity of the expanded MSC was 85%–97%.
Samples of the HAM, approximately 1.5 cm2, were placed into individual wells of a six-well plate. Custom-made surgical steel seeding rings were placed onto the surface of the tissue to define the area of material to be seeded. At 3 h prior to transfection, the medium was removed from the MSC and replace with fresh growth medium. The cells were transfected with the pEGFP-N1 vector by using calcium phosphate. DNA, CaCl2, and enough distilled water were added together to a sterile tube, and the specified amount of 2× HEPES-buffered saline was added to another tube and continually vortexed while the prepared CaCl2/DNA solution was slowly being added to it. When the DNA addition was complete, the solution was incubated at room temperature for 30 min and then vortexed again, just prior to being added to the cells. Finally, the solution was added to the plate on which the HAM had been attached via its basement membrane and was swirled to distribute the precipitate evenly. The MSC (2 × 105) were loaded onto the HAM immediately by using lyophilized fibrin adhesive (Greenplast) for cell attachment and incubated at 37°C, under 5% CO2. The preparations were harvested 48 h later, and MSC loading onto the surface of the HAM was verified by GFP visualization under a fluorescence microscope (Fig. 2).
Confirmation of transfection of stem cell factor into mesenchymal stem cells loaded on the surface of human amniotic membrane. Microscopic images obtained by phase-contrast and fluorescence microscopy before transfection (a, b) and post-transfection at day 7 (c, d). Green fluorescent protein (GFP) fluorescence, i.e., GFP expression, can be seen in d. × 200
Surgical procedure
Twenty rabbits were divided into four groups, each containing five animals. In an aseptic surgical room, a full-thickness skin defect about 1.5 × 1.5 cm was excised, by means of sterile surgical instruments, from the back of each animal. The wound was covered with the designated HAM and sutured by using 5–0 nylon at 0.75 cm intervals. Among 10 of the skin defects, five defects each were grafted with HAM that was previously loaded with autologous MSCs (group A) or allologous MSCs (group B) at about 2 × 105 cells. Among another 10 defects, five defects each were grafted with HAM and subsequently injected with autologous MSCs (group C) or allologous MSCs (group D) in about 0.5 ml (6.8 × 106 cells) beneath the HAM by using a syringe. The wound was covered with a standard wet compress to prevent HAM detachment and desiccation, and then each animal was housed in its own cage to avoid damage to the wound.
Wound-size measurement and histological assessment
The wounds were photographed with the rabbits in a standard prone position by using a 400 million pixel digital camera (Olympus), and an image analyzer (Adobe Photoshop, ver. 5.5) was used for wound-size measurements. The wounds were almost completely covered with scar tissue by 15 days after grafting; the macroscopic size of the remaining wounds was calculated three times for each group at 7, 12, and 15 days after grafting. The wounds were harvested at 25 days after grafting for histological assessment. The harvested wound area included a borderline of normal tissue, and the wounds were divided into two segments (10 mm wide, 10 mm long, and a depth of 5 mm). The tissue was fixed in 10% formalin and embedded in paraffin. Sections stained with hematoxylin and eosin were used to determine the quality of wound healing as determined by the characteristics and amount of granulation of the epidermal and dermal layers.
Statistical analysis
Wound-healing rates were expressed as the mean±SD. Group comparisons were made by using an analysis of variance with the application of the Bonferroni correction for multiple comparisons. In all cases, a P-value of less than 0.05 was defined as being statistically significant.
Results
Animals
All rabbits were kept in individual cages during the experiments. A healthy wound healing process was observed throughout the experimental period. After 25 days, all wounds in all groups had adequately healed and demonstrated re-epithelialization.
Wound-size measurement
Groups A and B showed the most pronounced effect on wound closure, with statistically significant improvements in wound healing being seen at 7, 12, and 15 days after grafting (P < 0.05; Fig. 3). The earliest difference was observed at 7 days after grafting when groups A and B demonstrated 45.3 ± 7.2% and 43.7 ± 2.8% wound closure, respectively, whereas both group C (16.2 ± 4.9%) and group D (14.3 ± 6.7%) were still poorly healed (P < 0.05; Fig. 4). Although a slight trend toward improved wound healing was noted in group A relative to group B, no statistically significant differences were found at any time period between the two groups (Figs. 3, 4). Furthermore, no statistically significant differences were apparent at any time period between group C and group D (Figs. 3, 4).
Comparison of wound closures among the four groups at 7 days after grafting. Groups A and B demonstrated significant improvement in wound healing compared with groups C and D (*P < 0.05 for group A or B compared with group C or D; † P > 0.05 for group A compared with group B; ‡ P > 0.05 for group C compared with group D)
Histological assessment
Histological examination of wounds in group A at 25 days after grafting revealed well-recovered epidermal and dermal layers. The epidermis was thin and showed mature differentiation, and the dermal layer showed thick collagen bundle deposition and well-recovered skin appendages at this time point. Group B showed similar histological features to that of group A, but multifocal fibrosis was noted in the dermal layer; this was not seen in group A. In contrast to groups A and B, groups C and D demonstrated a reduced amount of epidermis and dermis recovery. Group C showed slightly thickened epidermis with immature epithelial cells, and increased fibroblast proliferation was seen in the dermal layer. Moreover, the inflammatory reaction was mild and a slightly increased vascularity was noted. Group D also revealed a focally thickened epidermis and diffuse fibrotic changes in the dermis; increased fibroblast proliferation with capillary formation and mild inflammation were also noted. Skin appendages were partially recovered in both groups C and D (Fig. 5).
Comparison of sections stained with hematoxylin and eosin among the four groups at 25 days after grafting. a–d Groups A and B showed thin epidermis with mature differentiation (black arrows) and collagen bundle deposition (stars) with recovered skin appendages (open arrows) in the dermal layer. e–h Unlike groups A and B, groups C and D showed thickened epidermis with immature epithelial cells (arrowhead) and increased fibroblast proliferation with only partially recovered skin appendages in the dermal layer (boxed areas). ×40 (a, c, e, g), ×100 (b, d, f, h)
Discussion
HAM, a thin semi-transparent tissue forming the innermost layer of the fetal membrane (van Herendael et al. 1978), is composed of three major layers: a single epithelial layer, a thick basement membrane, and an avascular mesenchyme layer (Benirschke and Kaufman 2000). The basement membrane consists of a full complement of collagen types IV and V and laminin (Modesti et al. 1989). As laminin is one of the main components of the amniotic basement membrane, it contributes critically to cell differentiation, cell shape and movement, the maintenance of tissue function, and the promotion of tissue survival (Akashi et al. 1999). According to a previous report detailing the constituents of HAM, the specialized arrangement of the intracellular cytoskeletal filaments of the epithelial layer (such as actin, α-actinin, spectrin, ezrin, cytokeratins, vimentin, and desmoplakin) has a role in the structural integrity and modulation of cell shape and of junctional permeability (Wolf et al. 1991). Because of these properties, HAM has been used clinically to promote epithelialization for the treatment of burns and skin ulcers, as a dressing for wounds or skin grafts (Prasad et al. 1986; Subrahmanyam 1995; Trelford and Trelford-Sauder 1979), and for ocular surface reconstitution. It has been considered a suitable tissue for stem-cell culture and allotransplantation based on its anti-inflammatory effects, low immunogenicity, non-tumorigenicity, and the few ethical problems involved with its use (Lwebuga-Mukasa et al. 1984; Toda et al. 2007; van der Linden et al. 1996).
Allologous HAM has been used in a number of surgical procedures to cure disease or replace damaged tissue and as a dressing to cover chronic wounds of the skin, cornea, and conjunctiva (Devine et al. 2001; Dinicola et al. 2002; McIntosh and Bartholomew 2000). Despite the beneficial properties of amniotic membrane, such as its immune privilege, antimicrobial effect, and ability to reduce pain upon application, it is not always suitable for clinical use (Dinicola et al. 2002; Koizumi et al. 2000; Mohamad 2001; Talmi et al. 1991; Ueta et al. 2002). The immune privilege of amniotic membrane is thought to be site-specific, and it has been used extensively in ophthalmologic applications, such as in the cornea, another site of immune privilege (Devine et al. 2001; Dinicola et al. 2002; McIntosh and Bartholomew 2000). Allologous amniotic membrane has also been used in other applications, but the possibility of immune recognition and subsequent response remains (Wilshaw et al. 2006). As previously reported, in pre-sensitized recipient mice that have undergone repeated amniotic membrane implantation, graft survival rates are markedly decreased compared with unsensitized recipients, suggesting that fresh allologous amniotic membrane expresses immunogenicity (Wang et al. 2006). Nevertheless, at present, amniotic membrane and the cells derived from it seem to be a suitable tissue for transplantation because of their low immunogenic characteristics. In support of this, clinical signs of acute rejection have not been observed when amniotic membrane has been transplanted into volunteers (Akle et al. 1981). The expression level of the major histocompatibility complex class I antigens is still controversial. Although human leukocyte antigens A, B, C, and DR have not been detected in cultured amniotic epithelium (Adinolfi et al. 1982), the presence of class I antigen has been documented in almost all cells of the amniotic membrane (Hammer et al. 1997; Kubo et al. 2001). In contrast, the class II antigen is expressed only in some fibroblasts within HAM (Kubo et al. 2001). However, in clinical applications, the risk of immunogenicity or rejection should not be ignored and might be influenced by the transplantation site or repeated amniotic membrane transplantation from the same donor.
MSCs are multipotent cells that can differentiate into a variety of cell types (including osteoblasts, chondrocytes, myocytes, adipocytes, and beta-pancreatic islets cells) by the action of various members of the transforming growth factor-β family (Deng et al. 2005; Fathke et al. 2004; Lee et al. 2005; Nakagawa et al. 2005; Orlic et al. 2001; Ortiz-Urda et al. 2003; Pittenger et al. 1999; Sheridan and Tompkins 1990). Preliminary clinical results indicate that autografting with MSCs might improve heart function after myocardial infarction (Korbling et al. 2002; Mangi et al. 2003; Okamoto et al. 2002; Satoh et al. 2004; Stamm et al. 2003). Although the process of plasticity or transdifferentiation of stem cells is poorly understood, it remains a focus of study in the fields of developmental biology, cell biology, and stem-cell biology. MSCs are currently thought to be a source of cells for regenerative medicine. Nevertheless, despite their therapeutic potential, stem cells present a number of challenges associated with their clinical application. Adult bone marrow MSCs might serve as a resource to provide skin progenitor cells. However, the microenvironment might play a decisive role for the induction of cutaneous appendage differentiation of MSCs (Badiavas et al. 2003; Kataoka et al. 2003).
In cases of allologous stem cell transplantation, the donors and recipients are fully immunologically unrelated. This gives rise to the issue of possible adverse immune reaction and possible rejection of allogenic MSCs by the recipients. The results of previous studies suggest that MSCs might be immune-privileged cells (Bartholomew et al. 2002; Dinicola et al. 2002). The surface characteristics of MSCs are believed to allow them to avoid rejection. MSCs have been shown not express MSC class-II molecules or co-stimulatory molecules (B7 and CD40), which are necessary for the full activation of the T cells responsible for transplant rejection (Devine et al. 2001; McIntosh and Bartholomew 2000). Previous in vitro immunological studies have established that MSCs do not provoke immune reaction if combined with allologous lymphocytic cells (Bartholomew et al. 2002; Dinicola et al. 2002).
In this study, no histopathologic features of graft rejection have been observed in any of the groups, and our results suggest that the most rapid wound healing, as determined by measurement of wound size and histological pathology, occurs in groups A and B rather than in groups C and D. Moreover, no significant difference has been found between the autologous and allologous graft groups. Any differences in wound healing between autologous and allologous transplantation in this study are thus unclear, and the use of allologous stem cells for human transplantation remains difficult. However, autologous MSCs loaded on the grafted HAM might indeed be able to facilitate wound healing and differentiate of epithelium at wound sites, as observed in this study. Yan et al. (2004) have reported that the graft of HAM loaded with stem cells plays an effective role in promoting high quality wound healing. Fu et al. (2006) have further reported that autologous MSCs can enhance wound-healing quality and generate de novo intact skin, resulting in excellent skin regeneration after full-thickness injury. This suggests that HAM and the autologous MSCs used in this experiment could be approved for clinical use and may accelerate wound healing in humans.
This study has shown that HAM loaded with MSCs improves wound healing, whereas HAM injected with MSCs offer poorer improvement in rabbits, but with uncertain stem cell function. Further studies are needed to investigate the mechanism of action of these stem cells, including their terminal cellular differentiation pattern and the potential for plasticity of these stem cells in a non-hematopoietic tissue.
In conclusion, the graft of HAM loaded with MSC plays an effective role in the healing of skin defects in rabbits. Furthermore, no significant differences have been found in wound healing between autologous and allologous MSC transplants.
References
Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, et al (1982) Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature 295:325–327
Akashi T, Miyagi T, Ando N, Suzuki Y, Nemoto T, Eishi Y, et al (1999) Synthesis of basement membrane by gastrointestinal cancer cell lines. J Pathol 187:223–228
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I (1981) Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet II:1003–1005
Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P (2003) Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 196:245–250
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, Mcintosh K, Patil S, et al (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30:42–48
Benirschke K, Kaufman P (2000) Pathology of the human placenta. Springer, New York, pp 273–281
Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, et al (2005) Engrafted bone marrow-derived FIK-11 mesenchymal stem cells regenerate skin tissue. Tissue Eng 11:110–119
Devine SM, Peter S, Martin BJ, Barry F, Mcintosh KR (2001) Mesenchymal stem cells: stealth and suppression. Cancer J 2:76–82
Dinicola M, Carlo-Stella C, Magni M, Milanesi IM, Longoni PD, Matteucci P, et al (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843
Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al (2004) Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 22:821–822
Fu X, Fang L, Li X, Cheng B, Sheng Z (2006) Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen 14:325–335
Hammer A, Hutter H, Blaschitz A, Mahnert W, Hartmann M, Uchanska-Ziegler B, et al (1997) Amnion epithelial cells, in contrast to trophoblast cells, express all classical HLA class I molecules together with HLA-G. Am J Reprod Immunol 37:161–171
Herendael B van, Oberti C, Brosens I (1978) Microanatomy of the human amniotic membranes. A light microscopic, transmission, and scanning electron microscopic study. Am J Obstet Gynecol 131:872–880
Kataoka K, Medina RJ, Kageyama T, Miyazaki M, Yoshino T, Makino T, et al (2003) Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol 163:1227–1231
Kim CH, Kim SS, Sohn SK, Kim DH, Song CG, Kim HJ (2008) The effect of human amniotic membrane, epidermal cells and marrow mesenchymal stem cells in healing a skin defect. J Korean Orthop Assoc 43:276–286
Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S (2000) Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res 20:173–177
Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, et al (2002) Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 346:738–746
Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42:1539–1546
Lee JH, Kosinski PA, Kemp DM (2005) Contribution of human bone marrow stem cells to the individual skeletal myotubes followed by myogenic gene activation. Exp Cell Res 307:174–182
Linden PJ van der, Groetj AF de, Dunselman GA (1996) Endometrial cell adhesion in an in vitro model using intact amniotic membranes. Fertil Steril 65:76–80
Lwebuga-Mukasa JS, Thulin G, Madri JA, Barrett C, Warshaw JB (1984) An acellular human amnionic membrane model for in vitro culture of type II pneumocytes: the role of the basement membrane in cell morphology and function. J Cell Physiol 121:215–225
Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al (2003) Mesenchymal stem cells modified with Akt provent remodeling and restore preference of infarcted hearts. Nat Med 9:1195–1201
McIntosh K, Bartholomew A (2000) Stromal cell modulation of the immune system. A potential role for mesenchymal stem cells. Graft 3:324–328
Modesti A, Scarpa S, D’Orazi G, Simonelli L, Caramia FG (1989) Localization of type IV and V collagens in the stroma of human amnion. Prog Clin Biol Res 296:459–463
Mohamad H (2001) Anatomy and embryology of human placenta. Amnion and chorion. World Scientific, London
Nakagawa H, Akita S, Fukui M, Fujii T, Akino K (2005) Human mesenchymal stem cells successfully improve skin-substitute wound healing. Cutan Biol 153:2–36
Okamoto R, Yajima T, Yamazki M, Kanai T, Mukai M, Okamoto S, et al (2002) Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med 8:1011–1017
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410:701–705
Ortiz-Urda S, Lin Q, Green CL, Keene DR, Marinkovich MP, Khavari PA (2003) Injection of genetically engineered fibroblasts corrects regenerated human epidermolysis bullosa skin tissue. J Clin Invest 111:251–255
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Prasad J, Feller I, Thomson P (1986) Use of amnion for the treatment of Stevens-Johnson syndrome. J Trauma 26:945–946
Satoh H, Kishi K, Tanaka T, Kubota Y, Nakajima T, Akasaka Y, et al (2004) Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transplant 13:405–412
Sheridan RL, Tompkins RG (1990) Skin substitutes in burns. Burns 25:97–103
Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al (2003) Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 361:45–46
Subrahmanyam M (1995) Amniotic membrane as a cover for microskin grafts. Br J Plast Surg 48:477–478
Suzuki S, Matsuda K, Isshiki N, Tamada Y, Ikada Y (1990) Experimental study of a newly developed bilayer artificial skin. Biomaterials 11:356–360
Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y (1991) Antibacterial properties of human amniotic membranes. Placenta 12:285–288
Toda A, Okabe M, Yoshida T, Nikaido T (2007) The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 105:215–228
Trelford J, Trelford-Sauder M (1979) The amnion in surgery, past and present. Am J Obstet Gynecol 134:833–845
Ueta M, Kweon MN, Sano Y, Sotozono C, Yamada J, Koizumi N, et al (2002) Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol 129:464–470
Wang M, Yoshida A, Kawashima H, Ishizaki M, Takahashi H, Hori J (2006) Immunogenicity and antigenicity of allogeneic amniotic epithelial transplants grafted to the cornea, conjunctiva, and anterior chamber. Invest Ophthalmol Vis Sci 47:1522–1532
Wilshaw SP, Kearney JN, Fisher J, Ingham E (2006) Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng 12:2117–2129
Wolf HJ, Schmidt W, Drenckhahn D (1991) Immunocytochemical analysis of the cytoskeleton of the human amniotic epithelium. Cell Tissue Res 266:385–389
Yan G, Su Y, Ai G (2004) Study on human amniotic membrane loaded with marrow mesenchymal stem cells and epidermis cells in promoting healing of wound combined with radiation injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 18:497–501
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by research funds from Dong-A University.
Rights and permissions
About this article
Cite this article
Kim, S.S., Song, C.K., Shon, S.K. et al. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res 336, 59–66 (2009). https://doi.org/10.1007/s00441-009-0766-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0766-1