Abstract
The human amniotic membrane (hAM) has been successfully used as a natural carrier containing amniotic mesenchymal stromal cells, epithelial cells and growth factors. It has a little or no immunogenicity, and possesses useful anti-microbial, anti-inflammatory, anti-fibrotic and analgesic properties. It has been used for many years in several indications for soft tissue repair. We previously reported that hAM represents a natural and preformed sheet containing highly potent stem cells, and could thus be used for bone repair. Indeed, native hAM possesses pre-osteoblastic potential that can easily be stimulated, even as far as mineralization, by means of in vitro osteogenic culture. However, cell culture induces damage to the tissue, as well as to cell phenotype and function. The aim of this study was to evaluate new bone formation by fresh and in vitro osteodifferentiated hAM, alone or associated with an additional scaffold presenting osteoinductive properties. Moreover, we also aimed to determine the effect of in vitro hAM pre-osteodifferentiation on its in vivo biocompatibility/tissue degradation. Results showed that neither fresh nor osteodifferentiated hAM induced ectopic bone formation, whether or not it was associated with the osteoinductive scaffold. Secondly, fresh and osteodifferentiated hAM presented similar in vivo tissue degradation, suggesting that in vitro hAM pre-osteodifferentiation did not influence its in vivo biocompatibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human amniotic membrane (hAM) is the innermost layer of the foetal membrane, and is a thin, highly flexible, translucent, and semipermeable membrane possessing interesting mechanical properties (Dua et al. 2004). Several beneficial properties of hAM have been described, including anti-inflammatory, anti-scarring, anti-fibrotic and pain-reducing effects (Mamede et al. 2012). Furthermore, no graft rejection has been observed after hAM transplantation in humans (Akle et al. 1981) or in animals (Kubo et al. 2001). hAM is routinely used in ophthalmology (Dua et al. 2004) and its benefits in oral, maxillofacial and orthopaedic surgery have been clearly highlighted (Fairbairn et al. 2014; Gindraux et al. 2013).
In bone repair, tissue engineering (TE) strategies usually require isolated cells combined with suitable carrier materials (Gindraux et al. 2010). hAM already possesses the relevant properties for TE since it represents a natural scaffold containing highly potent stem cells and growth factors that would probably improve current surgical approaches for the repair of large bone defects and bone non-union (Obert et al. 2009; Zappaterra et al. 2011; Zwetyenga et al. 2012). Proof of concept of hAM use for bone repair is supported by several observations. Lindenmair et al. reported successful osteodifferentiation of intact hAM in in vitro osteogenic conditions (Lindenmair et al. 2010), and the strategy was subsequently patented (Eibl and Redl 2011). Mohr et al. described the generation of an osteogenic composite graft using human amniochoronic membrane as a scaffold, which was able to improve osteogenic differentiation of chorionic membrane-derived cells (Mohr et al. 2010). Recently, Starecki et al. grafted bone autograft with the NuCel amniotic tissue preparation in an animal model of a critical size bone defect, and reported robust bone formation and complete bridging of the defect gap compared to control bone graft alone (Starecki et al. 2014). Finally, abundant literature exists on the in vitro osteogenic differentiation potential of amniotic epithelial cells (hAEC) and amniotic mesenchymal stromal cells (hAMSC) (Parolini et al. 2008), and several papers specify their in vivo osteogeny in ectopic or orthotopic models (Kmiecik et al. 2014; Si et al. 2015).
In previous studies, we summarized Lindenmair’s findings (Lindenmair et al. 2010), and specified that native hAM exhibited pre-osteoblastic potential. In addition, we reported that in vitro osteodifferentiation of hAM by culture induced a modification of tissue structure, cell phenotype and cell function (Gualdi et al. submitted; Laurent et al. 2014). Moreover, in these osteogenic conditions, we reported that hAEC presented a mesenchymal phenotype with (surprisingly) osteocyte function, associated with organic hydroxyapatite synthesis, focusing osteogenic potential mainly in this epithelial layer. Thus, we concluded that pre-osteodifferentiation of f-hAM by osteogenic culture does not appear to be necessary for its use in bone repair, and the next step consisted in comparing these in vitro osteogenic potential results with in vivo experiments.
In this context, the present work aimed to evaluate the ability of intact hAM to induce ectopic bone formation, using a common animal model in a subcutaneous site. For this purpose, we compared the bone formation capacity of fresh and in vitro osteodifferentiated hAM, associated or not with an additional scaffold, namely a bone substitute with demonstrated osteoinductive properties (Arinzeh et al. 2005; Miramond et al. 2014; Trojani et al. 2006). Similarly, we used this ectopic model to determine whether in vitro hAM pre-osteodifferentiation, showed in previous works as impacting on tissue structure as well as cell phenotype and function (Gualdi et al. submitted; Laurent et al. 2014), would also induce changes in in vivo tissue degradation and inflammation/biocompatibility.
Materials and methods
hAM collection and in vitro osteodifferentiation
hAM was collected by the local tissue bank from consenting healthy mothers (tested seronegative for HIV, cytomegalovirus, Toxoplasma gondii, hepatitis B and C, and syphilis) during routine Caesarean section births. Native hAM, hereafter called “fresh hAM” (f-hAM), was peeled off the chorionic membrane by blunt dissection. As previously described (Gualdi et al. submitted; Laurent et al. 2014), pieces of 5 cm in diameter were cut and then cultured in Petri dishes for 3 weeks in either: (a) osteogenic medium (OM) adapted from Pittenger et al. (Pittenger et al. 1999) (called “pOM”) and composed of α-minimal essential medium (α-MEM, Gibco, Grand Island, NY, USA), 10% foetal bovine serum (FBS) (Hyclone, Logan, UT, USA), 1% antibiotics, penicillin–streptomycin (PS) supplemented by 2.16 mg/ml β-glycerophosphate (Sigma-Aldrich, Saint Louis, MO, USA), 50 µg/ml ascorbic acid (Sigma-Aldrich, Saint Louis, MO, USA) and 20 µg/ml dexamethasone (Sigma-Aldrich, Saint Louis, MO, USA); OR (b) in commercial OM (StemPro Osteogenesis Differentiation Kit, Gibco, Grand Island, NE, USA) (called “cOM”); OR (c) or in control medium (CM) composed of α-MEM, FBS and 1% antibiotics, PS. We named the culture of intact hAM in these media respectively: OM-hAM (pOM-hAM and cOM-hAM) and CM-hAM, and the culture of hAM in CM constituted a negative control of hAM osteodifferentiation. Six intact hAM were investigated and for each hAM, five patches were used per condition (pOM, cOM and CM).
Histological and immunolabelling controls of hAM in vitro osteodifferentiation
Controls were performed on intact f-hAM, OM-hAM (pOM-hAM and cOM-hAM) and CM-hAM placed on sterile discs of nitrocellulose (Sartorius, Stedim Biotech GmbH, Goettingen, Germany), fixed in 4% formalin, embedded in paraffin and 5 µm sectioned using a microtome. Tissue sections were stained by von Kossa and Alizarin red staining to highlight mineralization. Immunohistochemistry was carried out with rabbit polyclonal anti-bovine type I collagen (Novotec, 20121) and anti-human osteocalcin (AbDSerotec, sc-30044) and mouse monoclonal anti-human alkaline phosphatase (ALP) (Santa Cruz Biotechnology Inc, 0300-0430). After dewaxing, antigenic site retrieval was carried out using hyaluronidase 0.5% solution (Sigma, H3506) for matricial labelling or heat-pretreatment in buffer citrate pH 6 for cellular labelling. After having incubated sections in the respective antibody solution overnight at 4 °C, endogenous activity was blocked with hydrogen peroxide. After incubation with peroxydase conjugates antibody Rabbit/Mouse HRP Envision (Dako, K4002/K4000), antigen–antibody complexes were revealed by reaction with tetrahydrochloride diaminobenzidine (Dako, K3468) and sections were slightly counterstained with Mayer’s hematoxylin. Negative controls were carried out with phosphate buffered saline buffer (working as a first antibody). Microscopic observations were done by light microscope, which is connected to digital camera system controlled by image acquisition and analysis software (Leica Microsystems).
Subcutaneous implantation
Experimentation was approved by the local ethics committee (CEBEA, Franche-Comte, France). Female Balc/cJ mice (Janvier Laboratories, Genest Saint Isle, France) from 6 to 10 weeks old were used. Animals were placed under general anaesthesia with intraperitoneal injection of 0.1 mg/g ketamine (Clorkétam 1000, Vétoquinol) and 0.01 mg/g xylazine (Rompum 2%, Bayer). Surgical implantations of hAM [f-hAM (n = 3), OM-hAM (n = 3) and CM-hAM (n = 3)] were performed in subcutaneous pockets on the caudal part of the back. Two pieces of tissue of approximately 250 × 250 × 200 mm were grafted (one on the right and one on the left) on each mouse. Positive control was performed with human skin. Four mice were used per condition (total = 60 mice) and one mouse was sacrificed after each of 1, 2, 4 and 8 weeks of implantation. Grafts and adjacent tissues were explanted to perform histological analyses.
In a parallel study, an additional scaffold, namely a synthetic biphasic calcium phosphate bone substitute [MBCP, Biomatlante, France; for reference, see Miramond et al. (2014)] was associated with hAM grafting. To this end, hAM was wrapped just before implantation around one granule of MBCP (5 × 5 × 2 mm = 0.05 cm3) using a resorbable suture and grafted (one on the right and one on the left).
Histological and immunolabelling analysis of grafts
Bone formation was highlighted by Masson’s trichrome staining according to Goldner’s technique. Microscopic observations were obtained by light microscope-digital camera system (Leica, DM2000-DFC420C) and photographs were analysed by image edition software (Photoshop CS6).
Results
hAM in vitro osteogenic potential
Figure 1a–t reports on histological staining and immunolabelling of OM-hAM, CM-hAM and f-hAM. Von Kossa and Alizarin red staining were positive for osteogenic conditions (Fig. 1a, b, e, f), with better potential observed with pOM (Fig. 1a, e), but were negative for CM-hAM (Fig. 1c, g) and f-hAM (Fig. 1d, h). Immunolabelling for collagen type I, osteocalcin and ALP were predominantly localized in the hAEC layer in f-hAM (Fig. 1i, p, t) and showed a decreasing trend across the culture conditions, from CM (Fig. 1k, o, s) to pOM (Fig. 1i, m, q) to cOM (Fig. 1j, n, r).
Histological staining and immunolabelling of OM-hAM cultured in pOM (a, e, i, m, q) and cOM (b, f, j, n, r), CM-hAM (c, g, k, o, s) and f-hAM (d, h, l, p, t). a–d Von Kossa staining. e–h Alizarin red staining. i–l Anti-human type I collagen. m–p Anti-human osteocalcin. q–t Anti-human alkaline phosphatase (ALP). Scale bar 50 µm.
Adapted from Gualdi et al. (submitted)
In vivo implantation
Following 8-weeks implantation, all samples could be located. For all timepoints, explanted f-hAM and CM-hAM samples were pliable to the touch, whereas OM-hAM were slightly rigid. One of the pOM-hAM samples presented sporadic white calcifications. No other visual difference could be observed.
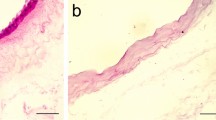
Masson’s trichrome staining (Fig. 2a–p) did not show any bone formation in any of the tested conditions over time (from 1 to 8 weeks): f-hAM (Fig. 2a, e, i, m), or cultured in CM (Fig. 2b, f, j, n) or in both OM (only pOM is represented: Fig. 2c, g, k, o).
Masson’s trichrome staining of subcutaneous grafted f-hAM (a, e, i, m), CM-hAM (b, f, j, n) and cOM-hAM (c, g, k, o) and human skin (d, h, l, p) in Balc/cJ mice after 1 week of graft (a–d), 2 weeks (e–h), 4 weeks (i–l) and 8 weeks (m–p). Fibrocellular tissue (*fc) can be seen after 1 week which infiltrates the grafts (*gft) after 2 weeks. In contrast, whereas human skin dermis (*D) and epidermis (*E) could no longer be seen after 8 weeks (p), the integrity of grafted hAM was better preserved (m–o). Scale bar 200 µm
One week after implantation, all the hAM grafts were surrounded by granulation tissue, specific to a foreign body reaction, which was mostly filled with spindle-shaped cells (fibroblasts and myofibroblasts) and macrophages. The acute immune reaction was very limited, and only a few granulocytes and lymphocytes could be seen in this fibrocellular peripheral tissue (noted “fc” in Fig. 2). No cell infiltrated the grafts. At 2 weeks after implantation, the immune reaction remained stable for all types of implant (Fig. 2e–h), with a moderate foreign body reaction and reduced acute immune reaction. A few cells infiltrated the grafts, and limited damage of the tissue graft (noted “gft” in Fig. 2) was observed. A shift in the tissue reaction was noted at 4 weeks after implantation: in the control graft of human skin (Fig. 2l), the macrophagic reaction significantly increased (many histiocytes and giant cells), associated with substantial loosening of the dermis (noted “D” in Fig. 2), significant cell necrosis and dissociation of the epidermis (noted “E” in Fig. 2). On the contrary, in hAM grafts (Fig. 2i–k), the macrophagic reaction remained quite stable and the tissue damage was moderate and highly individual-dependent. Acute inflammatory response decreased to become negative in f-hAM, CM-hAM and pOM-hAM, but, for human skin graft, remained comparable to the 2-week faint reaction. At 8 weeks after implantation, cell infiltration in f-hAM (Fig. 2m) and CM-hAM (Fig. 2n) grafts increased, as well as epithelial cell necrosis and destruction of the extracellular matrix. Both hAM cultured in OM (only pOM-hAM is represented: Fig. 2o) showed a similar level of resorption but in a different manner, namely the integrity of the epithelium remained well preserved or even fragmented, but the extracellular matrix was fully resorbed. No difference could be observed between the two OM-hAM (cultured in pOM and cOM). Resorption of the human skin graft was complete after 8 weeks (Fig. 2p), only cell and tissue fragments could be noted, associated with the noticeable presence of macrophages and giant cells. Granulation tissue still surrounded all the graft locations. No acute inflammatory signs were noted at this stage (Fig. 2m–o).
As shown in Fig. 3a–f, additional bone substitute grafted with f-hAM, CM-hAM or OM-hAM did not improve bone formation over time (from 1 to 8 weeks). Moreover, no amniotic cell migration could be observed on MBCP. As there was no significant difference between times and conditions, only MBCP (Fig. 3a, b) + f-hAM (Fig. 3c, d) or pOM-hAM (Fig. 3e, f) conditions after respectively 8, 2 and 8 weeks of implantation are shown in Fig. 3.
Discussion
In a previous work, we first aimed to evaluate the in vitro osteogenic potential of intact hAM, with a view to using it for bone repair. We reported the pre-osteoblastic potential of the hAEC layer of f-hAM, and the complete osteodifferentiation capacity of native tissue when cultured in OM (Gualdi et al. submitted). The present work consisted in comparing these in vitro results regarding osteogenic potential with the ability to induce bone formation in a common mice ectopic model. When we implanted fresh or osteodifferentiated hAM (whose osteodifferentiation was controlled, Fig. 1), neither osteoblast nor mineral deposition was observed (Fig. 2). Moreover, contrary to the in vitro results showing greater osteogenic potential with pOM (Fig. 1) (Gualdi et al. submitted), no difference was observed subcutaneously between the two OM (only pOM results are presented in Fig. 2). The association of hAM with an additional scaffold presenting osteoinductive properties also failed to induce ectopic bone formation (Fig. 3) after 8 weeks of implantation, the usual time required to observe bone formation in an ectopic model (Kuznetsov et al. 1997; Piersanti et al. 2006; Trojani et al. 2006). There are two potential explanations for our results. Firstly, the osteoinductive capacity of hAM was not sufficient to allow ectopic bone formation. Indeed, in the literature, in immunocompetent mice like Balb/c, ectopic bone formation is often due to the addition of growth factors [such as bone morphogenetic protein (BMP)] with osteoinductive properties (Chung et al. 2013; Kishimoto et al. 2002; Maeda et al. 2004). Thus, in our study, we might have expected the use of osteoinductive MBCP (Arinzeh et al. 2005; Trojani et al. 2006) to improve bone formation potential. However, we actually observed an encapsulation of the biomaterial by hAM, and no amniotic cells migrated on the scaffold. Ideally, the hAM should have been slightly torn or slit, to improve contact between the MBCP and the tissue. Secondly, the Balb/c strain may not have been the most appropriate model. Indeed, it has been reported that hAM transplantation in animals does not involve graft rejection (Kubo et al. 2001). Based on previous works performed in Balb/c mice (Chung et al. 2013), we selected this strain instead of the immunocompromised mice usually used for human MSC xenograft (Krebsbach et al. 1997; Kuznetsov et al. 1997; Piersanti et al. 2006) and which might have been more suitable for ectopic bone formation.
The second objective of the study was to use this animal model, as in previous biocompatibility studies (Gholipourmalekabadi et al. 2015; Kamarul et al. 2014; Wilshaw et al. 2008), to determine whether the modifications in tissue structure, cell phenotype and function observed after in vitro hAM osteodifferentiation (Gualdi et al. submitted; Laurent et al. 2014) would induce changes in the in vivo biocompatibility/degradation. Results were similar between fresh and osteodifferentiated hAM, and showed no acute inflammatory reaction over the duration of implantation, as compared to the control, i.e. human skin, which presented a limited acute reaction (Fig. 2). Moreover, the hAM resorption process had approximately the same kinetics for all samples, i.e. slow, as compared to human skin. Finally, we noted a slight difference in tissue degradation between non-osteodifferentiated hAM (f-hAM and CM-hAM) and OM-hAM, probably due to a mineralized hAEC layer. We stopped our investigations based on Masson’s trichrome staining observations, completed by hematoxylin, eosin and saffron staining (data not shown), because the degradation of f-hAM and OM-hAM was similar, and evaluation of detailed host response with specific immunolabelling would not have yielded any additional information. We thus concluded that hAM pre-osteodifferentiation does not appear to influence its in vivo biocompatibility. Our results are supported by a convincing work performed in a rat ectopic model reporting good in vivo biocompatibility of f-hAM by complete blood count, clinical chemistry measurements and immunohistochemical analysis (Kamarul et al. 2014).
In conclusion, in the chosen ectopic model (Balb/c strain), neither hAM pre-osteodifferentiation, nor the use of an additional scaffold with osteoinductive properties, induced bone formation. Regarding the results of hAM in vivo tissue degradation, in vitro hAM pre-osteodifferentiation did not influence its in vivo biocompatibility in this model.
References
Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I (1981) Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2:1003–1005
Arinzeh TL, Tran T, McAlary J, Daculsi G (2005) A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 26:3631–3638
Chung EJ, Chien KB, Aguado BA, Shah RN (2013) Osteogenic potential of BMP-2-releasing self-assembled membranes. Tissue Eng Part A 19:2664–2673. doi:10.1089/ten.TEA.2012.0667
Dua HS, Gomes JA, King AJ, Maharajan VS (2004) The amniotic membrane in ophthalmology. Surv Ophthalmol 49:51–77
Eibl J, Redl H (2011) Process for differentiating stem cells of the amniotic membrane. WO2011151043
Fairbairn NG, Randolph MA, Redmond RW (2014) The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg 67:662–675. doi:10.1016/j.bjps.2014.01.031
Gholipourmalekabadi M et al (2015) Development of a cost-effective and simple protocol for decellularization and preservation of human amniotic membrane as a soft tissue replacement and delivery system for bone marrow stromal cells. Adv Healthc Mater 4:918–926. doi:10.1002/adhm.201400704
Gindraux F, Obert L, Laganier L, Barnouin L (2010) Industrial approach in developing an advanced therapy product for bone repair. J Tissue Eng Regener Med 4:194–204
Gindraux F et al (2013) Human amniotic membrane: clinical uses, patents and marketed products. Rec Patents Regener Med 3:193–214
Gualdi T et al (submitted) Need for and consequences of in vitro osteodifferentiation of human intact amniotic membrane with a view to use for bone repair
Kamarul T, Krishnamurithy G, Salih ND, Ibrahim NS, Raghavendran HR, Suhaeb AR, Choon DS (2014) Biocompatibility and toxicity of poly(vinyl alcohol)/N,O-carboxymethyl chitosan scaffold. Sci World J. doi:10.1155/2014/905103
Kishimoto KN, Watanabe Y, Nakamura H, Kokubun S (2002) Ectopic bone formation by electroporatic transfer of bone morphogenetic protein-4 gene. Bone 31:340–347
Kmiecik G, Spoldi V, Silini A, Parolini O (2014) Current view on osteogenic differentiation potential of mesenchymal stromal cells derived from placental tissues. Stem Cell Rev. doi:10.1007/s12015-014-9569-1
Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG (1997) Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63:1059–1069
Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42:1539–1546
Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, Robey PG (1997) Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res 12:1335–1347
Laurent R, Nallet A, Obert L, Nicod L, Gindraux F (2014) Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank 15:267–275. doi:10.1007/s10561-014-9437-x
Lindenmair A et al (2010) Osteogenic differentiation of intact human amniotic membrane. Biomaterials 31:8659–8665
Maeda H, Sano A, Fujioka K (2004) Controlled release of rhBMP-2 from collagen minipellet and the relationship between release profile and ectopic bone formation. Int J Pharm 275:109–122. doi:10.1016/j.ijpharm.2004.01.040
Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF (2012) Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res 349:447–458
Miramond T, Corre P, Borget P, Moreau F, Guicheux J, Daculsi G, Weiss P (2014) Osteoinduction of biphasic calcium phosphate scaffolds in a nude mouse model. J Biomater Appl 29:595–604. doi:10.1177/0885328214537859
Mohr S, Portmann-Lanz CB, Schoeberlein A, Sager R, Surbek DV (2010) Generation of an osteogenic graft from human placenta and placenta-derived mesenchymal stem cells. Reprod Sci 17:1006–1015. doi:10.1177/1933719110377471
Obert L, Lepage D, Gindraux F, Garbuio P (2009) Bone morphogenetic proteins in soft-tissue reconstruction Injury 40(Suppl 3):S17–S20
Parolini O et al (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 26:300–311
Piersanti S et al (2006) Lentiviral transduction of human postnatal skeletal (stromal, mesenchymal) stem cells: in vivo transplantation and gene silencing. Calcif Tissue Int 78:372–384
Pittenger MF et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Si J et al (2015) Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Intl. doi:10.1155/2015/565732
Starecki M, Schwartz JA, Grande DA (2014) Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Adv Orthop Surg. doi:10.1155/2014/572586
Trojani C et al (2006) Ectopic bone formation using an injectable biphasic calcium phosphate/Si-HPMC hydrogel composite loaded with undifferentiated bone marrow stromal cells. Biomaterials 27:3256–3264
Wilshaw SP, Kearney J, Fisher J, Ingham E (2008) Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A 14:463–472
Zappaterra T et al (2011) Induced membrane technique for the reconstruction of bone defects in upper limb. A prospective single center study of nine cases. Chir Main 30:255–263
Zwetyenga N, Fricain JC, De Mones E, Gindraux F (2012) Induced membrane technique in oral and maxillofacial reconstruction. Rev Stomatol Chir Maxillofac 113:231–238. doi:10.1016/j.stomax.2012.05.008
Acknowledgements
The authors thank Fiona Ecarnot (EA3920, University Hospital Besancon, France) for editorial assistance. This work was supported by the Région of Franche-Comté, France and the Foundation of Transplantation (FDTSFV), Saint Apollinaire, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have are no conflict of interest.
Rights and permissions
About this article
Cite this article
Laurent, R., Nallet, A., de Billy, B. et al. Fresh and in vitro osteodifferentiated human amniotic membrane, alone or associated with an additional scaffold, does not induce ectopic bone formation in Balb/c mice. Cell Tissue Bank 18, 17–25 (2017). https://doi.org/10.1007/s10561-016-9605-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-016-9605-2