Abstract
Different biomaterials have been used as biological dressing for wound regeneration. For many decades, human amniotic membrane graft (AM) has been widely applied for treating acute and chronic wounds. It has minimal toxicity and immunogenicity, supports mesenchymal cell in-growth, improves epidermal cell adherence and proliferation, and finally is inexpensive and readily available. Enrichment of tissue grafts with the stem cells is a new approach to improve their regenerative effects. This animal study aimed at investigating feasibility, safety, and efficacy of tissue-engineered dressings composed of AM and two different types of mesenchymal stem cells (MSCs) in the excisional wound model in rats. Human adipose–derived MSCs (ADMSCs) and placenta-derived MSCs (PLMSCs) were manufactured from the donated adipose and placenta tissues respectively. After cell characterization, MSCs were seeded on acellular AM (AAM) and cultivated for 5 days. Excisional wound model was developed in 24 male Wistar rats that were randomly classified into four groups including control, AAM, ADMSCs + AAM, and PLMSCs + AAM (n = 6 in each group). Tissue-engineered constructs were applied, and photographs were taken on days 0, 7, and 14 for observing the wound healing rates. In days 7 and 14 post-treatment, three rats from each group were euthanized, and wound biopsies were harvested, and histopathologic studies were conducted. The results of wound closure rate, re-epithelialization, angiogenesis, and collagen remodeling demonstrated that in comparison with the control groups, the MSC-seeded AAMs had superior regenerative effects in excisional wound animal model. Between MSCs group, the PLMSCs showed better healing effect. Our data suggested that seeding of MSCs on AAM can boosts its regenerative effects in wound treatment. We also found that PLMSCs had superior regenerative effects to ADMSc in the rat model of excisional wound.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound, which is defined as a pathological disruption in normal architecture of the skin, always drowns global attention because of its clinical and economic burden imposes on healthcare systems all over the world. Annually, millions of people are involved with various kinds of wounds that are extremely increasing and affect their quality of life. Despite several efforts have been made for wound management, no definitive cure is identified yet [1]. Accordingly, finding an efficient novel treatment presents great importance especially for chronic and refractory wounds.
Recently, regenerative medicine (RM) has been introduced as a promising approach for wound treatment, and several preclinical and clinical studies have been conducted in this field with promising results. In this concept, cell-based therapies have been extensively studied [2,3,4]. Stem cells have valuable self-renewal characteristics and could differentiate into multiple cell lineages [5].
The most prevalent stem cell types used for treating a variety of diseases are mesenchymal stem cells (MSCs). Remarkably, they are capable to promote healing in all phases of wound repair. Although MSCs in the normal skin participate in wound repair, it has been shown that using MSCs derived from other sources like bone marrow (BM), adipose tissue, placenta, cord blood, hair follicle, and umbilical cord can be helpful [6, 7]. MSCs produce growth factors, cytokines, and extracellular matrix and promotes the migration of other cells [8]. BM is considered as the most commonly used source for MSC derivation, but there are some limitations to its accessibility and the quantity of sample and the resulting expanded cells [9]. Therefore, some alternative sources have been introduced recently. Adipose tissue is nominated as one of the more accessible and less invasive sources that can be harvested in large quantities during lipoaspiration surgery. Adipose tissue–derived MSCs (ADMSCs) are multipotent stem cells with the least ethical concerns and possess characteristics similar to those derived from BM. A high yield of stem cells with minimal donor morbidity during the harvest is the plus points of the ADMSCs [9,10,11]. Perinatal tissues which are another valuable source of potent MSCs can be used for allogeneic transplantation. They can be harvested in large quantities through a non-invasive procedure from waste products of pregnancy [12]. PLMSCs have been shown to provide a more homogeneous and primitive population with a higher proliferative rate compared with other sources. A greater number of PLMSCs can be obtained in fewer subcultures which is a remarkable characteristic. It can cause a reduction in the risk of cell senescence and the occurrence of the aging phenotype. Actually, cell senescence is an essential parameter that should be taken into account in cell therapy [13]. These allogeneic sources of stem cells allow manufacturing “off-the-shelf” cell-based products to treat more patients with lower costs and easier commercialization. Despite the remarkable characteristics of stem cells, their function can be improved by combination with the tissue-engineered scaffolds.
Tissue-engineered skin substitutes have suggested a new treatment modality for chronic and refractory wounds. Different natural and synthetic biomaterials have been successfully used alone or in combination with stem cells as dermal matrix substitutes [14]. AM is a natural biomaterial that has been widely applied for accelerating the wound healing process. Despite the medical application of AM dates back to the year 600 BC, in 1910, J.S. Davis published the first report of fresh AM transplantation in wound treatment [15]. According to the more recent publications, anti-inflammatory characteristics of the AM are demonstrated for both cellular and acellular form. In inflammatory conditions like wounds, secretion of different factors by amniotic epithelial cells cause inhibitory effects on T and B lymphocyte proliferation and neutrophil and macrophage chemotactic properties. Moreover, AM has been shown to bind the T lymphocytes along with other leukocytes to prevent them from participating in the inflammatory process [16]. AM is a biodegradable scaffold that can facilitate tissue-formation process and stimulate cell attachment, proliferation, and differentiation. It has minimal toxicity and immunogenicity, supports mesenchymal cell in-growth, improves epidermal cell adherence and proliferation, and finally an inexpensive and available source. It has been suggested that the addition of MSCs to AM can improve its regenerative properties [17,18,19,20].
It is worthy to note that majority of wound healing researches have investigated the allogeneic and autologous transplantation of MSCs from more conventional sources like bone marrow [21]. Herein, we studied the xenotransplantation of MSCs derived from two different sources as more available and non-invasive ones. Accordingly, the present study is aimed at a comparative assessment of feasibility, safety, and efficacy of a tissue-engineered product with combination of AM and two different types of MSCs in the excisional wound model in rats.
Materials and methods
Ethical considerations
Animal studies and stem cell experiments were fulfilled according to the codes of ethics, declared by Iran National Committee for Ethics in Biomedical Research (https://ethics.research.ac.ir). Written informed consent was obtained from adipose and placenta tissue donors according to procedures approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran. Because of minimizing animal suffering, we tried to reduce the number of animals used in this research. Furthermore, to facilitate future clinical translation, good manufacturing practice (GMP) compliant protocols were applied for PLMSCs, ADMSCs, and AM manufacturing [22,23,24].
Tissue procurement
All tissues were procured from healthy donors according to our donor’s eligibility criteria. In addition to routine donor screening, at the time of donation, a blood sample was taken for laboratory tests. The presence of HIV, HBV, HCV, CMV, EBV, HTLV, and venereal diseases was assessed by appropriate laboratory tests. Adipose tissue was harvested from residues of lipoaspiration surgery (< 40 years old), and placenta tissue was harvested from full-term (> 38 weeks) caesarian section delivery.
MSC isolation and expansion
Isolation and culture of ADMSCs
ADMSCs were isolated and cultured according to our previously established protocol with some modifications [23]. Briefly, adipose tissues were washed thoroughly with Dulbecco’s phosphate-buffered saline (DPBS; Biowest, France) and then cut into small pieces. Tissue fragments were digested using Collagenase CLSAFA/AF (Worthington, USA) at 37 °C for 60 to 90 min. Tissue digest was washed twice in sterile DPBS, and the stromal vascular fraction (SVF) isolated by centrifugation (400×g for 10 min). After counting and viability assessment, the SVF was seeded into culture flasks containing low-glucose Dulbecco modified Eagle medium (DMEM-LG; Biowest, France), supplemented with 5% human platelet lysate (hPL; PLBioscience, Germany) and were incubated in CO2 incubator (Memmert, Germany) at 37 °C, 5% CO2, and 95% humidity. To remove non-adherent cells, after 48 h of incubation, the culture media were changed. Culture media were refreshed twice per week. At 80 to 90% confluency, the ADMSCs were subcultured using TrypLE express (Invitrogen, USA) as dissociating enzyme. ADMSCs were expanded up to five subcultures and were cryopreserved for future applications.
Isolation and culture of PLMSCs
PLMSCs were manufactured using our previously established protocol with some modifications [24]. Briefly, the fetal membrane was separated from the placenta and chorionic plate was cut to expose the placenta tissue. Pieces of chorionic villi and its surrounding tissue were harvested and thoroughly washed with DPBS (Biowest, France) to eliminate blood, then cut into small fragments and digested by Collagenase CLSAFA/AF (Worthington, USA) at 37 °C for 90 min. Tissue digest was diluted with cold DPBS and passed through a 100-µm cell strainer (Corning, USA). Centrifugation was performed to precipitate the isolated cells (300 g for 10 min). To enrich the mononuclear cell (MNC) fraction, the cell pellet was layered on Ficoll Paque Plus (GE Healthcare, USA) and centrifuged at 500×g for 20 min. MNCs were seeded into culture flasks containing culture media (DMEM + 5% hPL) and incubated in CO2 incubator at 37 °C, 5% CO2, and 95% humidity. After 72 h of incubation, non-adherent cells were washed out with changing the culture media. Culture media were changed twice per week and PLMSCs were subcultured at 70 to 80% confluency using TrypLE express (Invitrogen, USA). PLMSCs were expanded up to five subcultures and were cryopreserved for future applications.
Cryopreservation of MSCs
MSCs, at 90% confluency, were collected by enzymatic dissociation, centrifuged (200×g/5 min), resuspended in cryopreservation media (DMEM-LG + 5% hPL + 10% DMSO (WAK-Chemie, Germany)), and aliquoted into 2 ml cryovials (Corning, USA). The cryovials were put in a pre-cooled Mr. Frosty freezing container (Nalgene, Thermo Fisher Scientific, USA) and stored in an ultralow temperature freezer (−80 °C, New Brunswick, USA) overnight. Then, the cryovials were removed from the container and transferred to the vapor phase of a liquid nitrogen tank (Statebourne Cryogenics, UK).
MSC characterization
Multilineage differentiation of MSCs
Osteogenic and adipogenic differentiation were induced by StemPro® Osteogenesis and Adipogenesis differentiation kits (Invitrogen, USA) according to the manufacturer’s protocols. To demonstrate adipogenesis, MSCs were fixed after 14 days, in freshly prepared 4% paraformaldehyde solution (PFA) for 20 min at room temperature. Oil Red O (Sigma, USA) staining was performed to identify lipid deposition in differentiated cells. For osteogenic induction, calcium deposition was examined in the PFA-fixed MSCs after 21 days with Alizarin Red S (Sigma, USA) staining.
CD markers expression
Surface marker expression (CD45, CD34, HLADR, CD105, CD73, and CD90) was evaluated by flow cytometry method (FACS Calibur, BD Biosciences, USA). MSCs at 5th subculture (0.8–1 × 106 cells) were harvested, centrifuged, and re-suspended in 1 ml cold DPBS. The samples were transported on ice to the flow cytometry department of SABA Biomedical Co. (Tehran, Iran). Further procedures were performed based on their protocols.
Preparation of AM scaffold
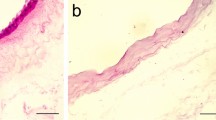
The acellular AM (AAM) was used to providing scaffold for MSCs seeding. AM was dissected from the harvested fetal membranes and washed three times with cold DPBS containing antibiotic-antimycotic solution (Biowest, France). AM processing was done according to our previously published protocol [22]. Briefly, AM was spread over a sterile nitrocellulose sheet (epithelial side up) and cut into 3 × 3 cm pieces. Each piece was transferred to a sterile polypropylene tube containing DMEM and glycerol (50% v/v) and cryopreserved at −80°C for future application. At the time of experiment, cryopreserved AM was thawed and washed three times with cold DPBS. To detach the epithelial cell layer, AM was incubated in 0.05% Trypsin-EDTA (Invitrogen, USA) at 37 °C for 30 min. Then, epithelial cells were removed by cell scraper (SPL, Korea) and multiple washing with DPBS. To neutralize the remaining trypsin, the prepared AAM was transferred to a six-well culture plate containing DMEM + 5% hPL and incubated at 37 °C until the MSCs were ready for seeding. To confirm decellularization process, a control sample was fixed with 4% PFA and stained with hematoxylin.
Tissue-engineered graft preparation
ADMSCs and PLMSCs were thawed rapidly at 37 °C water bath and washed two times with culture media (centrifuged at 200×g for 5 min). The six-well culture plate was removed from the incubator, and AAMs were washed with DPBS. The MSCs were counted and resuspended in fresh culture media at a concentration of 3 × 104 viable cells/ml. Each piece of AAM was seeded with 3 ml of cell suspension and incubated at 37 °C, 5% CO2, and 95% humidity. After 24 h, the culture media were changed, and samples of old media from each group were sent for microbiological studies. At day 5, the prepared tissue-engineered grafts were ready for transplantation. The grafts were packed in sterile polypropylene bottles containing cold PBS. Then, samples were transported on ice and maintained in wet ice temperature condition for maximum of 6 h. Before releasing the grafts, representative samples of each group were stained with hematoxylin and examined under microscope (Nikon, Japan) to confirm MSC adhesion. Furthermore, a representative sample of PLMSCs seeded AM was examined in each step of processing with scanning electron microscopy (SEM). Briefly, the samples (intact AM, AAM, and PLMSCs + AAM) were immersed in Karnovsky’s fixative at 4 °C for 90 min, washed twice in DPBS, dehydrated with increasing concentration of ethanol solution, and sent to the SEM laboratory for further processing.
Animal experimentation
Excisional wound splinting model
Twenty-four male Wistar rats (Pasteur institute, Tehran, Iran; 9–10 weeks old, weighing 180–200 g) were enrolled in this study. They were put in individual cages at controlled temperature and humidity with a 12-h light-dark cycle, and also free availability to food and tap water were provided. Animal anesthesia was performed with an intraperitoneal injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1) that were purchased from Sigma-Aldrich (USA). After anesthesia, animals were randomly classified into four groups, and the excisional wound splinting model was created as previously described [25]. In brief, the hairs of dorsal surface of rats were shaved after anesthetizing. A 10-mm Acu-Punch kit (Acuderm Inc., USA) was used to create a full-thickness excisional skin wounds on the dorsum of each rat. To prevent contraction, a donut-shaped silicone splint was fixed around the wounds with four interrupted sutures (6‐0 Ethilon Nylon Suture).
Tissue-engineered graft transplantation
After the preparation of wound healing model, rats were randomly divided into four groups including silicon sheet dressing as control (n = 6), AAM (n = 6), ADMSCs + AAM (n = 6), and PLMSCs + AAM (n = 6).
Wound healing assessment
Rate of wound contraction
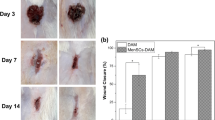
Photographs were taken using a mounted digital camera (COOLPIX B700, Nikon, Japan) on days 0, 7, and 14 post-intervention. Wound closure kinetics were identified using computer software Image-Pro Plus® V.6 (Media Cybernetics Inc., USA). Percentage wound contraction was calculated as previously described [26] and shown as mean percentage of each group (± SD).
Histopathologic studies
Wound biopsies were collected from each group on 7 (n = 3) and 14 days (n = 3) after intervention. Animals were euthanized, and biopsies were harvested and then quickly fixed in 10% neutral-buffered formalin. The fixed tissue samples processed, embedded in paraffin, and sectioned to 5-μm thickness. Finally, sections were stained with hematoxylin and eosin (H&E) and Masson’s trichrome (MT). MT staining was used to identify the progression of collagen synthesis and matrix remodeling. Histopathological changes were examined under light microscope (Olympus, Japan) by independent observer. Epithelialization, collagen synthesis, neovascularization, granulation tissue formation, and inflammatory cell infiltration were evaluated in different groups, comparatively. The number of blood vessels and inflammatory cells (lymphocytes and neutrophils) was counted (For this reason, 5HPF-Magnification ×400 was counted, and the mean result provided). Regarding collagen content and related quantification method, the MT-stained tissue samples from the control and experimental groups were compared at day 14 post-treatment; the color settings in the Image-Pro Plus® V.6 (Media Cybernetics Inc., USA) was maintained between the samples to identify the green (mature collagen type 1-treatment groups) areas. Magnification ×200 was employed for evaluating the samples for this criterion, and the calculation was repeated for six microscopic fields. Finally, the average number of collagen content or collagen density (%) for these fields was then recorded. Other collagen containing tissues (e.g., blood vessels) were not quantified by image processing software (area and percentage) and excluded by automatic color picker based on the color density. Epithelialization was also evaluated semi-quantitatively on 5-point scale including 0 (without new epithelialization), 1 (25%), 2 (50%), 3 (75%), and 4 (100%). The results were confirmed by a comparative analysis of one independent observer blinded to the treatment groups.
Statistical analysis
Kruskal-Wallis analysis was used for comparing all results (SPSS 20.0 software, IBM, USA). Results with P values <0.05 were considered to be statistically significant.
Results
MSCs culture and characterization
PLMSCs and ADMSCs were successfully isolated and expanded in vitro up to 5th subculture. Both MSCs showed typical spindle-shaped fibroblast-like morphology at first subculture (Fig. 1a).
Differentiation capacity
PLMSCs and ADMSCs could be successfully differentiated to adipocytes and osteocytes using appropriate induction media. Figure 1b illustrates the results of Oil Red O and Alizarin Red staining in both MSCs.
Expression of CD markers
Flow cytometry analysis of the MSCs surface markers demonstrated that both PLMSCs and ADMSCs were positive for CD105, CD73, and CD90 and negative for CD45, CD34, and HLA-DR (Fig. 1c).
MSCs seeding on acellular amniotic membrane
The decellularization process of AMs was confirmed by hematoxylin staining. As shown in Fig. 2a, the epithelial cells have been successfully removed which resulted in acellular AM scaffold. PLMSCs and ADMSCs were successfully seeded on AAM and reached to appropriate confluency after 5 days. Figure 2 demonstrates a representative sample of PLMSC-seeded AM in each step of processing stained with hematoxylin (Fig. 2a) and examined with SEM (Fig. 2b).
Wound healing assessment
Rate of wound contraction
Macroscopic changes of wound area in different groups from baseline (day 0) are shown in Fig. 3a. On days 7 and 14, the highest wound contraction percentage was seen in PLMSCs + AAM group and the lowest in control group. Figure 3b illustrates the wound contraction percentage in all study groups at different time intervals. These results were not statically significant (P value > 0.05).
Histopathological analysis
Histopathologic study of the wounds was performed by H&E and TM staining, and representative microscopic images of each group are illustrated in Fig. 4. The histopathological assessment of control group on day 7 post-intervention presented polymorphonuclear inflammatory cell (PMNs) infiltration and granulation tissue formation; however, wound covered by a crusty scab and epidermal layer has not been made. Analysis of the AAM group on day 7 showed a close similarity to the control group, i.e., a crusty scab covered the wound area without the epidermal formation, and the presence of inflammation in the wound area was evident. Both ADMSCs + AAM and PLMSCs + AAM showed less inflammation and higher collagen synthesis than the other groups. On day 14, islets of epithelial cells formed in the control group without forming an epithelial layer. While the thickness of the epithelial layer has not demonstrated significant changes on the day7, all treatment groups (AAM, ADMSCs + AAM, and PLMSCs + AAM) have shown a significant increase on day 14, but no significant alterations in the thickness of the dermis layer. The number of inflammatory cells on day 14 was lower than day 7, and more collagen synthesis was seen. In AAM group, a thin layer of epithelium was formed, and mild inflammation was observed. Histopathological assessments of ADMSCs + AAM revealed epidermal proliferation and forming epidermal layer on 14 days post-intervention. In PLMSCs + AAM, a considerable inflammation reduction was seen in comparison with the others. Moreover, the micrographs of this group demonstrated regeneration of other skin appendages such as sebaceous glands (Fig. 4, black arrow head). This group presented more similarity to normal skin, by the thin epidermis and the appearance of normal rete ridges, normal thickness of skin layers, and the appearance of skin appendages. Table 1 demonstrates the histomorphometric analysis results of epithelialization, angiogenesis, collagen deposition, thickness of epithelial and dermis layers, and number of inflammatory cells in study groups. The lowest epithelialization score was seen in the control group and the highest in PLMSCs + AM-treated group. Collagen fibers were stained blue-green in the MT staining, and the intensity of this color matches the relative amount of deposited collagen fibers which reveals the promotion of collagen synthesis and regeneration. The results demonstrated that among the experimental groups, human PLMSCs + AM group had the greatest collagen synthesis. In contrast, the lowest collagen synthesis and deposition was seen in the control group. New blood vessel formation was more prominent in PLMSCs + AM and ADMSCs + AM than the other groups.
Discussion
Extensive deep dermal and full-thickness skin injuries are important challenges in dermatology and plastic surgery. They are usually involved in severe complications that need appropriate wound closure. However, current treatment modalities like different advanced dressing and skin grafts are accompanied to some difficulties due to the lack of suitable donor sites, poor skin quality, high costs, and scar formation. To facilitate wound healing process, some new regenerative methods such as stem cell transplantation, tissue-engineered skin substitutes, and bioactive dressing have been suggested. Recent studies demonstrated that applying MSCs may have beneficial effects on wound healing process [9, 11, 12, 27]. Wu et al. noted that BM-derived mesenchymal stem cells (BM-MSCs) increase the wound healing process in non-diabetic and diabetic mice by enhancing angiogenesis, re-epithelialization, and cell infiltration [28]. Sasaki et al. suggested that MSC therapy accelerates skin wound healing especially refractory, common therapy-resistant skin ulcers [29]. Majority of cell-based studies have demonstrated that MSCs are safe and efficient cell sources for wound treatment with no need for immunosuppression which make it suitable for allotransplantation [30]. More recent, allogeneic MSCs have been center of attraction as suitable source for off-the-shelf products that are easier to scale up and commercialization [31]. In current study, we applied two sources of human MSCs in a xenotransplantation model without immunosuppression. Tissue engineering is a new approach to boost cell transplantation effects. Combination of scaffold with stem cells improves its viability, proliferation, regenerative effects, and applicability. Tissue-engineered products could be a suitable solution to several kinds of skin defects that are refractory to standard therapies. They produce different cytokines and growth factors which stimulate cell proliferation and collagen synthesis and reduce pain, inflammation, infection, and scaring. These grafts can also serve as a biodegradable scaffold which provides a matrix to induce cell attachment and proliferation and protects wound bed against infection. Furthermore, these scaffolds could be a good carrier for delivery and maintenance of cells in the site of transplantation [11, 32, 33]. Fresh and preserved AM has been applied for decades as a biological wound dressing. According to the previous studies, AM effects on wound healing are attributed to its anti-inflammatory, angiogenic, and antifibrotic properties [34,35,36,37]. AM can synthesize and release growth factors and cytokines such as transforming growth factors (TGFα, TGFβ), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), hepatic growth factor (HGF), keratinocyte growth factor (KGF), interleukin-4 (IL-4), IL-6, IL-8, β-defensins, prostaglandins, and natural inhibitors of matrix metalloproteases that all are involved in different stages of wound healing. Recently, some studies have used AAM as a natural scaffold for producing tissue-engineered grafts. Different cell types such as fibroblasts, mesenchymal stem cells, limbal stem cells, hair follicle stem cells, and keratinocytes have been cultured on AAM scaffold [38,39,40,41,42]. Fatemi et al. demonstrated that transplantation of ADMSC-seeded AAM in mouse model of third degree burn can accelerate wound healing and improve fibroplasia, epithelialization, and neovascularization of wounds [43]. In another study by Mahmoudi-Rad et al., AAM was applied as a substrate for human fibroblast culture and then transplanted in rat model of full-thickness wound. They showed that transplantation of fibroblast + AAM can significantly improve wound contraction, epithelialization, and matrix remodeling compared with the control group [44]. A study conducted by Chehelcheraghi et al. demonstrated that in comparison with the control group BM, MSC-seeded AAM transplantation significantly improved survival of random skin flap in rats [45]. The results of our experiment were similar to the aforementioned studies. The AAM could strongly support attachment and expansion of ADMSCs and PLMSCs. The resulting constructs considerably improved healing process in rat model of excisional wound. In histopathology assessment, neovascularization, re-epithelialization, and collagen remodeling were significantly better than the AAM and control group. In the current study, we compared the effect of two different sources of MSCs on wound healing and found that PLMSCs + AAM yielded the best results including the normal thickness of the epidermal layer and regeneration of skin appendages. It can be attributed to the fetal origin of PLMSCs that causes its phenotypic plasticity, immunomodulatory, and angiogenic properties [46]. The unique angiogenic and immunomodulatory effect of PLMSCs has been reported in different clinical trials [47,48,49]. Few studies have reported clinical applications of cell-seeded AAM in different skin lesions. Moravvej et al. studied the effect of fibroblast-seeded AAM for the treatment of dystrophic epidermolysis bullosa. Significant decrease in qualitative wound scores and wound size were observed in their study [50]. In a clinical trial reported by Hashemi et al., AAM seeded with Wharton’s jelly MSC was used in 5 patients with chronic diabetic wound. They concluded that this tissue-engineered graft could significantly accelerate the wound healing process comparing with the control group [51]. Osman et al. investigated the effect of autologous BMMSC injection in combination with AM transplantation in 6 patients with leg ulcer. They found that this combination is a safe and effective treatment for non-healing chronic leg ulcer [52]. The future perspective of our study was clinical application of stem cell–seeded AAM in wound management. In this preliminary study, we assessed feasibility of manufacturing a clinically applicable tissue-engineered product using GMP-friendly protocols. Considering the importance of ancillary materials in safety and efficacy of the cell therapy products [53], we used reagents that could be easily replaced by their clinical or GMP-grade counterparts. To improve the safety of our cell products, some modifications were made in our previously published protocol [24, 54]. We used animal origin free collagenase (Collagenase CLSAFA/AF, Worthington, USA) and replaced FBS with human platelet lysate. Furthermore, we compared two commonly used sources of allogenic MSCs to find the best candidate for future studies in field of wound regeneration. Although some confounding factors such as donor variation, small number of animals in each study group, and induced wound model might be responsible for differences between ADMSCs and PLMSCs groups, we concluded that PLMSCs are more suitable candidate for our future studies. We used cryopreserved MSCs and AM to produce tissue engineered dressing, but the final products were applied as fresh grafts. To manufacture an off-the-shelf product, further studies are suggested to find the best cryopreservation protocol and packaging materials for the final product. This approach enhances the safety of the final product and makes it more accessible for the patients.
Conclusion
This animal study demonstrated that seeding of ADMSCs and PLMSCs on AAM can considerably boost its effect in wound healing. Furthermore, PLMSCs showed more beneficial regenerative effects than ADMSCs in rat model of excisional wound.
References
Tonnesen MG, Feng X, Clark RAF. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5(1):40–6.
Hausherr T, Nuss K, Thein E, Krähenbühl S, Applegate L, Pioletti D. Effect of temporal onsets of mechanical loading on bone formation inside a tissue engineering scaffold combined with cell therapy. Bone Rep. 2018;8:173–9.
Thomas D, O’Brien T, Pandit A. Toward customized extracellular niche engineering: progress in cell-entrapment technologies. Adv Mater. 2018;30(1):1703948.
O’Rourke C, Day A, Murray-Dunning C, Thanabalasundaram L, Cowan J, Stevanato L, et al. An allogeneic ‘off the shelf’therapeutic strategy for peripheral nerve tissue engineering using clinical grade human neural stem cells. Sci Rep. 2018;8(1):2951.
Arjmand B, Goodarzi P, Aghayan HR, Payab M, Rahim F, Alavi-Moghadam S, et al. Co-transplantation of human fetal mesenchymal and hematopoietic stem cells in type 1 diabetic mice model. Front Endocrinol. 2019;10:761.
Goodarzi P, Falahzadeh K, Nematizadeh M, Farazandeh P, Payab M, Larijani B, et al. Tissue engineered skin substitutes. Adv Exp Med Biol. 2018;1107:143–88.
Zeng X, Tang Y, Hu K, Jiao W, Ying L, Zhu L, et al. Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: a case report. Medicine (Baltimore). 2017;96(51):e9212-e.
Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–43.
Lei Z, Singh G, Min Z, Shixuan C, Xu K, Pengcheng X, et al. Bone marrow-derived mesenchymal stem cells laden novel thermo-sensitive hydrogel for the management of severe skin wound healing. Mater Sci Eng C. 2018;90:159–67.
Caruana G, Bertozzi N, Boschi E, Grieco MP, Grignaffini E, Raposio E. Role of adipose-derived stem cells in chronic cutaneous wound healing. Ann Ital Chir. 2015;86:1–4.
Goodarzi P, Alavi-Moghadam S, Sarvari M, Tayanloo Beik A, Falahzadeh K, Aghayan H, et al. Adipose tissue-derived stromal cells for wound healing. Adv Exp Med Biol. 2018.
Isakson M, De Blacam C, Whelan D, McArdle A, Clover A. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells Int. 2015;2015.
Human placenta-derived mesenchymal stem/stromal cells. The Biology and Therapeutic Application of Mesenchymal Cells 2016. p. 32–8.
Virador GM, de Marcos L, Virador VM. Skin wound healing: refractory wounds and novel solutions. In: Turksen K, editor. Skin Stem Cells: Methods and Protocols. New York, NY: Springer New York; 2019. p. 221–41.
Yusof N, Hilmy N. Historical development of amnion. Human Amniotic Membrane: World Scientific; 2017. p. 73–86.
Mrugala A, Sui A, Plummer M, Altman I, Papineau E, Frandsen D, et al. Amniotic membrane is a potential regenerative option for chronic non-healing wounds: a report of five cases receiving dehydrated human amnion/chorion membrane allograft. Int Wound J. 2016;13(4):485–92.
Chehelcheraghi F, Eimani H, Homayoonsadraie S, Torkaman G, Amini A, Alavi Majd H, et al. Effects of acellular amniotic membrane matrix and bone marrow-derived mesenchymal stem cells in improving random skin flap survival in rats. Iran Red Crescent Med J. 2016;18(6):e25588-e.
Kong P, Xie X, Li F, Liu Y, Lu Y. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun. 2013;438(2):410–9.
Castellanos G, Bernabé-García Á, Moraleda JM, Nicolás FJ. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta. 2017;59:146–53.
Baradaran Rafiei AR, Aghayan HR, Arjmand B. Amniotic membrane transplantation: Javadi MA; 2007.
Kim SS, Song CK, Shon SK, Lee KY, Kim CH, Lee MJ, et al. Effects of human amniotic membrane grafts combined with marrow mesenchymal stem cells on healing of full-thickness skin defects in rabbits. Cell Tissue Res. 2009;336(1):59–66.
Aghayan HR, Goodarzi P, Baradaran-Rafii A, Larijani B, Moradabadi L, Rahim F, et al. Bacterial contamination of amniotic membrane in a tissue bank from Iran. Cell Tissue Bank. 2013;14(3):401–6.
Aghayan HR, Goodarzi P, Arjmand B. GMP-compliant human adipose tissue-derived mesenchymal stem cells for cellular therapy. Methods Mol Biol (Clifton, NJ). 2015;1283:93–107.
Aghayan HR, Payab M, Mohamadi-Jahani F, Aghayan SS, Larijani B, Arjmand B. GMP-compliant production of human placenta-derived mesenchymal stem cells. 2020.
Wang X, Ge J, Tredget EE, Wu Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc. 2013;8(2):302–9.
Sardari K, Kakhki EG, Mohri M. Evaluation of wound contraction and epithelialization after subcutaneous administration of Theranekron® in cows. Comp Clin Pathol. 2007;16(3):197–200.
Abd-Allah SH, El-Shal AS, Shalaby SM, Abd-Elbary E, Mazen NF, Abdel Kader RR. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life. 2015;67(9):701–9.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–59.
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–7.
Kastrup J, Haack-Sørensen M, Juhl M, Harary Søndergaard R, Follin B, Drozd Lund L, et al. Cryopreserved off-the-shelf allogeneic adipose-derived stromal cells for therapy in patients with ischemic heart disease and heart failure—a safety study. Stem Cells Transl Med. 2017;6(11):1963–71.
Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63(11):1886–92.
Miyazaki H, Tsunoi Y, Akagi T, Sato S, Akashi M, Saitoh D. A novel strategy to engineer pre-vascularized 3-dimensional skin substitutes to achieve efficient, functional engraftment. Sci Rep. 2019;9(1):7797.
Kallis PJ, Friedman AJ, Lev-Tov H. A guide to tissue-engineered skin substitutes. Journal of drugs in dermatology : JDD. 2018;17(1):57–64.
Li Q, Radenbaugh P, Moroi S. Evidence of Anti–Inflammatory and Anti–Fibrotic Effects of Cryo–Preserved Human Amniotic Membrane. Invest Ophthalmol Vis Sci. 2006;47(13):47-.
Faraj LA, Stewart EA, Albert R, Allen CL, Petrovski G, Dua HS, et al. In vitro anti-angiogenic effects of cryo-preserved amniotic membrane and the role of TIMP2 and thrombospondin. Journal of EuCornea. 2018;1(1):3–7.
Mamede KM, SANT’ANNA LB. Antifibrotic effects of total or partial application of amniotic membrane in hepatic fibrosis. An Acad Brasil Ciênc. 2019;91(3).
ElHeneidy H, Omran E, Halwagy A, Al-Inany H, Al-Ansary M, Gad A. Amniotic membrane can be a valid source for wound healing. Int J Womens Health. 2016;8:225–31.
Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng A. 2008;14(4):463–72.
Koizumi N, Inatomi T, Quantock AJ, Fullwood NJ, Dota A, Kinoshita S. Amniotic Membrane as a Substrate for Cultivating Limbal Corneal Epithelial Cells for Autologous Transplantation in Rabbits. Cornea. 2000;19(1):65–71.
Wilshaw S-P, Kearney J, Fisher J, Ingham E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A. 2008;14(4):463–72.
Liu P, Guo L, Zhao D, Zhang Z, Kang K, Zhu R, et al. Study of human acellular amniotic membrane loading bone marrow mesenchymal stem cells in repair of articular cartilage defect in rabbits. Genet Mol Res. 2014;13(3):7992–8001.
Liu F, Zhou H, Du W, Huang X, Zheng X, Zhang C, et al. Hair follicle stem cells combined with human allogeneic acellular amniotic membrane for repair of full thickness skin defects in nude mice. J Tissue Eng Regen Med. 2020;14(5):723–35.
Fatemi MJ, Khajerahimi AA, Nikoumaram B, Sakhaei M, Mostafavi S, Atashi A, et al. Amniotic membrane seeded with mesenchymal adipose-derived stem cell for coverage of wound in third degree burn: an experimental study. Tehran University Medical Journal. 2014;72(6):367–78.
Mahmoudi Rad M, Talebpour Amiri F, Mirhoseini M, Ghasemi M, Mirzaei M, Mosaffa N. Application of allogeneic fibroblast cultured on acellular amniotic membrane for full-thickness wound healing in rats. Wounds : a compendium of clinical research and practice. 2016;28(1):14–9.
Chehelcheraghi F, Eimani H, Homayoonsadraie S, Torkaman G, Amini A, Alavi Majd H, et al. Effects of acellular amniotic membrane matrix and bone marrow-derived mesenchymal stem cells in improving random skin flap survival in rats. Iran Red Crescent Med J. 2016;18(6):e25588.
Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–11.
Norgren L, Weiss N, Nikol S, Hinchliffe RJ, Lantis JC, Patel MR, et al. PLX-PAD cell treatment of critical limb ischaemia: rationale and design of the PACE trial. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2019;57(4):538–45.
Sadeghi B, Remberger M, Gustafsson B, Winiarski J, Moretti G, Khoein B, et al. Long-term follow-up of a pilot study using placenta-derived decidua stromal cells for severe acute graft-versus-host disease. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2019;25(10):1965–9.
Ringden O, Baygan A, Remberger M, Gustafsson B, Winiarski J, Khoein B, et al. Placenta-derived decidua stromal cells for treatment of severe acute graft-versus-host disease. Stem Cells Transl Med. 2018;7(4):325–31.
Moravvej H, Abdollahimajd F, Naseh M, Piravar Z, Abolhasani E, Mozafari N, et al. Cultured allogeneic fibroblast injection vs. fibroblasts cultured on amniotic membrane scaffold for dystrophic epidermolysis bullosa treatment. Br J Dermatol. 2018;179(1):72–9.
Hashemi SS, Mohammadi AA, Kabiri H, Hashempoor MR, Mahmoodi M, Amini M, et al. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: a randomized clinical trial. J Cosmet Dermatol. 2019;18(6):1961–7.
Osman A, El Ansary M, Gabr H, Gad A, Al-Inany H, El-badawy A. Using amniotic membrane graft together with autologous stem cells in treatment of leg ulcers. Regen Med. 2015;10(7):206.
Solomon J, Csontos L, Clarke D, Bonyhadi M, Zylberberg C, McNiece I, et al. Current perspectives on the use of ancillary materials for the manufacture of cellular therapies. Cytotherapy. 2016;18(1):1–12.
Aghayan H-R, Goodarzi P, Arjmand B. GMP-compliant human adipose tissue-derived mesenchymal stem cells for cellular therapy. Stem Cells and Good Manufacturing Practices: Springer; 2014. p. 93–107.
Acknowledgements
The authors would like to acknowledge Dr. Mohsen Khorshidi and Shokouh Salimi for their kind support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghayan, H.R., Hosseini, M.S., Gholami, M. et al. Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv. and Transl. Res. 12, 538–549 (2022). https://doi.org/10.1007/s13346-021-00952-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00952-3