Abstract
TIFY, previously known as ZIM, comprises a plant-specific family annotated as transcription factors that might play important roles in stress response. Despite TIFY proteins have been reported in Arabidopsis and rice, a comprehensive and systematic survey of ZmTIFY genes has not yet been conducted. To investigate the functions of ZmTIFY genes in this family, we isolated and characterized 30 ZmTIFY (1 TIFY, 3 ZML, and 26 JAZ) genes in an analysis of the maize (Zea mays L.) genome in this study. The 30 ZmTIFY genes were distributed over eight chromosomes. Multiple alignment and motif display results indicated that all ZmTIFY proteins share two conserved TIFY and Jas domains. Phylogenetic analysis revealed that the ZmTIFY family could be divided into two groups. Putative cis-elements, involved in abiotic stress response, phytohormones, pollen grain, and seed development, were detected in the promoters of maize TIFY genes. Microarray data showed that the ZmTIFY genes had tissue-specific expression patterns in various maize developmental stages and in response to biotic and abiotic stresses. The results indicated that ZmTIFY4, 5, 8, 26, and 28 were induced, while ZmTIFY16, 13, 24, 27, 18, and 30 were suppressed, by drought stress in the maize inbred lines Han21 and Ye478. ZmTIFY1, 19, and 28 were upregulated after infection by three pathogens, whereas ZmTIFY4, 13, 21, 23, 24, and 26 were suppressed. These results indicate that the ZmTIFY family may have vital roles in response to abiotic and biotic stresses. The data presented in this work provide vital clues for further investigating the functions of the genes in the ZmTIFY family.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
TIFY family, previously known as ZIM (zinc-finger protein expressed in inflorescence meristem; Nishii et al. 2000), is one of the most vital transcription factor families in higher plants and plays important roles in the abiotic and biotic stress response (Zhang et al. 2012; Aparicio et al. 2013). The TIFY family is named for a conserved core motif TIF [F/Y] XG, located within a TIFY domain about 28 amino acids in length. This family of TFs can be divided into four subfamilies: TIFY, PPD, JAZ, and ZML, according to their distinct domain structures (Vanholme et al. 2007; Bai et al. 2011).
All TIFY proteins contain a TIFY domain. In addition, the ZML subfamily, including ZIM and ZIM-like (ZML) TFs, contains C2C2-GATA and CCT domains (CONSTANS, CO-like, TOC1). The PPD and JAZ subfamilies lack GATA and CCT domains (Chung et al. 2009). In addition to the TIFY domain, the JAZ subfamily also contains a conserved, C-terminus sequence Jas motif. This motif contains the characteristic sequence SLX2FX2KRX2RX5PY, consisting of approximately 27 amino acids, and is similar in sequence to the CCT domain (Staswick et al. 2008; Chung et al. 2009). PPD proteins, including a unique N-terminal PPD domain and TIFY subfamily, contain only the TIFY domain (Staswick et al. 2008; Zhang et al. 2012).
Jasmonate (JA) is a pivotal regulator of plant responses to both biotic and abiotic stresses, such as wounding, pathogen infection, drought, and salinity stress (Devoto et al. 2005; Wasternack 2007). JAZ proteins are targets of the SCFCOI1 complex and function as negative regulators in the JA signaling pathway (Zhang et al. 2012).
Recent discoveries have shown that TIFY genes play a vital role in JA signaling and the response to various abiotic stresses. In Arabidopsis thaliana L., ZIM (At3g17860) is induced by NaCl treatment (Jiang and Deyholos 2006) and several TIFY genes are highly upregulated by ozone treatment (Rao et al. 2000) and wounding (Major and Constabel 2006). Most OsTIFY genes are responsive to at least one abiotic stress, including drought, salinity, and low temperature (Ye et al. 2009). GsTIFY10 is induced by salinity and bicarbonate stress, and the plant hormone JA (Zhu et al. 2011). Some of the grape TIFY genes (VvTIFY) are regulated by osmotic, cold, drought, salinity, JA, and abscisic (ABA) treatments (Zhang et al. 2012). The PvTIFY genes might play a vital role in regulating bean (Phaseolus vulgaris L.) adaptation to phosphorus (P)-starvation, mediated by JA signaling (Aparicio et al., 2013).
These studies in plants indicated that TIFY family genes might be vital not only for plant development but also for the response and adaptation to stresses, but a comprehensive survey of ZmTIFY proteins has not yet been conducted. To investigate the functions of ZmTIFY genes in this family, transcript levels of the genes were measured in various maize tissues and seedling leaves under various abiotic and biotic stresses. The results presented herein provide a reference for future studies of TIFY family genes in maize.
Materials and methods
Isolation of TIFY family genes in maize
Sequences of 18 Arabidopsis and 20 rice (Oryza sativa L.) TIFY proteins were obtained from the TAIR (http://www.arabidopsis.org) and TIGR (http://rice.plantbiology.msu.edu) databases (Ye et al. 2009). To acquire all maize TIFY genes, BLASTP searches were carried out in the Gramene database (http://www.gramene.org/Multi/blastview) with the Arabidopsis and rice TIFY proteins as queries. All homologous protein sequences of the predicted TIFY family members were downloaded if satisfied with E < 10−10 (Zhang et al. 2014). The full-length cDNA sequences of maize ZmTIFY genes were downloaded from the Gramene database.
Structural analysis of maize TIFY genes
Information on all ZmTIFY genes, including ORF length, chromosomal location, full-length cDNA and DNA sequences, were obtained from the B73 maize-sequencing database (http://www.ensembl.gramene.org/Zea_mays/Info/Index). Exon and intron structures of ZmTIFY genes were visualized using GSDS (http://www.gsds.cbi.pku.edu.cn/; Guo et al. 2007).
Motif display and phylogenetic analysis of ZmTIFY proteins
The multiple expectation maximization for motif elicitation (MEME) utility software (Bailey et al. 2009) was used to display domains of ZmTIFY proteins. The matrix for phylogenetic analysis included 18, 20, and 30 TIFY genes from Arabidopsis, rice, and maize, respectively. Amino acid sequences of all proteins were aligned using ClustalX2.0 (Larkin et al. 2007) and the unrooted phylogenetic tree was generated using the MEGA4 software, with the neighbor-joining method and bootstrap values from 1000 replicates at each branch (Tamura et al. 2007).
Promoter regions analysis of ZmTIFY genes
To explore cis-elements in promoter sequences of ZmTIFY genes, 2000 bp of B73 genomic DNA upstream of the initiation codon (ATG) were downloaded from the National Center for Biotechnology Information. The promoter structure was predicted using Promoter 2.0 (http://www.cbs.dtu.dk/services/Promoter/) and PLACE (Plant cis-acting regulatory DNA elements, with more than 6 bp; http://www.dna.affrc.go.jp/PLACE/) software (Higo et al. 1999).
Microarray data collection and analyses of expression profiles
The spatial and temporal specific expression behaviors of ZmTIFY genes were analyzed in a set of maize transcriptome data at PLEXdb (http://www.plexdb.org). The microarray data, from genome-wide gene expression analysis of the maize inbred line B73 (GSE27004) and transcriptomic analysis of induced maize senescence, was provided by Dr. Shawn Kaeppler of the University of Wisconsin (Sekhon et al. 2011, 2012). The Affymetrix GeneChip array data of gene expression during infection with three fungi and drought treatment were downloaded from GEO with the accession numbers GSE31188, GSE19501, GSE29747, and GSE16567 (Zheng et al. 2010; Ghareeb et al. 2011; Voll et al. 2011). The data were analyzed using the GeneSpring 12.5 software. A heat map was used to show the number of ZmTIFY genes, and the map was generated using the MultiExperiment viewer (MeV, version 4.8.1) software. The data were adjusted by median centering of genes. The data were clustered by a complete linkage clustering method, using a Euclidean distance metric (Zhang et al. 2014).
Results
Identification of TIFY family genes in maize
Thirty ZmTIFY genes were identified in the maize genome (1 TIFY, 3 ZML, and 26 JAZ; Table 1). The maize TIFY genes were named ZmTIFY1 to 30, according to their order in chromosomes 1–10. The length of ZmTIFY proteins ranged from 110 amino acids (aa) to 426 aa (Table 1). BLAST analysis against the Pfam and InterPro databases showed that all of the genes included a TIFY domain (accession no. PF06200) and a Jas domain (accession no. PF09425).
The 30 ZmTIFY genes were distributed on eight maize chromosomes: 11 genes on chromosome 1, 5 on chromosome 2, 4 on chromosomes 5 and 7, 2 on chromosomes 6 and 9, and 1 on chromosomes 4 and 10 (Fig. 1). Chromosome 1 harbored two ZmTIFY gene clusters, ZmTIFY1, 2, 3, 4 and ZmTIFY8, 9, 10.
The full-length cDNA sequences were compared with the corresponding genomic DNA sequences to determine the numbers and positions of exons and introns within each ZmTIFY gene using GSDS (http://www.gsds.cbi.pku.edu.cn/chinese.php; Guo et al. 2007). Most ZmTIFY genes had two to eight exons, except for ZmTIFY1, 2, 4, 9, 10, 20, 28, and 29, which had one exon (Fig. 2). Based on phylogenetic results (Supplementary Fig. 1), 11 paralogs were identified in ZmTIFY genes: ZmTIFY1/ZmTIFY29, ZmTIFY2/ZmTIFY3, ZmTIFY5/ZmTIFY27, ZmTIFY6/ZmTIFY7, ZmTIFY8/ZmTIFY19, ZmTIFY9/ZmTIFY20, ZmTIFY12/ZmTIFY30, ZmTIFY15/ZmTIFY25, ZmTIFY17/ZmTIFY24 ZmTIFY21/ZmTIFY23, and ZmTIFY22/ZmTIFY26 (Fig. 1).
Motif analysis and protein architecture
To identify the conserved domain distribution in TIFY proteins, the MEME web server was employed to analyze the sequences of Arabidopsis, rice, sorghum (Sorghum bicolor L.), and maize proteins. Three putative conserved domains were detected in the ZmTIFY family, including the TIFY domain (accession no. PF06200), Jas domain (accession no. PF09425,) and GATA zinc finger (accession no. PF00320) (Fig. 3). The TIFY domain generally contained 36 amino acids, with a highly conserved pattern of TIFYXG, T [L/I] SFXG, SILYKG, and SNGSEG. In addition, all of the ZmTIFY proteins included a TIFY domain and a Jas domain, with a conserved motif SLX2FX2KRX2RX5PY (Staswick 2008). Moreover, three maize TIFY proteins (ZmTIFY18, 21, 23) contained an additional GATA zinc finger, which was a marker for group II in the phylogenetic tree.
Phylogenetic analysis
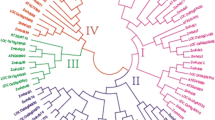
To investigate the evolutionary pattern and phylogenic relationships among TIFY in maize (30 genes), rice (20 genes), and Arabidopsis (18 genes), the predicted amino acid sequences of TIFY genes were aligned by ClustalX (1.83) and a phylogenetic tree was constructed using the neighbor-joining (NJ) method by MEGA4 (Fig. 4). All of the proteins fell into two major groups (I and II), with the exceptions of TIFY8 and OsTIFY8, which contained only the TIFY domain. Three proteins contained the TIFY, GATA zinc-finger, and Jas domains (ZmTIFY18, 21, 23) and were clustered together in group I. Proteins that contained TIFY and Jas domains, but not GATA zinc-finger domains, constituted the second major group (II). This group contained all JAZ proteins of Arabidopsis, rice, and putative maize JAZ homologs (ZmTIFY1-17, 19, 20, 22, 23-30).
Cis-element analysis
By searching the PLACE database, promoter regions (2-kb range B73 genomic DNA sequences upstream of the translation start site) of maize TIFY genes were analyzed. In this study, a series of cis-elements involved in the abiotic stress response, phytohormone, pollen-specific and quantitative (Zhang et al. 2014), seed development and germination were identified. The cis-elements included the ABRE (ACGTG), DRE (GCCGCC), LTRE (CCGAC; Haberer et al. 2006), LTRE (CCGAC; Hamilton et al. 1998) and SEF (RTTTTTR; Allen et al. 1989) motifs (Supplementary Table 1). In silico sequence analysis showed that 30 annexin genes contain at least three of the five putative cis-elements. Cis-elements play an essential function in the regulation of gene expression by controlling promoter efficiency. Studies of cis-elements could provide vital information for further research into the functions of the ZmTIFY gene family.
Expression profiles of ZmTIFY family genes in various tissues and organs
To identify the spatial and temporal expression patterns of ZmTIFY genes, we used microarray data of the gene expression levels of 60 tissues from varying developmental stages of maize (Sekhon et al. 2011). The heat map indicated that all of the 22 detected genes were involved in various biological processes, and expressed in the majority of tissues, but their individual expression levels were diverse (Fig. 5). ZmTIFY8 and 19 had higher expression levels in reproductive organs, such as whole seed (DAP), embryo (DAP), endosperm (DAP), and pericarp. The ZmTIFY15 and 25 transcript levels had higher expression levels mainly in vegetative organs such as seedlings, various leaves, stem, SAM, and outer husk. ZmTIFY4, 9, 14, 20, 26, and 28 had higher expression levels in both vegetative and reproductive organs, such as the primary root, seedling, leaves, whole seed (DAP), embryo (DAP), endosperm (DAP), and pericarp. ZmTIFY1, 10, 12, 13, 17, 24, 27, and 29 were detected at higher levels in vegetative and early reproductive organs such as seedlings, various leaves, stem, shoot tip, immature tassel, and 2 DAP whole seeds. ZmTIFY6, 21, 23, and 30 were expressed at lower levels in vegetative reproductive organs (Fig. 5).
Expression profiles of ZmTIFY genes under abiotic and biotic stresses
Many plant gene families are involved in both stress and development responses. To determine whether ZmTIFY genes were responsive to drought stress, GEO data GSE16567 (Zheng et al. 2010) was analyzed using the GeneSpring 12.5 software. The results indicated that ZmTIFY4, 5, 8, 26, and 28 were induced, while ZmTIFY16, 13, 24, 27, 18, and 30 were suppressed, in the maize inbred lines Han21 and Ye478. ZmTIFY4, 5, 8, 26, and 28 were induced and reached the highest expression levels in response to severe drought stress (53.1 % of Han21 and 41.4 % of Ye478 leaves; Zheng et al. 2010). ZmTIFY20, 21, and 23 were induced in Han21, while ZmTIFY12, 15, 19, and 25 were induced in Ye478. ZmTIFY1, 15, and 25 were suppressed only in Han21. ZmTIFY6, 9, 10, and 17 were not induced in either of the two inbred lines (Fig. 6).
To investigate the ZmTIFY (23 genes were detected) genes involved in biotic stresses, we identified differentially expressed genes using microarray data. Data from three experiments involving infection by the pathogens F. moniliforme, Sphacelotheca reiliana, and Colletotrichum graminicola were analyzed. As shown in Fig. 7, ZmTIFY1, ZmTIFY19, and ZmTIFY28 were upregulated after infection by the three pathogens, while ZmTIFY4, 13, 21, 23, 24, and 26 were suppressed. ZmTIFY5, 17, 18, and 20 were suppressed, while ZmTIFY 6 and 16 were upregulated by F. moniliforme. ZmTIFY8, 12, 15, 18, and 25 were induced, while ZmTIFY 5, 17, and 20 were suppressed by S. reiliana. ZmTIFY3 was upregulated, while ZmTIFY 9, 10, and 25 were suppressed, by C. graminicola.
Expression levels of ZmTIFY genes under abiotic stresses. Log2 ratios of expression were used to produce this heat map. Red indicates higher expression, while green signifies lower expression. CK1: Samples from uninfected control plants were taken at the same time points of T1. T1: Samples from infected leaves were taken at 36 h post-infection. CK2: Samples from uninfected control plants were taken at the same time points of T2. T2: Samples from infected leaves were taken at 96 h post-infection (color figure online)
Discussion
TIFY is a large, plant-specific, TF gene family. In this study, 30 genes belonging to the TIFY family in maize were identified. Among the more extensively analyzed plant TIFY families, Arabidopsis harbored 18 TIFY genes, at least 20 TIFY genes were expressed in rice (Ye et al. 2009), and least 19 TIFY genes in sorghum (http://www.gramene.org/Multi/blastview; Bai et al. 2011). The 30 ZmTIFY genes were distributed on eight maize chromosomes and 11 paralogs were identified in these genes. According to the B73 maize genome annotation results, ZmTIFY2/ZmTIFY3 or ZmTIFY6/ZmTIFY7 was located in one tandem duplicate. While segmental duplications might contribute to other nine paralogous genes. The genome structure of the ZmTIFY genes showed that most contained one to seven introns, with the exceptions of ZmTIFY1, 2, 4, 9, 10, 20, 28, and 29, which lacked introns (Fig. 2). Three genes lack introns and the other members have one to seven introns (Ye et al. 2009). The Arabidopsis TIFY genes have one to eight introns (Bai et al. 2011). All of the 30 proteins (1 TIFY, 3 ZML, and 26 JAZ) contained two putative conserved domains, including TIFY and Jas. Three of the thirty proteins—ZmTIFY18 (ZmZML1), ZmTIFY21 (ZmZML2), and ZmTIFY23 (ZmZML3)—contained an additional GATA zinc finger, which functioned as a transcriptional activator and a DNA-binding domain (Merika and Orkin 1993; Shikata et al. 2003).
There is increasing evidence that some gene families play vital roles both in development and the stress response (Qin et al. 2009). The members of the TIFY gene family in rice and Arabidopsis showed tissue-specific expression patterns and functions. Most of the OsTIFY genes were expressed at very high levels in leaves. OsTIFY3 showed a constitutively high expression level in vegetative organs (Ye et al. 2009). In Arabidopsis, AtTIFY1/ZIM (AT4G24470) was highly expressed in reproductive and vegetative organs, such as the inflorescence meristem (Nishii et al. 2000), petiole and hypocotyl elongation (Shikata et al. 2004). AtTIFY4a (PPD1) and AtTIFY4b (PPD2) play a role in the coordination of leaf growth (White 2006). Regarding rice, the transcript levels of the 10 ZmTIFY genes differed in reproductive and vegetative organs (Fig. 5). We noted that ZmTIFY8 and ZmTIFY19, which were closely grouped in the phylogenetic tree, had similar expression patterns and were expressed at higher levels in reproductive organs. An additional two genes, ZmTIFY15 and ZmTIFY25, were grouped together in the phylogenetic tree and showed similar expression levels in vegetative organs.
Recent discoveries have shown that the TIFY family may play important roles in the response to abiotic stresses. Arabidopsis ZIM (At3g17860) was induced after 6 h of NaCl treatment (Jiang and Deyholos 2006). Most OsTIFY genes were responsive to at least one abiotic stress, such as drought, salinity, or low temperature. Overexpression of OsTIFY11a significantly increased the tolerance of rice transgenic lines to salt and dehydration stresses (Ye et al. 2009). GsTIFY10 was induced by salinity, bicarbonate stress, and the plant hormone JA. Overexpression of GsTIFY10 in Arabidopsis enhanced plant tolerance to bicarbonate stress during most developmental stages (Zhu et al. 2011). Some grape TIFY genes (VvTIFY) were regulated by osmotic, cold, drought, salinity, JA, and ABA treatments. Only a single VvTIFY gene responded to heat stress and no VvTIFY gene responded to biotic stress (Zhang et al. 2012). PvTIFY genes might play a vital role in bean adaptation to P-starvation mediated by JA signaling (Aparicio et al. 2013). Our findings indicated that ZmTIFY4, 5, 8, 26, and 28 were induced, while ZmTIFY16, 13, 24, 27, 18, and 30 were suppressed, by drought stress in the maize inbred lines Han21 and Ye478 (Fig. 6). ZmTIFY1, ZmTIFY19, and ZmTIFY28 were upregulated after infection by three pathogens (F. moniliforme, S. reiliana and C. graminicola), while ZmTIFY4, 13, 21, 23, 24, and 26 were suppressed (Fig. 7).
These studies in plants indicated that TIFY family genes might be vital not only for plant development but also for the response and adaptation to stresses. In this study, we have systematically investigated the putative TIFY gene family and revealed that the maize genome contained 30 gene members encoding TIFY transcription factors. The detailed information on the genomic structures, chromosomal locations, protein architecture, promoter component, and phylogenetic analysis among TIFY genes in maize, Arabidopsis, and rice were presented. In addition, the expression profiles of the genes were measured in various maize tissues and seedling leaves under various abiotic and biotic stresses. The results presented herein provide vital clues for further investigating the functions of the genes in the ZmTIFY family.
Abbreviations
- ABA:
-
Abscisic acid
- CCT:
-
CONSTANS, CO-like, TOC1
- JA:
-
Jasmonic acid
- JAZ:
-
JASMONATE ZIM-DOMAIN
- PPD:
-
PEAPOD
- ZIM:
-
Zinc-finger protein expressed in inflorescence meristem
References
Allen RD, Bernier F, Lessard PA, Beachy RN (1989) Nuclear factors interact with a soybean beta-conglycinin enhancer. Plant Cell 1:623–631
Aparicio-Fabre R, Guillen G, Loredo M, Arellano J, Valdes-Lopez O, Ramirez M, Iniguez LP, Panzeri D, Castiglioni B, Cremonesi P, Strozzi F, Stella A, Girard L, Sparvoli F, Hernandez G (2013) Common bean (Phaseolus vulgaris L.) PvTIFY orchestrates global changes in transcript profile response to jasmonate and phosphorus deficiency. BMC Plant Biol 13:26
Bai Y, Meng Y, Huang D, Qi Y, Chen M (2011) Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 98:128–136
Bailey TL, Boden M, Buske FA, Martin F, Grant CE, Clernenti L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucl Acids Res 37:W202–W208
Chung HS, Niu Y, Browse J, Howe GA (2009) Top hits in contemporary JAZ: an update on jasmonate signaling. Phytochemistry 70:1547–1559
Devoto A, Ellis C, Magusin A, Chang HS, Chilcott C, Zhu T, Turner JG (2005) Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol Biol 58:497–513
Ghareeb H, Becker A, Iven T, Feussner I, Schirawski J (2011) Sporisorium reilianum infection changes inflorescence and branching architectures of maize. Plant Physiol 156:2037–2052
Guo AY, Zhu QH, Chen X, Luo JC (2007) GSDS: a gene structure display server. Yi Chuan 29:1023–1026
Haberer G, Mader MT, Kosarev P, Spannagl M, Yang L, Mayer KF (2006) Large-scale cis-element detection by analysis of correlated expression and sequence conservation between Arabidopsis and Brassica oleracea. Plant Physiol 142:1589–1602
Hamilton DA, Schwarz YH, Mascarenhas JP (1998) A monocot pollen-specific promoter contains separable pollen-specific and quantitative elements. Plant Mol Biol 38:663–669
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucl Acids Res 27:297–300
Jiang Y, Deyholos MK (2006) Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol 6:25
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Major IT, Constabel CP (2006) Molecular analysis of poplar defense against herbivory: comparison of wound- and insect elicitor-induced gene expression. New Phytol 172:617–635
Merika M, Orkin SH (1993) DNA-binding specificity of GATA family transcription factors. Mol Cell Biol 13:3999–4010
Nishii A, Takemura M, Fujita H, Shikata M, Yokota A, Kohchi T (2000) Characterization of a novel gene encoding a putative single zinc-finger protein, ZIM, expressed during the reproductive phase in Arabidopsis thaliana. Biosci Biotechnol Biochem 64:1402–1409
Qin Y, Ye H, Tang N, Xiong L (2009) Systematic identification of X1-homologous genes reveals a family involved in stress responses in rice. Plant Mol Biol 71:483–496
Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12:1633–1646
Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR, de Leon N, Kaeppler SM (2011) Genome-wide atlas of transcription during maize development. Plant J 66:553–563
Sekhon RS, Childs KL, Santoro N, Foster CE, Buell CR, de Leon N, Kaeppler SM (2012) Transcriptional and metabolic analysis of senescence induced by preventing pollination in maize. Plant Physiol 159:1730–1744
Shikata M, Takemura M, Yokota A, Kohchi T (2003) Arabidopsis ZIM, a plant-specific GATA factor, can function as a transcriptional activator. Biosci Biotechnol Biochem 67:2495–2497
Shikata M, Matsuda Y, Ando K, Nishii A, Takemura M, Yokota A, Kohchi T (2004) Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J Exp Bot 55:631–639
Staswick PE (2008) JAZing up jasmonate signaling. Trends Plant Sci 13:66–71
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G (2007) The tify family previously known as ZIM. Trends Plant Sci 12:239–244
Voll LM, Horst RJ, Voitsik AM, Zajic D, Samans B, Pons-Kuhnemann J, Doehlemann G, Munch S, Wahl R, Molitor A, Hofmann J, Schmiedl A, Waller F, Deising HB, Kahmann R, Kamper J, Kogel KH, Sonnewald U (2011) Common motifs in the response of cereal primary metabolism to fungal pathogens are not based on similar transcriptional reprogramming. Front Plant Sci 2:39
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
White DW (2006) PEAPOD regulates lamina size and curvature in Arabidopsis. Proc Natl Acad Sci USA 103:13238–13243
Ye H, Du H, Tang N, Li X, Xiong L (2009) Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol Biol 71:291–305
Zhang Y, Gao M, Singer SD, Fei Z, Wang H, Wang X (2012) Genome-wide identification and analysis of the TIFY gene family in grape. PLoS One 7:e44465
Zhang Z, Chen Y, Zhao D, Li R, Wang H, Zhang J, Wei J (2014) X1-homologous genes family as central components in biotic and abiotic stresses response in maize (Zea mays L.). Funct Integr Genomics 14:101–110
Zheng J, Fu J, Gou M, Huai J, Liu Y, Jian M, Huang Q, Guo X, Dong Z, Wang H, Wang G (2010) Genome-wide transcriptome analysis of two maize inbred lines under drought stress. Plant Mol Biol 72:407–421
Zhu D, Bai X, Chen C, Chen Q, Cai H, Li Y, Ji W, Zhai H, Lv D, Luo X, Zhu Y (2011) GsTIFY10, a novel positive regulator of plant tolerance to bicarbonate stress and a repressor of jasmonate signaling. Plant Mol Biol 77:285–297
Acknowledgments
We are grateful to our editors and reviewers for their helpful comments and the groups who submitted the microarray data to the public databases. This work was supported in part by the Beijing Nova Program (No. Z121105002512031), the National Special Program of Transgenic Research (2014ZX08003-003B), the Youth Foundation of Beijing Academy of Agriculture and Forestry Sciences (No. QNJJ201303), and the Beijing Academy of Agriculture and Forestry Sciences (No. KJCX20140202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Lai.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Z., Li, X., Yu, R. et al. Isolation, structural analysis, and expression characteristics of the maize TIFY gene family. Mol Genet Genomics 290, 1849–1858 (2015). https://doi.org/10.1007/s00438-015-1042-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-1042-6