Abstract
Developmental mutants with defects in fruiting body formation are excellent resources for the identification of genetic components that control cellular differentiation processes in filamentous fungi. The mutant pro4 of the ascomycete Sordaria macrospora is characterized by a developmental arrest during the sexual life cycle. This mutant generates only pre-fruiting bodies (protoperithecia), and is unable to form ascospores. Besides being sterile, pro4 is auxotrophic for leucine. Ascospore analysis revealed that the two phenotypes are genetically linked. After isolation of the wild-type leu1 gene from S. macrospora, complementation experiments demonstrated that the gene was able to restore both prototrophy and fertility in pro4. To investigate the control of leu1 expression, other genes involved in leucine biosynthesis specifically and in the general control of amino acid biosynthesis (“cross-pathway control”) have been analysed using Northern hybridization and quantitative RT-PCR. These analyses demonstrated that genes of leucine biosynthesis are transcribed at higher levels under conditions of amino acid starvation. In addition, the expression data for the cpc1 and cpc2 genes indicate that cross-pathway control is superimposed on leucine-specific regulation of fruiting body development in the leu1 mutant. This was further substantiated by growth experiments in which the wild-type strain was found to show a sterile phenotype when grown on a medium containing the amino acid analogue 5-methyl-tryptophan. Taken together, these data show that pro4 represents a novel mutant type in S. macrospora, in which amino acid starvation acts as a signal that interrupts the development of the fruiting body.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In filamentous fungi, meiospores develop in fruiting bodies, which can be considered as aggregations of differentiated hyphae. During the meiotic cycle in ascomycetes and basidiomycetes, sexual spores within sporangia arise from dikaryotic hyphae, which carry two genetically distinct nuclei per septum. In basidiomycetes, the dikaryotic hyphae participate in fruiting body formation. In contrast, fruiting bodies in ascomycetes arise from non-dikaryotic, sterile hyphae that surround the dikaryotic cells (Braus et al. 2002; Moore and Frazer 2002).
Previous genetic analyses have provided considerable evidence for polygenic control of fruiting body development in mycelial fungi and, consequently, developmental mutants with defects in fruiting body formation have proven to be excellent material for the identification of components that control fungal morphogenesis (Moore 1998). A forward genetic approach was therefore chosen to probe the developmental pathway for fruiting body formation in the pyrenomycetous ascomycete Sordaria macrospora (Nowrousian et al. 1999; Kück and Pöggeler 2004). At least 114 genes are now known to be involved in the formation of the multicellular fruiting bodies, or perithecia, which arise during sexual propagation (S. Masloff and U. Kück, unpublished data). One major step in this differentiation process is the morphological transition from spherical pre-fruiting bodies (protoperithecia) to flask-like fruiting bodies (perithecia). In our case, we selected developmental mutants which arrest after protoperithecia formation and labelled these with the prefix “pro”. Recently, we were able to characterize two mutants of this type. One lacks the gene for a C6 binuclear zinc-finger transcription factor and is directly involved in the developmental control of fruiting body formation (Masloff et al. 1999, 2002), the other has a defect in a gene for a WD-repeat protein that can be functionally replaced by its mammalian homologue (Pöggeler and Kück 2004).
In this report, the molecular analysis of a further “pro”-type mutant, designated pro4, is described. Like other S. macrospora mutants with defects in fruiting body formation, pro4 is sterile and thus unable to form ascospores (Masloff et al. 1999; Nowrousian et al. 1999). However, this strain is the only one in our collection of developmental mutants that also shows an amino acid auxotrophy. pro4 is shown here to lack the leu1 gene for β-isopropylmalate dehydrogenase, and further expression data indicate that genes encoding leucine pathway-specific enzymes, as well as those involved in CPC, play a role in fruiting body development (Braus et al. 2004).
Materials and methods
Plasmids and gene probes
Plasmid pleu11, which carries the leu1 gene from Neurospora crassa, was a generous gift from George Marzluf (Ohio State University, Columbus, OH). Cosmid G2 was isolated from our indexed cosmid library (Pöggeler et al. 1997) and contains a 40-kb insert encompassing the leu1 gene region from S. macrospora. A 400-bp fragment of the coding region of the S. macrospora gpd gene (M. Nowrousian, personal communication) was used as a loading control in northern hybridization experiments. All other hybridization probes were generated by PCR amplification using specific primers (listed in Table 1), as detailed in the text.
Strains and culture conditions
The wild-type strain K of Sordaria macrospora (S 17736), the fertile mutant fus1 (which produces brownish ascospores), and the sterile mutant pro4 were obtained from our laboratory collection. The developmental mutant pro4 was generated as described previously (Masloff et al. 1999): for morphological analysis and mRNA isolation, strains were grown on rich corn meal (CM) medium with 0.8% malt extract (Esser 1982). Prototrophic and auxotrophic strains were differentiated on minimal (MM) medium (11.5 mM glucose, 1.8 mM KH2PO4, 1.8 mM K2HPO4, 8.3 mM urea, 1 mM MgSO4, 5 μM biotin, and trace elements, pH 6.7). For growth of the auxotrophic mutant pro4 on MM, the medium was supplemented with 10 mM leucine. Starvation for single amino acids was induced by the addition of 12 or 24 mM 3-amino-1,2,4-triazole (3AT) or 4 mM 5-methyl-tryptophan (5MT) to liquid or solid complete medium (CM). The fertile/sterile phenotype was monitored after growth for at least 2 weeks on supplemented medium. RNA, for northern hybridizations and 9RT-PCR experiments, was isolated after strains had been grown for 2–7 days under conditions of amino acid starvation.
Crosses and spore analysis
Crosses were set up by inoculating a plate containing solid CM medium with the sterile pro4 mutant and a second strain, fus1, carrying a spore colour mutation. Perithecia containing recombinant asci that form in the zone of contact between the mycelia can be recognized because they contain four black and four brown ascospores. Tetrad analysis, random spore analysis, and ascospore germination on sodium acetate medium were conducted as described by Esser (1982).
Nucleic acid analysis
Protoplast preparation and isolation of nucleic acids were carried out as described by Pöggeler et al. (1997). Restriction digests and mRNA preparations were fractionated by gel electrophoresis, transferred onto nylon membranes, and hybridized with radioactively labelled dsDNA probes, according to conventional methods (Sambrook et al. 1989). 32P-labelled hybridization probes used for colony blots and Southern hybridizations were generated by random oligonucleotide priming (Feinberg and Vogelstein 1984) or by PCR amplification as indicated. All other recombinant DNA techniques were applied according to standard protocols.
DNA-mediated transformation of fungal strains
Transformation of S. macrospora was carried out as described by Masloff et al. (1999), with some modifications. Using a glass pipette, suspensions of transformed protoplasts were spread on plates containing MM supplemented with 10.8% sucrose (MMS medium). In the absence of nutritional supplements, only primary transformants which were complemented to prototrophy were able to grow on this medium. Subsequently, individual transformants were recovered and maintained on either solid or liquid MM.
Isolation and sequencing of genomic and cDNA clones
A PCR amplification strategy, using two sets of oligonucleotide primers (Table 1), was employed to amplify fragments of the leu1 gene region and adjacent DNA sequences. For the isolation of gene fragments from S. macrospora, primers based on the N. crassa genome sequence (available at http://www-genome.wi.mit.edu/annotation/fungi/neurospora/) were used in PCRs together with genomic DNA from the wild-type strain of S. macrospora as template. The resulting PCR products were cloned into the vector pDrive (Qiagen, Hilden, Germany) and further analysed by DNA sequencing, which was performed by the custom-sequencing services of Qiagen and MWG Biotech (Ebersberg, Germany). FASTA (Pearson 1990) was used for comparisons of nucleotide and amino acid sequences. Alignments were made using the CLUSTAL W program (Thompson et al. 1994) provided by the European Bioinformatics Institute. The nucleotide sequences have been deposited in GenBank under the Accession no. AY386218. S. macrospora protein sequences were used as query sequences in BLASTP searches.
Quantitative RT-PCR
Quantification of mRNAs derived from the leu1,leu2, leu4,leu6, cpc1, and cpc2 genes was done in an Opticon 2 (MJ Research, Watertown, MA) as described recently (Nowrousian et al. 2005). Mean Ct values (threshold cycles) for an amplicon derived from the SSU rRNA were used as a reference for normalization. The oligonucleotide primers listed in Table 2 were used for 9RT-PCR.
Results
The developmental mutant pro4 is sterile and auxotrophic for leucine
As previously reported (Masloff et al. 1999), conventional mutagenesis has been used to generate developmental mutants of S. macrospora that show defects in fruiting body formation. During this programme, we have isolated more than 100 sterile mutants, each of which is defective in a single genetic locus. This collection of sterile mutants includes a class of strains that is unable to execute the transition from protoperithecium (the immature pre-fruiting body) to perithecium (the mature fruiting body). These mutants were labelled with the prefix “pro”. Protoperithecia and perithecia differ in size and shape, and can therefore be distinguished quite easily. While protoperithecia are about 30–70 μm in size, perithecia are always larger than 150 μm. Protoperithecia can be viewed as an aggregation of hyphae and are of spherical shape, whereas perithecia form a neck, giving them a beacon-like form. According to this classification, pro4 belongs to the “pro” category of mutants. pro4 can only be propagated on MM if the medium is supplemented with 10 mM leucine. On CM medium, pro4 shows a growth rate of 1.6 cm/day, like the wild-type strain. With an average size of about 70 μm, the protoperithecia in pro4 are larger than those observed in other pro-mutants, which are about 40 μm in diameter (Masloff et al. 1999; S. Pöggeler and U. Kück, unpublished results).
Tetrad analysis indicates that the loci responsible for sterility and leucine auxotrophy in pro4 are closely linked
To determine whether the auxotrophy and sterility phenotypes are genetically linked, tetrad analysis was performed using ten tetrads from a cross between pro4 and the fus1 marker strain. The fus1 mutant produces brownish ascospores, but shows normal fertility. The use of the fus1 mutant in genetic crosses enables one to easily distinguish between recombinant and non-recombinant asci. In parallel analysis, 100 randomly isolated ascospores were tested for prototrophy and fertility. None of the tested ascospores gave rise to isolates that were sterile and prototrophic or fertile and auxotrophic. This finding implies that the genetic determinants responsible for the sterile and auxotrophic phenotypes are tightly linked. In addition, the fus phenotype (brownish ascospores) showed the expected random segregation in all ascospore isolates.
The pro4 mutation is a deletion that includes the leu1 gene encoding β-isopropylmalate dehydrogenase
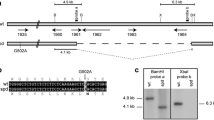
The genetic analyses described above indicate that pro4 carries a mutation in a gene involved in leucine biosynthesis, which is also responsible for the developmental defect. Therefore, a Southern analysis was performed using the leu1 gene encoding β-isopropylmalate dehydrogenase from N. crassa as a heterologous probe (Jarai et al. 1990; Li et al. 1993). Genomic DNAs from wild-type S. macrospora and the pro4 mutant were digested with EcoRI, BamHI, SacI, XbaI, and NcoI. As a control, DNA from the cosmid clone G2, which shows homology to the leu1 gene from N. crassa, was isolated from an indexed S. macrospora library (Pöggeler et al. 1997) using the leu1 gene from N. crassa as the probe. Wild-type and pro4 genomic DNAs showed different hybridization patterns. Some fragments were of identical size in both, whereas others were clearly different. As an example, the patterns generated by digestion with SacI and PstI are shown in Fig. 1. Interestingly, nearly all of the hybridizing fragments from the wild-type and pro4 were also detectable in the cosmid clone G2. Analysis of the Southern data revealed a size difference of about 6 kb between the wild-type and the mutant strain, indicating that pro4 carries a genomic deletion. This deletion was further characterized with three different probes generated by PCR using S. macrospora genomic DNA as the template. Due to the high degree of sequence similarity and conservation of overall genomic organization (Nowrousian et al. 2004), the N. crassa sequence was used to design two primer pairs to amplify probes specific for genes that are adjacent to leu1 in the S. macrospora genome. Southern hybridization with both probes revealed that pro4 lacked leu1 and the adjacent gene smu06233, but not the smu06231 gene (data not shown).
Determination of the extent of the deletion in pro4. DNA from the wild-type or pro4 strain was digested with the indicated restriction enzymes and blotted for Southern hybridization. The radiolabelled DNA from cosmid G2 was used, carrying the leu1 gene region, as the probe. For comparison, DNA from cosmid G2 was used in parallel
Isolation and sequencing of the leu1 gene from S. macrospora
Using primers 1759 and 1764 (Table 1), a 5.5-kb DNA fragment was amplified from the wild-type strain, and sequence analysis demonstrated that it carried the complete leu1 gene including upstream and downstream sequences. Using RT-PCR with the oligonucleotides 1761 and 1762 (Table 1), we also generated a 1.1-kb cDNA clone, pLeu1c (Fig. 2). Both primers are homologous to the N. crassa leu1 sequence and contain the start and termination codons, respectively. Comparison of the genomic and cDNA sequences revealed that the S. macrospora leu1 ORF encodes 368 amino acids and is interrupted by three introns located at the same positions as those in the leu1 gene of N. crassa (Li et al. 1993). The predicted gene product shows a high degree of identity to other fungal proteins involved in leucine biosynthesis, including the N. crassa LEU1 (98.4%), Saccharomyces cerevisiae Leu2p (63.6%) and the Phanerochaete chrysosporium LEU2 (53.8%). The gene isolated from S. macrospora was designated leu1 because of its close resemblance to the leu1 gene from N. crassa. Upstream of the S. macrospora leu1 ORF we found a 1,340-bp sequence that includes a predicted promoter sequence, which is 71.4% identical to that adjacent to N. crassa leu1.
Physical and genetic map of the leu1 gene region from S. macrospora. The DNA fragments generated by PCR amplification and used for DNA-mediated transformation and sequencing are indicated below the map. The cDNA, generated by RT-PCR, was used to determine exon/intron boundaries and this clone was also used to test for complementation of the pro4 mutant. The location of all oligonucleotide primers used for PCR amplifications are indicated by the numbered arrows
leu1 is required for both prototrophy and fertility in S. macrospora
To verify the dual function of the leu1 gene with respect to amino acid biosynthesis and fertility, cosmid G2 and several of its subclones were tested for the ability to complement the pro4 mutant. This analysis demonstrated that all clones containing the full-length ORF of the leu1 gene are able to restore fertility in pro4 (Fig. 2). The corresponding transformants formed wild-type fruiting bodies containing mature ascospores. In addition, all fertile transformants showed a prototrophic phenotype on MM. Even the full-length cDNA clone of the leu1 gene was able to restore the wild-type phenotype, despite lacking its promoter sequence. Most probably, ectopic integration of the transformed DNA occurred, as at least some transformants could transcriptionally express the transformed leu1 gene fragment. As expected from the high degree of sequence identity between the S. macrospora and N. crassa leu1 genes, the N. crassa gene could also be used to restore the wild-type phenotype in pro4 (data not shown). These genetic data were confirmed by feeding experiments. While pro4 generates only sterile protoperithecia on CM medium, supplementation of the medium with at least 10 mM leucine resulted in the formation of perithecia, and thus in the restoration of fertility. Conversely, wild-type strains kept on media containing 4 mM 5MT, an amino acid analogue that induces starvation for tryptophan, showed a sterile phenotype, which resembled that of the mutant pro4 insofar as only protoperithecia were formed. However, feeding with 12–36 mM 3AT (which induces starvation for histidine) did not result in a sterile phenotype, most probably due to limited uptake of this amino acid analogue (data not shown). The effect of 5MT can also be observed when the levels of leu1 and leu4 transcripts are measured (see the following section). From the above data, it may be concluded that, as in Aspergillus nidulans (Eckert et al. 1999; Hoffmann et al. 2000), starvation for single amino acids causes the arrest of sexual development in S. macrospora.
Transcriptional expression of genes involved in amino acid biosynthesis
In yeast, leucine biosynthesis is controlled by the general control (GC) of amino acid biosynthesis, which is analogous to the cross-pathway control (CPC) of amino acid biosynthetic pathways seen in filamentous fungi (Kohlhaw 2003). In A. nidulans, both amino acid biosynthesis and sexual development are controlled by the CPC, which is activated during amino acid starvation by the transcription factor CPCA (syn. CPC1). Conversely, in the presence of amino acids, the network is repressed by transcription factor CPCB (syn. CPC2) (Braus et al. 2004).
The expression of leucine pathway-specific and cross-pathway-specific genes was therefore investigated at the transcriptional level in S. macrospora. Using the above-mentioned N. crassa genomic sequence, PCR primers (Table 1) were designed to generate amplicons (for use as hybridization probes) encoding leucine pathway or cross-pathway-specific polypeptides from S. macrospora: leu2 (β-isopropylmalate isomerase), leu4 (α-isopropylmalate synthase), leu6 (cytoplasmic leucyl tRNA synthetase), cpc1 (activator of CPC of amino acid biosynthesis) and cpc2 (repressor of CPC of amino acid biosynthesis).
mRNA derived from the wild-type or the pro4 strain was isolated at different times (2–7 days) after plating and subjected to northern analysis. For mRNA quantification, all experiments were conducted at least three times and blots were reprobed with a gpd probe to check for equality of loading. For optimal detection of the various transcripts, mRNA was isolated after incubation for 2 or 5 days on CM medium, or for 5–7 days on MM.
Although the corresponding cDNA could be generated, the leu1 transcript, like the leu2 and leu4 RNAs, was barely detectable in the wild-type strain under the growth conditions described above. However, when pro4 was grown on supplemented MM, leu2 and leu4 transcripts (Fig. 3) and, to a much lesser extent, leu6 transcripts (data not shown) were clearly detectable. When the wild-type strain was starved for amino acids by plating in the presence of the amino acid analogue 3AT (which is expected to deplete histidine levels), the leu4 transcript was induced to detectable levels after 5 days on CM medium (Fig. 3). These data were verified and extended by examining gene expression using quantitative RT-PCR (qRT-PCR). These experiments were performed with RNA from cultures which had been kept for 5 days under conditions of amino acid starvation. As can be seen in Fig. 4, levels of the leu1,leu2, and leu4 transcripts were increased at least twofold under amino acid starvation. Note that, in Fig. 4, the relative expression levels (presence/absence of analogue) are plotted as log2 values (i.e. a value of 1 on the histogram corresponds to a twofold increase in expression in the presence of analogue). In the case of the leu4 transcript, the dramatic up-regulation in the presence of either analogue confirms the results of the northern analysis shown in Fig. 3. These results provide clear evidence that the leucine biosynthesis genes assayed specifically respond to the imposition of starvation for other amino acids.
Northern analysis of the expression of genes involved in leucine biosynthesis. In all cases, mRNA was used. For quantification of transcript levels, all filters were re-probed with a gpd probe, and the corresponding hybridization signal is shown as a reference. Genes and their products: gpd glyceraldehyde-3-phosphate dehydrogenase, leu1 isopropylmalate dehydrogenase, leu2 β-isopropylmalate isomerase, leu4 α-isopropylmalate synthase, 1×3AT, 2×AT and 1% gluc indicate that the CM medium was supplemented with 12 mM or 24 mM 3-aminotriazole, or with 1% glucose, respectively
Quantitative RT-PCR analysis of leu gene expression in the wild-type strain grown in the presence/absence of the amino acid analogue of 5MT or 3AT. Transcription of the genes leu1,leu2, and leu4, which are involved in leucine biosynthesis, was examined with gene-specific primers (see Table 2). Data were normalized to an internal control (SSU rRNA) and relative expression levels for each gene were calculated as described previously (Nowrousian et al. 2005). The mean relative change in expression in wild-type cells grown in the presence versus absence of amino acid analogue is plotted in log2 values. The analogues were added to CM medium at a final concentration of 24 mM (3AT) or 4 mM (5MT)
Analyses of the expression of cpc1 and cpc2 mRNAs have demonstrated that both genes are part of a CPC of amino acid biosynthesis that induces the transcription of several amino acid biosynthesis genes upon starvation for any single amino acid in A. nidulans, N. crassa and S. cerevisiae (Sachs 1996; Braus et al. 2004). Interestingly, in yeast, the target genes include those involved in branched amino acid biosynthesis except the gene encoding β-isopropylmalate dehydrogenase (the leu1 homologue in yeast). In order to provide a detailed analysis of cpc1 and cpc2 expression in S. macrospora, both quantitative northern hybridization and qRT-PCR were performed. As can be seen in Fig. 5, the cpc1 transcript shows an almost threefold increase after growth of the wild-type for 4 days under conditions of amino acid starvation. For comparison, when the wild-type strain was kept on glucose-containing medium, no change in transcript levels was seen (Fig. 5b). This difference in mRNA levels was also observed after 7 days on medium supplemented with 24 mM 3AT (data not shown). On supplemented MM or CM medium, no significant differences in levels of cpc1 could be detected between the wild-type and pro4 strains. However, an effect of the leu1 deletion on the expression of cpc2 became apparent when the strains were grown for 4 days on CM medium supplemented with 10 mM leucine. Under these conditions, the cpc2 mRNA level was fivefold higher in the wild-type than in pro4 (Fig. 5c). When pro4 was grown for a prolonged period (5–7 days) on CM medium, with or without leucine supplementation, the cpc2 mRNA level increased significantly (data not shown). Overall, these data suggest that leu1 expression is also subject to CPC. To further confirm these data, the wild-type strain was grown for 4 days on four different media, and mRNA was isolated and used for qRT-PCR experiments. Primer sets corresponding to the sequences of cpc1 and cpc2 (Table 2) were used to amplify the specific cDNAs from strains grown on CM without supplements or on media supplemented with either 24 mM 3AT or 4 mM 5MT. cpc1 was found to be up-regulated in response to the amino acid analogues, while the level of the cpc2 transcript was only marginally increased in the presence of either of the amino acid analogues, in agreement with the northern data (data not shown).
a–c Quantitative northern analysis of cpc1 and cpc2 transcripts. a Northern analysis with the indicated probes of mRNAs isolated from the indicated strains grown under the indicated conditions. b, c Relative levels of cpc1 (b) and cpc2 (c) transcripts (normalized relative to the gpd signal). Strains (black bars, WT; grey bars, pro4) were grown for 2 (CM without 3AT) or 4 days on MM or CM medium supplemented with 3AT or 1% glucose. Genes and their products: cpc1, activator of cross-pathway control of amino acid biosynthesis; cpc2, repressor of cross-pathway control of amino acid biosynthesis; all other abbreviations are explained in the legend to Fig. 3
Discussion
Our forward genetic approach to S. macrospora has facilitated the isolation of developmental genes and helped to elucidate a developmental pathway for fruiting body formation (Masloff et al. 1999; Nowrousian et al. 1999; Pöggeler and Kück 2001, 2004). pro4 is the second developmental mutant found to have a defect in a gene involved in basic metabolism. Previously, we showed that a mutant lacking ATP citrate lyase (ACL) activity is sterile due to defects in perithecium formation (Nowrousian et al. 1999). ACL is specifically induced at the beginning of the sexual cycle, and produces acetyl-CoA which is used mainly in fatty acid and sterol biosynthesis. These compounds are essential for fruiting body formation. The defect in amino acid biosynthesis in pro4 is another example for signals derived from basic metabolism that regulate sexual development in S. macrospora. pro4 lacks the leu1 gene that codes for β-isopropylmalate dehydrogenase. The function of this enzyme in leucine biosynthesis has been intensively investigated in a broad range of microbes (Kohlhaw 2003). Mutations in leu genes usually result in auxotrophic strains, which are ideal tools for genetic investigations. A prominent example for this is the first DNA-mediated transformation of a eukaryotic organism, which was conducted with a leucine auxotrophic mutant (leu2-2) of S. cerevisiae showing a defect in the β-isopropylmalate dehydrogenase gene (Hinnen et al. 1978).
Fungal leucine biosynthesis has been intensively studied in N. crassa. Several leucine auxotrophic mutants have been described (Perkins et al. 1982), but a sterility phenotype in a leu1 mutant has previously only been mentioned once and very briefly (cited as a personal communication in Perkins et al. 1982). Levels of β-isopropylmalate dehydrogenase have been observed to be proportional to the amount of the leu4 transcript; however, mutants with a defective β-isopropylmalate dehydrogenase gene produce large amounts of leu2 and leu4 gene products (Kohlhaw 2003). This is consistent with our measurement of leu2 and leu4 mRNA levels in pro4 and in the wild-type strain. In the latter, no leu2 transcript was detectable under the conditions tested. In N. crassa, the expression of the structural genes leu1,leu2, and leu4 of the leucine biosynthetic pathway is controlled by the product of the regulatory gene leu3 (Jarai et al. 1990; Li et al. 1993). It is believed that isopropylmalate, which is an inducer of leu1 expression, forms a complex with LEU3 (which has a DNA-binding domain) that then interacts with different target genes, including the leu1 gene.
In yeast, the leucine pathway is controlled by the Leu3p–isopropylmalate complex. This pathway also controls the isopropylmalate dehydrogenase gene. However, superimposed on this regulation is the general amino acid control mechanism mediated by Gcn4p. Gcn4p is functionally equivalent to the cpc1 and cpcA gene products in N. crassa and A. nidulans, respectively. Leucine pathway-specific genes—with the exception of the isopropylmalate dehydrogenase gene—are directly or indirectly regulated by the general amino acid control system (Kohlhaw 2003). Our data, however, allow the conclusion that, in S. macrospora, the leu1 gene is also under CPC, since the level of cpc2 mRNA increases many fold in the wild-type if leucine is present in the medium. The same effect can be seen also in the pro4 mutant if it is kept for at least 5 days on CM or MM supplemented with leucine (data not shown). Thus, the leucine starvation signal seems to regulate fruiting body formation in S. macrospora through the CPC that also regulates genes involved in the biosynthesis of other amino acids. This proposal is further supported by the expression data obtained with the highly sensitive 9RT-PCR analysis, in which several independent samples were used for the quantification of leucine gene expression.
The involvement of amino acid biosynthesis genes in fungal fruiting body development has been well documented for A. nidulans (Hoffmann et al. 2000). Auxotrophic strains showing defects in fruiting body formation can be rescued by feeding with the appropriate amino acids. Braus and co-workers have investigated mutant strains of A. nidulans that are auxotrophic for histidine, tryptophan, or arginine; all show a sterile phenotype when grown under conditions of amino acid limitation (Eckert et al. 1999, 2000; Busch et al. 2001). Under these growth conditions, the auxotrophic strains form only microcleistothecia, which can be considered equivalent to protoperithecia in pyrenomycetes such as S. macrospora or N. crassa.
Although pro4 is sterile on CM, the formation of perithecia and ascospores can at least partially be restored when the medium is supplemented with excess leucine. This may be explained by the fact that biosynthesis of branched-chain amino acids is mainly controlled by pathway-specific regulation.
A. nidulans grown under amino acid starvation conditions, which activate CPC, has been shown to initiate the sexual developmental programme, but fruiting body formation is blocked before the completion of meiosis. In all filamentous fungi investigated to date, the cross-pathway network is specifically repressed by the product of the cpc2 (cpcB) gene and, consequently, knock-out strains lacking the cpc2 gene are unable to undergo the sexual cycle in A. nidulans (Hoffmann et al. 2000). Remarkably, in N. crassa, deletion of the cpc2 gene even prevents the formation of any protoperithecia (Müller et al. 1995). In agreement with this finding, inhibition of fruiting body formation occurs when the cpc1 gene, an activator of CPC, is overexpressed in strains grown on media supplemented with amino acids. The same phenotype was observed when 3AT, a histidine analogue, was added to the culture medium. This developmental arrest is also seen in the wild-type strain of S. macrospora, when it is kept under conditions of amino acid starvation.
In summary, in our collection of strains, pro4 represents a novel mutant type in which leucine starvation acts as a signal that affects fruiting body development. So far, pro4 is the only one among 114 developmental sterile mutants examined (S. Masloff and U. Kück, unpublished) that shows amino acid auxotrophy. Moreover, pro4 can be used as a recipient strain for DNA-mediated transformation in S. macrospora, making it an excellent investigational tool. To date, only limited data are available for S. macrospora describing auxotrophic strains that are suitable as recipient strains for transformation. Finally, the leucine auxotrophic markers present an important alternative when, for example, a second recombinant gene is to be introduced into recombinant strains, which already carry the frequently used hygromycin B resistance gene.
References
Braus GH, Krappmann S, Eckert SE (2002) Sexual development in Ascomycetes. Fruit body formation of Aspergillus nidulans. In: Osiewacz HD (eds), Molecular biology of fungal development. Marcel Dekker, New York, pp 215–244
Braus GH, Pries R, Düvel K, Valerius O (2004) Molecular biology of fungal amino acid biosynthesis regulation. In: Kück U (eds) The Mycota II: genetics and biotechnology, 2nd edn. Springer, Berlin Heidelberg New York, pp 239–269
Busch S, Hoffmann B, Valerius O, Starke K, Düvel K, Braus GH (2001) Regulation of the Aspergillus nidulans hisB gene by histidine starvation. Curr Genet 38:314–322
Eckert SE, Hoffmann B, Wanke C, Braus GH (1999) Sexual development of Aspergillus nidulans in tryptophan auxotrophic strains. Arch Microbiol 172:157–166
Eckert SE, Kübler E, Hoffmann B, Braus GH (2000) The tryptophan synthase-encoding trpB gene of Aspergillus nidulans is regulated by the cross-pathway control system. Mol Gen Genet 263:867–876
Esser K (1982) Cryptogams. Cyanobacteria, algae, fungi, lichens. Cambridge University Press, Cambridge
Feinberg AP, Vogelstein B (1984) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 137:266–267
Hinnen A, Hicks JB, Fink GR (1978) Transformation of yeast. Proc Natl Acad Sci USA 75:1929–1933
Hoffmann B, Wanke C, Lapaglia SK, Braus GH (2000) c-Jun and RACK1 homologues regulate a control point for sexual development in Aspergillus nidulans. Mol Microbiol 37:28–41
Jarai G, Yagmai B, Fu YH, Marzluf GA (1990) Regulation of branched-chain amino acid biosynthesis in Neurospora crassa: cloning and characterization of the leu-1 and ilv-3 genes. Mol Gen Genet 224:383–388
Kohlhaw GB (2003) Leucine biosynthesis in fungi: entering metabolism through the back door. Microbiol Mol Biol Rev 67:1–15
Kück U, Pöggeler S (2004) Sordaria macrospora. In: Gellissen G (ed) Production of recombinant proteins. Novel microbial and eucaryotic expression systems. Wiley VCH, Weinheim, pp 215–231
Li Q, Jarai G, Yaghmai B, Marzluf GA (1993) The leu-1 gene of Neurospora crassa: nucleotide and deduced amino acid sequence comparisons. Gene 136:301–305
Masloff S, Pöggeler S, Kück U (1999) The pro1 gene from Sordaria macrospora encodes a C6 zinc finger transcription factor required for fruiting body development. Genetics 152:191–199
Masloff S, Jacobsen S, Pöggeler S, Kück U (2002) Functional analysis of the C6 zinc finger gene pro1 involved in fungal sexual development. Fungal Genet Biol 36:107–116
Moore D (1998) Fungal morphogenesis. Cambridge University Press, Cambridge
Moore D, Frazer LN (2002) Essential fungal genetics. Springer, Berlin Heidelberg New York
Müller F, Krüger D, Sattlegger E, Hoffmann B, Ballario P, Kanaan M, Barthelmess IB (1995) The cpc-2 gene of Neurospora crassa encodes a protein entirely composed of WD-repeat segments that is involved in general amino acid control and female fertility. Mol Gen Genet 248:162–173
Nowrousian M, Masloff S, Pöggeler S, Kück U (1999) Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol Cell Biol 19:450–460
Nowrousian M, Würtz C, Pöggeler S, Kück U (2004) Comparative sequence analysis of Sordaria macrospora and Neurospora crassa as a means to improve genome annotation. Fungal Genet Biol 41:285–292
Nowrousian M, Ringelberg C, Dunlap J, Loros J, Kück U (2005) Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol Genet Genomics 273:137–149
Pearson WR (1990) Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol 183:63–98
Perkins DD, Radford A, Newmeyer D, Björkman M (1982) Chromosomal loci of Neurospora crassa. Microbiol Rev 46:426–570
Pöggeler S, Kück U (2001) Identification of transcriptionally expressed pheromone receptor genes in filamentous ascomycetes. Gene 280:9–17
Pöggeler S, Kück U (2004) A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin. Eukaryot Cell 3:232–240
Pöggeler S, Nowrousian M, Jacobsen S, Kück U (1997) An efficient procedure to isolate fungal genes from an indexed cosmid library. J Microbiol Methods 29:49–61
Sachs MS (1996) General and cross-pathway controls of amino acid biosynthesis. In: Marzluf GA (ed) The Mycota III: biochemistry and molecular biology. Springer, Berlin Heidelberg New York, pp 315–345
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Acknowledgements
I thank Ingeborg Godehardt and Susanne Schlewinski for their excellent technical assistance, Edda Jung for her help in preparing the manuscript, Dr. S. Masloff for generating mutant pro4, Prof. G. Marzluf for providing the leu1 gene from N. crassa, Prof. G. Braus for his advice on analysing the transcriptional expression of cross-pathway-specific genes, Dr. M. Nowrousian for her advice and help in performing qRT-PCR experiments, and Dr. S. Pöggeler for her comments on the manuscript. We are grateful to an anonymous reviewer, who suggested the use of 5-methyl-tryptophan as an alternative amino acid analogue to induce the general control of amino acid biosynthesis. This work is supported by Deutsche Forschungsgemeinschaft (SFB 480, project A1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C.P. Hollenberg
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kück, U. A Sordaria macrospora mutant lacking the leu1 gene shows a developmental arrest during fruiting body formation. Mol Genet Genomics 274, 307–315 (2005). https://doi.org/10.1007/s00438-005-0021-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-0021-8