Abstract
The filamentous fungus Sordaria macrospora forms complex three-dimensional fruiting bodies that protect the developing ascospores and ensure their proper discharge. Several regulatory genes essential for fruiting body development were previously isolated by complementation of the sterile mutants pro1, pro11 and pro22. To establish the genetic relationships between these genes and to identify downstream targets, we have conducted cross-species microarray hybridizations using cDNA arrays derived from the closely related fungus Neurospora crassa and RNA probes prepared from wild-type S. macrospora and the three developmental mutants. Of the 1,420 genes which gave a signal with the probes from all the strains used, 172 (12%) were regulated differently in at least one of the three mutants compared to the wild type, and 17 (1.2%) were regulated differently in all three mutant strains. Microarray data were verified by Northern analysis or quantitative real time PCR. Among the genes that are up- or down-regulated in the mutant strains are genes encoding the pheromone precursors, enzymes involved in melanin biosynthesis and a lectin-like protein. Analysis of gene expression in double mutants revealed a complex network of interaction between the pro gene products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The formation of complex three-dimensional structures is a key feature during sexual development in many multicellular eukaryotes. The fruiting bodies of filamentous ascomycetes present an excellent example of such a developmental process. Fruiting body morphogenesis is controlled by exogenous factors as well as endogenous developmental programs (Moore-Landecker 1992). Besides the cells that directly participate in karyogamy and meiosis, many more specialized cell types are formed that comprise the mature fruiting body. Of the 28 recognized cell types in Neurospora crassa, 15 arise only during fruiting body formation (Bistis et al. 2003). Despite the well-characterized morphology and physiology of fruiting bodies, little is known about the molecular networks that direct their differentiation. Several genes essential for fruiting body formation have been identified, mostly in the model organisms Aspergillus nidulans, N. crassa, Podospora anserina and Sordaria macrospora (Moore 1998; Pöggeler et al. 2005). However, differentiation of the many cell types of the fruiting body requires complex patterns of gene expression which are coordinated in time and space and which remain to be characterized. Array techniques offer an attractive means for parallel investigation of the expression of multiple genes. In recent years, cDNA microarrays have been developed for several filamentous fungi and have provided a method for investigating fungal gene expression on a large scale (Nowrousian et al. 2004a). Microarrays have been used for example to analyze circadian clock-regulated gene expression in N. crassa (Lewis et al. 2002; Correa et al. 2003; Nowrousian et al. 2003), biomass degradation in Trichoderma reesei (Foreman et al. 2003), hypovirulence in Cryphonectria parasitica (Allen et al. 2003; Allen and Nuss 2004a, b), mycotoxin production in Fusarium verticillioides (Pirttila et al. 2004) and nutrient dependent gene expression in N. crassa, A. nidulans and A. oryzae (Aign and Hoheisel 2003; Maede et al. 2004; Sims et al. 2004).
Here, we present microarray analyses of gene expression in S. macrospora developmental mutants. Several sterile mutants of S. macrospora have already been used to identify molecular components essential for fruiting body development (Masloff et al. 1999; Nowrousian et al. 1999; Pöggeler and Kück 2004). Several of these mutants are blocked at the stage of protoperithecium formation and hence are designated ‘pro’ mutants. As the corresponding pro genes are essential for fruiting body formation, it might be expected that they are directly or indirectly involved in the regulation of gene expression during sexual development, and microarray analysis of mutant gene expression might help to identify genes that are part of downstream developmental pathways. S. macrospora is an eminently suitable model organism for array analyses of fruiting body formation, because, in contrast to A. nidulans and N. crassa, it does not produce any asexual spores. Therefore, changes in patterns of gene expression during sexual development are not obscured by changes related to asexual sporulation. Microarrays have not yet been developed for S. macrospora, but several EST libraries are available for N. crassa, from which cDNA microarrays have been prepared (Nelson et al. 1997; Dolan et al. 2000; Zhu et al. 2001; Lewis et al. 2002; Aign and Hoheisel 2003; Nowrousian et al. 2003). S. macrospora and N. crassa are closely related species which share a high degree of genomic sequence identity (Nowrousian et al. 2004b), and we have therefore used N. crassa cDNA microarrays for hybridization with probes prepared from S. macrospora. These cross-species hybridizations yielded significant signals, and expression patterns were verified independently by Northern blot analysis and quantitative real time PCR. Here, we present the results of microarray experiments using three S. macrospora pro mutant strains, as well as an analysis of gene regulation in double mutants.
Materials and methods
Strains, growth conditions and transformation
The S. macrospora strains S48977 (wild type), M8871 (mutant pro1), S24117 (mutant pro11), and S22528 (mutant pro22) were from our laboratory collection. The double mutants S54284 (pro1/11), S60752 (pro1/22), and S54477 (pro11/22) were obtained from crosses of single mutant strains. Double mutant genotypes were verified by crosses with single mutants and molecular analysis by Southern hybridization or PCR. Unless stated otherwise, standard growth conditions and DNA-mediated transformation were performed as described previously (Masloff et al. 1999; Nowrousian et al. 1999). For RNA extraction, S. macrospora was grown at 25 °C in constant light in a medium derived from synthetic crossing medium (Davis and de Serres 1970). This medium contains KNO3 (1 g/l), KH2PO4 (1 g/l), MgSO47 H2O (0.5 g/l), NaCl (0.1 g/l), CaCl2 (0.1 g/l), trace elements (0.1 ml/l), arginine (1 g/l), glucose (20 g/l), soluble starch (40 g/l; Difco) and biotin (0.1 mg/l). For inoculation, an agar plug (0.7 cm in diameter) bearing mycelium was placed in the center of a petri dish containing 20 ml of medium.
PCR amplification and cloning of S. macrospora gene fragments
Segments of S. macrospora genes were amplified from genomic DNA using HotMaster DNA polymerase (Eppendorf) and oligonucleotides derived from the corresponding N. crassa ORFs. Oligonucleotides were chosen in regions that are conserved between N. crassa and Magnaporthe grisea or Fusarium graminearum by comparing available genomic sequences (Galagan et al. 2003; http://www.broad.mit.edu/annotation/fungi/fgi/index.html). Partial sequences of the following S. macrospora genes have been deposited in the EMBL database under the Accession Nos. indicated: SMU4370 (AJ575136), SMU4533 (AJ575138), SMU7280 (AJ575150), parts of SMU9390 (AJ575161 and AJ575162), parts of SMU1747 (AJ575165 and AJ575166), SMU1835 (AJ575168), SMU2131 (AJ575170), SMU3387 (AJ575176), SMU3584 (AJ575177), SMU3600 (AJ781426), and SMU5651 (AJ781427). Gene names comprise the prefix ’SMU’ followed by a number corresponding to the number of the orthologous N. crassa ORF in the genome annotation of Galagan et al. (2003).
Preparation and analysis of RNA
RNA was prepared as described previously (Yarden et al. 1992), and poly(A) RNA was isolated from total RNA with a polyATtract kit according to the manufacturer’s protocol (Promega). The integrity of RNAs was verified by agarose gel electrophoresis and Northern blot analysis prior to extraction of poly(A) RNA. Northern blots were prepared and hybridized according to standard techniques (Maniatis et al. 1982) using 32 P-labeled DNA probes.
Microarray hybridization experiments and data analysis
The N. crassa microarrays used in these experiments were prepared from two unigene cDNA libraries described previously (Dvorachek et al. 2001; Nowrousian et al. 2003). The total number of clones used for array preparation was 2,880. Inserts of cDNA clones were amplified by PCR and spotted onto GAPSII slides (Corning) as described previously (Nowrousian et al. 2003). Microarray probes were made from 1 μg aliquots of S. macrospora poly(A) RNA by reverse transcription in the presence of aminoallyl-dUTP (Sigma) using Superscript II reverse transcriptase (Gibco), followed by coupling of Cy3 or Cy5 dye and hybridization as described previously (Nowrousian et al. 2003). For each mutant strain (pro1, pro11 and pro22), two independent experiments were carried out; in the first, the mutant RNA was labeled with Cy3 and the wild type with Cy5; in the second experiment, the dyes were switched.
Analysis of TIFF files from arrays was performed with ScanAlyze (written by Michael Eisen, Stanford-University; http://rana.lbl.gov/EisenSoftware.htm) or GeneTraffic (Iobion Informatics). The resulting data files were further analyzed with GeneTraffic (Iobion Informatics), Excel (Microsoft) or using Cluster and Treeview (Eisen et al. 1998). Thresholds for CH1GTB1 and CH2GTB1 values calculated by ScanAlyze were set to ≥0.55 or ≥0.65 to eliminate spots that had signals not significantly above background levels. To correct for differences between slides or for uneven loss of samples during target preparation, the following normalization method was employed: the average fluorescence value for the whole slide was determined for each slide within an experimental series and a normalization factor was determined. The corrected values for each cDNA clone were then used for calculation of expression ratios of mutant vs. wild type by dividing the value for the mutant strain by the wild type value. This method of normalization allowed us to focus on changes in the expression of a given gene by eliminating information about absolute expression levels. Only those clones were included in the final analysis for which a ratio could be calculated in five out of six independent experiments (two experiments for each of the three mutant strains). Clones were defined as regulated differently in a mutant if they fulfilled one of the following criteria: (1) mutant/wild type expression ratio ≤ 0.5 or ≥2 in the two independent experiments for the mutant strain; (2) the mean ratio of two independent experiments was ≤0.5 or ≥2 with a coefficient of variance <0.5.
Quantitative real time PCR
For use in quantitative real time PCR, 1 μg aliquots of total RNA was treated with DNase I (Invitrogen) according to the manufacturer’s recommendations. Then the RNA was combined with 0.5 μg of oligo-dT (Invitrogen) and 1 μg of random hexamers (Amersham) in a volume of 15.5 μl, and incubated for 10 min at 70 °C. The sample was cooled on ice for 10 min and reverse transcribed with 600 U of Superscript II reverse transcriptase (Invitrogen) and 0.5 mM dNTPs in a total volume of 30 μl for 2 h at 42 °C. RNA was then hydrolyzed for 15 min at 65 °C after the addition of 10 μl of 1 M NaOH and 10 μl of 0.5 M EDTA (pH 7.5). After neutralization with 25 μl of 1 M TRIS-HCl (pH 7.4), samples were cleaned with Amicon YM-30 columns (Millipore), vacuum-dried, resuspended in 30 μl of water and used for real time PCR. As a control for successful DNase treatment, each reverse transcription was carried out twice, once with and once without reverse transcriptase. All samples were used for real time PCR and only samples where the control without reverse transcriptase did not yield a PCR product were used for further quantification analysis.
Real time PCR was performed in an ABI 5700 (Applied Biosystems) or a DNA Engine Opticon 2 (MJ Research) with qPCR MasterMix for SybrGreen (Eurogentec) in a volume of 20 μl. Each reaction was carried out in triplicate with each oligonucleotide primer at 0.3 μM. Oligonucleotide primers used for real time PCR are listed in Table 1. Primers were selected to have melting temperatures of 61–62 °C and to yield amplicons of 120–150 bp. PCR conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by a melting curve analysis. Primer pairs that produced dimers as revealed by the melting curve analysis were not used further. Amplicon size for each primer pair was verified by gel electrophoresis. The efficiency of each primer pair was calculated from standard curves (Pfaffl 2001) or using LinRegPCR (Ramakers et al. 2003). Mean Ct values (threshold cycles) were calculated from the triplicates and used for calculations of expression ratios according to Paffl (2001) with primer-specific efficiencies. The Ct values for an amplicon derived from the SSU rRNA were used as a reference for normalization. Real-time PCR experiments with each strain and primer pair were carried out at least twice with biologically independent samples, and the significance of differential expression was verified using REST (the pairwise fixed reallocation randomisation test; Pfaffl et al. 2002).
Results
Microarray analysis of S. macrospora developmental mutants
We compared the gene expression profiles of the three S. macrospora developmental mutants pro1, pro11 and pro22 with that of the wild type. Although the mutants have similar phenotypes—in that their development does not progress beyond the stage of protoperithecium formation—they carry mutations in different genes. The pro1 gene encodes a zinc cluster transcription factor (Masloff et al. 1999, 2002), pro11 codes for a membrane-associated WD40 repeat protein (Pöggeler and Kück 2004), and pro22 for a putative membrane protein (Rech and Kück, in preparation). Analyses of mutants with blocks at the same developmental stage might help to identify common downstream pathways of development. In addition, such analyses can be used to differentiate between pathways that are regulated specifically by only one or two of the mutated genes.
We used cross-species microarray hybridization, hybridizing S. macrospora targets to N. crassa cDNA microarrays . The targets were derived from S. macrospora mycelia grown for 4–5 days. At this stage wild-type protoperithecia begin to develop into mature perithecia, whereas the mutant protoperithecia do not develop any further. The N. crassa cDNA microarrays were hybridized with the S. macrospora targets under the same conditions of high stringency that were previously used for hybridization with N. crassa targets (Nowrousian et al. 2003). Comparative sequence analyses have shown that the average degree of sequence identity between coding regions of S. macrospora and N. crassa is close to 90% (Nowrousian et al. 2004b), and cross-species hybridizations with similarly related non-fungal species have been performed successfully by others (see Discussion). Under high stringency conditions, overall signal intensity may be lower but—more importantly—the incidence of unspecific hybridization is reduced. This is crucial, as cases of unspecific hybridization cannot easily be distinguished from specific hybridization events on cDNA microarrays. For each mutant strain, two hybridization experiments were performed, switching the dye labels in the second experiment. Of the 2880 cDNA clones on the arrays, 1420 gave hybridization signals that were significantly above background in at least five out of the six hybridizations. For these 1420 clones, the ratio of mutant to wild type expression could be determined for all three mutant strains, and only these clones were used for further analysis.
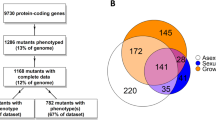
Since the clones on the arrays represent a combination of two independent unigene libraries (Dvorachek et al. 2001; Nowrousian et al. 2003), some genes are represented by more than one clone on the arrays. We performed an analysis of the EST sequences of the clones that were identified as differentially regulated in at least one of the mutant strains using the assembly program Phrap (P. Green, University of Washington, http://bozeman.mbt.washington.edu/phrap.docs/phrap.html). This analysis indicated that at least 5% of the genes were represented by clones from both libraries; elimination of these duplicates leaves 172 different genes that were up- or down-regulated in at least one of the mutant strains (Fig. 1). The 1,420 clones used in this analysis therefore most probably represent less than 1,350 different genes. However, our calculations were done using the conservative (uncorrected) number of 1,420 genes in total and the corrected number of 172 regulated genes. Thus, the 172 genes which were up- or down-regulated more than twofold in at least one of the mutants represent ~12% of all genes under investigation. Of these, 17 were up- or down-regulated in all three mutant strains, and an additional 24 genes were regulated differently in two out of the three mutants (Fig. 1).
Microarray analysis of gene expression in three developmental mutants. a Cluster analysis of gene expression in the three developmental mutants pro1, pro11 and pro22. Genes that are regulated differentially in at least one of the mutant strains are shown. Cluster analysis and visualization were done with Cluster and Treeview (Eisen et al. 1998). The graph shows logarithmic values of expression ratios (mutant/wild type) from two array experiments for each mutant strain. The number of the corresponding N. crassa ORF or EST and its GenBank Accession No. were determined by comparing the EST sequences of each of the spotted clones with the Neurospora genome sequence (http://www.broad.mit.edu/annotation/fungi/neurospora/) and with GenBank using BLASTX (Altschul et al. 1997). Homologues with known function are indicated; the Accession No. of the homologous sequence and the species of origin are given in cases where the organism is not N. crassa. Clusters (I–XII) of genes with different expression patterns in the three mutant strains are indicated on the left, the numbers indicate the following expression patterns: I, down-regulated in all three mutants; II, down-regulated in pro1 and pro11; III, down-regulated in pro1; IV, down-regulated in pro11 and pro22; V, up-regulated in pro1 and down-regulated in pro11, VI. down-regulated in pro11; VII, down-regulated in pro22; VIII, up-regulated in all three mutants; IX, up-regulated in pro11 and pro22; X, up-regulated in pro1; XI, up-regulated in pro11, XII, up-regulated in pro22. b Summary of genes that are up- or down-regulated in one or more of the mutants
Interestingly, only one gene (NCU01213.1, Fig. 1a, cluster V) was found to be up-regulated in one mutant (pro1) and down-regulated in another (pro11). All of the other 40 genes that were regulated differentially in more than one mutant strain showed the same tendency in all mutants. There are several possible explanations for this finding. It might be due to the fact that the only genes included in the analysis were those for which an expression ratio could be determined for all three mutant strains; but as there are up- as well as down-regulated genes in all mutant strains, this explanation does not seem very plausible. Instead, this finding might indicate that the three pro genes regulate at least some overlapping downstream developmental pathways in a similar manner.
To verify the results of the cross-species microarray hybridizations, we chose eleven genes with different expression patterns and isolated fragments from their corresponding S. macrospora orthologues by PCR using oligonucleotides derived from the N. crassa genome sequence (Galagan et al. 2003). The PCR fragments were cloned and sequenced, and used as probes for Northern blot analysis or to construct oligonucleotide primers for quantitative real time PCR. In addition, verification experiments were performed for ppg1, which was isolated in a previous study (Pöggeler 2000). The N. crassa ppg1 homologue, ccg-4, is represented on the arrays and was up-regulated in all three developmental mutants. For ppg1, as well as for 10 of the 11 newly identified S. macrospora genes, microarray expression patterns could be verified by Northern blot or real time PCR. The results for five of the genes are shown in Fig. 2, and the data for all 12 genes are summarized in Table 2. On the arrays, the genes SMU4533 and SMU9390 were found to be down-regulated in all three mutant strains, and this was verified by Northern analysis for SMU4533 (Fig. 2a) and by real time PCR for SMU9390 (Fig. 2d). Both SMU3600 and SMU1835 are down-regulated in pro1 and pro11, but not in the pro22 strain, and this could also be confirmed on Northern blots (Fig. 2b, c), whereas up-regulation of ppg1 was verified by real time PCR (Fig. 2e). These results demonstrate that overall the microarray data correctly reflect expression patterns. Expression ratios tend to be higher when using Northern blots or real time PCR, but this is a common finding in comparisons with microarray analyses. For one of the genes tested (SMU5651), the microarray data indicated down-regulation by more than twofold in pro1 and by less than twofold in pro11 and pro22. Real time PCR analysis showed greater than twofold down-regulation for the latter two mutants also (see below). These results indicate that the threshold of twofold up- or down-regulation is reasonable, as expression ratios which are determined using S. macrospora probes and primers tend to be higher than ratios derived from array data. Therefore, this threshold is more likely to yield false negatives than false positives; hence the overall numbers of regulated genes estimated from these experiments may be at the lower end of the true numbers.
Verification of microarray results by Northern analysis and quantitative real time PCR. The results obtained from the microarrays are shown in the panels on the left; the data shown on the right were obtained by Northern blot analysis (a–c) or real time PCR (d, e). Microarray and real time PCR data are given as logarithmic values of the mutant/wild type ratios (logarithm to the base 2 for the mean of at least two independent experiments). Real time PCR results were tested for the significance of differential expression at P=0.001 using REST (Pfaffl et al. 2002). Northern analyses (20 μg of total RNA per lane) were performed at least twice with RNAs prepared from independently grown mycelia. In each case data for only one experiment are shown
The EST sequences corresponding to the regulated genes on the arrays were compared to the Neurospora genome database (http://www.broad.mit.edu/annotation/fungi/neurospora/) and to GenBank (http://www.ncbi.nlm.nih.gov/BLAST/) using BLASTX (Altschul et al. 1997). Corresponding N. crassa genes and putative functional homologues are indicated in Fig. 1. About 65% of the regulated genes have homologues with known or putative function (Fig. 1, see also below). Among these genes is ppg1 which is one of the two pheromone precursor genes in S. macrospora (Pöggeler 2000). The N. crassa ppg1 homologue, ccg-4, and the second pheromone precursor gene mfa-1 are regulated differentially during sexual development of N. crassa (Bobrowicz et al. 2002), and mfa-1 has been shown to be essential for full fertility in N. crassa (Kim et al. 2002). mfa-1 was not represented on the arrays used in these experiments, but having discovered that ppg1 is up-regulated in the pro mutants (Figs. 1 and 2), we checked whether ppg2, the S. macrospora homologue of mfa-1 (Pöggeler 2000), is also up-regulated in the pro mutants. RNAs from developmental time courses from the pro1 mutant and the wild type were probed for ppg1 and ppg2, and both pheromone precursor genes turned out to be strongly up-regulated in the pro1 mutant, with transcript amounts increasing over time (Fig. 3). Northern analyses with RNAs from 4- to 5-day-old mycelia from mutants pro11 and pro22 also revealed up-regulation of ppg2 in these mutants (data not shown). These data provide the first link between the pro genes and pheromone precursor gene expression.
Expression analyses in double mutants
The microarray analyses identified a number of genes which are regulated differentially in more than one pro mutant strain. This finding indicates that the pro gene products have at least some downstream target genes in common. To further investigate genetic interactions between the pro genes, we investigated the expression of several of the regulated genes in all possible double mutants. The double mutant strains pro1/11, pro1/22 and pro11/22 were obtained from crosses between the single mutant strains. Like the single mutants, all double mutants are sterile and only produce protoperithecia. The results of the expression analyses were surprising in that expression profiles in double mutants did not follow a common pattern but were rather gene specific—and some of the genes were expressed at wild type levels in double mutants whereas they were down-regulated in each single mutant. Examples of this can be seen in the case of SMU4533 and SMU9390 (Fig. 4, Table 2). Both genes are down-regulated in all three single mutants, but have (almost) wild type transcript levels in the double mutants pro1/11 and pro1/22. Hence, combination of the mutant pro1 allele with either pro11 or pro22 restores transcript levels of these two genes to normal, whereas the combination of pro11 with pro22 does not. But this is not the case for all genes investigated, because SMU5651 is down-regulated in all single and double mutants.
Expression of regulated genes in double mutants. Northern hybridization (a) or quantitative real time PCR (b) analysis of gene expression in single and double mutants after 4 days of growth. Northern hybridization and real time PCR experiments were performed at least twice with RNAs from independently grown mycelia. Results for only one Northern experiment are shown in each case. Data for real time PCR are given as logarithmic values (base 2) of the mean expression ratio (mutant/wild type) from the different experiments; the results were tested for the significance of differential expression at P=0.001 using REST (Pfaffl et al. 2002). a Northern blots (loaded with 20 μg of total RNA per lane) were probed for SMU3600, SMU1835 or SMU4533. b Real time PCR for SMU5651 and SMU9390
SMU1835 exemplifies another kind of expression pattern. The gene is down-regulated in pro1, pro11 and in the pro1/11 double mutant, but is normal in pro22 and in both double mutants with pro22 (Fig. 4, Table 2). A different pattern again is observed for SMU3600, which is down-regulated in pro1 and pro11. The double mutants pro1/11 and pro1/22 have “intermediate” levels of the SMU3600 transcript compared to those in the respective single mutants. Taken together, these data indicate that several different pathways regulate the expression of downstream target genes and that each pro gene participates in more than one pathway.
A finding that might be related to this is that complemented transformants of the individual pro mutants show wild type-like expression patterns for most, but not all, of the genes investigated. Complemented transformants of pro1, pro11 or pro22 are fertile; therefore, one would expect expression patterns in general to be wild type-like; however, expression of SMU4533, for example, is not restored to fully wild type levels in any of the transformants, whereas expression of SMU5651 expression in pro11 transformants is even higher than in the wild type (data not shown). Some of the effects observed in the transformants might be due to position effects, although single-spore isolates from several independent transformants were investigated in all cases. A possible alternative explanation that might also be applicable to double mutants involves the assumption that the residual mutant proteins may have some effect, as discussed below.
Discussion
Identification of differentially regulated genes in S. macrospora developmental mutants
In this study, we have used cross-species microarray hybridization to identify developmentally regulated genes in S. macrospora. Studies of differential gene expression during fruiting body formation in filamentous fungi have been performed previously, mostly by EST sequencing or techniques based on differential hybridization. Such studies have been conducted with basidiomycetes like Agaricus bisporus (De Groot et al. 1997) and Agrocybe aegerita (Salvado and Labarere 1991) as well as ascomycetes like A. nidulans and N. crassa (Nelson and Metzenberg 1992; Lee et al. 1996), and in most cases led to the identification of 5–20 differentially expressed genes. Large scale EST sequencing of N. crassa yielded many genes derived from sexual differentiation-specific libraries (Nelson et al. 1997; Dolan et al. 2000). However, the proportion of the genes identified in differentiation-specific EST libraries that are preferentially expressed during fruiting body development has not yet been investigated on a larger scale. Initial small-scale array analyses of fungal fruiting body development have been performed with the ascomycete Tuber borchii (Lacourt et al. 2002). These authors compared gene expression in vegetative tissue and developing fruiting bodies at different stages using cDNA macroarrays with 171 genes. Although this study yielded a number of genes that were regulated differentially during fruiting body development, further experiments to elucidate the functions of these genes are hampered by the fact that truffle fruiting bodies cannot be grown under laboratory conditions and few molecular techniques are applicable to this species.
In our analyses, we used cross-species microarray hybridization, hybridizing S. macrospora targets to N. crassa cDNA microarrays. To the best of our knowledge, this is the first application of cross-species microarray hybridization to filamentous fungi. In recent years, it has been demonstrated that cross-species array hybridizations can be performed successfully with closely related species. Examples range from bacteria to plants and vertebrates, and both cDNA and oligonucleotide arrays have been used: There have been several reports of the use of human high-density oligonucleotide arrays with targets from non-human primates (chimpanzee, orangutan or rhesus monkey), but these species differ from each other in nucleotide sequence by less than 5% (Chismar et al. 2002; Enard et al. 2002). The slightly more divergent species Arabidopsis thaliana and Arabidopsis halleri share only 94% sequence identity; nevertheless, successful hybridization of high-density oligonucleotide A. thaliana arrays with targets derived from A. halleri is possible (Becher et al. 2004, Weber et al. 2004). Some 50–58% of the genes that gave a signal with A. thaliana targets also gave a signal with targets prepared from A. halleri. Borrelia hermsii targets were used successfully on B. burgdorferi nylon membrane arrays. The two Borrelia species share only 85% sequence identity within genomic ORFs, but cross-species array hybridizations yielded significant results for 72% of the chromosomal ORFs (Zhong and Barbour 2003). Similarly, human nylon membrane arrays have been used successfully with pig-derived targets, although sequence comparisons indicate that the overall sequence identity within coding regions between pig and human is only 84% (Medhora et al. 2002; Moody et al. 2002). Even hybridization of human cDNA nylon membrane arrays with opossum targets is possible, even though sequence comparison of 30 genes showed only 76% sequence identity (Wang et al. 2004). These reports indicate that species that show less than 20% nucleic acid sequence divergence can be used successfully for cross-species array hybridizations—at least with cDNA arrays, and our findings confirm that this applies to fungal species as well. S. macrospora and N. crassa show, on average, 89.5% sequence identity within coding regions (Nowrousian et al. 2004b) and, in agreement with reports on other species, our hybridizations yielded significant signals, and differential gene expression could be verified for most of the genes tested (Table 2). The genes for which expression was verified by Northern blot or real time PCR using S. macrospora probes and primers show on average 88.7% nucleic acid identity to their N. crassa orthologues within coding regions—close to the figure of 89.5% sequence identity found in the previous comparison of 85 genes (Nowrousian et al. 2004b). Our results indicate that, in some cases, even less than 80% sequence identity might be sufficient for cross-species hybridization: Expression of SMU4533 was found to be the same when determined with arrays and by Northern analysis (Fig. 2a), and this gene has only 78.9% nucleic acid identity to its N. crassa orthologue (Table 2). Two other genes, SMU5651 and ppg1, which have 80–85% nucleic acid identity to their N. crassa counterparts, also yielded reliable hybridization results on the arrays (Table 2). Altogether, there are 14 S. macrospora genes known that show less than 85% exon sequence identity to their N. crassa orthologues (Nowrousian et al. 2004b). Of these genes, five were represented on the arrays used here, and expression of three of them (SMU4533, SMU5651 and ppg1) was detected. The other two (NCU07908.1 and NCU09141.1 with 78.8% and 82.6% identity to S. macrospora, respectively) gave no significant signals, but as SMU4533 (78.9% sequence identity) was readily detected, this might be due to low expression levels rather than to insufficient homology. In summary, our data confirm that a level of ~80% sequence identity is sufficient for cross-species hybridizations using cDNA microarrays.
Differentially regulated genes might have functions in fruiting body development
In this study, we found 172 genes (12% of the total number investigated) to be differentially regulated in at least one of three developmental mutants of S. macrospora. The mutants carry mutations in three different genes, each of which is essential for fruiting body formation. Thus, it is conceivable that some of the genes that are regulated differentially in one or more of the mutant strains play important roles in fruiting body formation. BLASTX analysis of the 172 regulated genes showed that 112 genes (65%) have a homologue with a known or putative function (Fig. 1): 71 are up- and 41 are down-regulated, and these were sorted into functional classes (Fig. 5). Genes belonging to functional categories like metabolism and protein synthesis are present among both sets, but some functional categories are preferentially up- or down-regulated. Interestingly, among the down-regulated genes are genes involved in cell wall biogenesis and structure, as well as genes involved in secondary metabolism and genes which participate in developmental processes in other fungi (Fig. 5). Among the latter is SMU3600 (Fig. 2) which is an orthologue of the A. nidulans esdC gene. esdC (Accession No. AF532169.1) is annotated as a gene essential for sexual development in A. nidulans. Two other genes that are down-regulated are SMU3584 (NCU03584.1) and SMU9390 (NCU09390.1), and both are down-regulated in all three pro mutants (Figs. 1 and 2). They encode a putative polyketide synthase and a tetrahydroxynaphtalene reductase, respectively, both of which might be involved in melanin biosynthesis for ascospores and perithecial cell wall pigmentation. Another down-regulated gene is SMU5651 (Fig. 4), which shows highest similarity to a lectin isolated from fruiting bodies of Pleurotus cornucopiae (Iijima et al. 2002). Genes whose products participate in cell wall synthesis have also been identified as up-regulated in fruiting bodies of T. borchii compared to vegetative mycelium (Lacourt et al. 2002). In general, the process of fruiting body morphogenesis involves the differentiation of new cell wall structures and additional cell types with specialized functions (Moore-Landecker 1992; Bistis et al. 2003). Our data indicate that expression of many genes encoding factors for sexual development is dependent on the presence of functional pro genes, and that the three pro genes therefore represent positive regulators of a number of development-related genes.
Functional classification of differentially regulated genes. A total of 112 genes that have homologues with known or putative functions were regulated differentially in at least one of the three mutant strains. Of these, 71 genes were up-regulated and 41 genes down-regulated. Functional categories that are represented in both groups are indicated in shades of gray; categories that are represented in only one group are depicted in white
An obvious exception is the up-regulation of the pheromone precursor genes ppg1 and ppg2 in the pro mutants (Figs. 1 and 3); they were included in the class of genes involved in signal transduction (Fig. 5). One might speculate that the failure of protoperithecia to develop beyond a certain stage due to pro gene malfunction might lead to the loss of a signal which normally down-regulates the ppg genes and therefore to continued expression of the ppg genes. mfa-1, the N. crassa ppg2 orthologue, has been shown to be differentially expressed in a mating type-dependent manner and is required for fertility in N. crassa (Bobrowicz et al. 2002; Kim et al. 2002). The overexpression of the ppg genes in three developmental mutants might indicate that the ppg genes also play a role in S. macrospora development.
In general, most genes that were regulated differentially in more than one mutant were regulated similarly in both or all three mutants rather than being up-regulated in one and down-regulated in another mutant strain (Fig. 1). Taken together, these findings indicate that the three pro genes are involved in regulation of common downstream developmental pathways. The fact that several of the genes that were regulated differentially in one or more mutant strains are involved in developmental processes in other fungi is compatible with the idea that there is a core set of fungal developmental genes that can be identified by their expression patterns.
Several regulatory pathways form a complex network that regulates gene expression during development
As discussed above, pro1, pro11 and pro22 regulate the expression of a number of genes in a similar manner. Nevertheless, several different regulatory pathways can be distinguished, as shown by our analysis of double mutants (Fig. 4, Table 2). These results indicate that the genes necessary for sexual development regulate a complex genetic network, with each gene being involved in more than one regulatory pathway and some of the pathways converging on the same downstream targets. At this stage, it is difficult to offer any conclusive explanation for the different expression patterns at the molecular level. The gene products encoded by pro1, pro11 and pro22 are transcription factors or parts of signal transduction cascades, but evidence for direct interactions between them is lacking as yet. A fact which might further complicate the analysis is that pro11 and pro22 are not knockout mutants but carry point mutations, and residual mutant proteins might be present in the mutant strains. The pro11 mutant allele, for example, carries a point mutation which leads to a premature stop codon, truncating its product—a WD40 repeat protein (Pöggeler and Kück 2004). Most probably, the mutated PRO11 gene product still retains some function (Pöggeler and Kück 2004), and similar effects might occur in the pro22 mutant, as the pro22 mutant allele also contains a premature stop codon leading to a shorter ORF (Rech and Kück, in preparation). Homologues of both PRO11 and PRO22 have been shown to interact with a number of different regulatory proteins in yeast and other organisms (Kemp and Sprague 2003, Pöggeler and Kück 2004), and further analysis of putative interaction partners should help to elucidate their function and thereby explain the observed expression patterns in S. macrospora.
References
Aign V, Hoheisel JD (2003) Analysis of nutrient-dependent transcript variations in Neurospora crassa. Fungal Genet Biol 40:225–233
Allen TD, Nuss DL (2004a) Linkage between mitochondrial hypovirulence and viral hypovirulence in the chestnut blight fungus revealed by cDNA microarray analysis. Eukaryot Cell 3:1227–1232
Allen TD, Nuss DL (2004b) Specific and common alterations in host gene transcript accumulation following infection of the chestnut blight fungus by mild and severe hypoviruses. J Virol 78:4145–4155
Allen TD, Dawe AL, Nuss DL (2003) Use of cDNA microarrays to monitor transcriptional responses of the chestnut blight fungus Cryphonectria parasitica to infection by virulence-attenuating hypoviruses. Eukaryot Cell 2:1253–1265
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Becher M, Talke IN, Krall L, Krämer U (2004) Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J 37:251–268
Bistis GN, Perkins DD, Read ND (2003) Different cell types in Neurospora crassa. Fungal Genet Biol 50:17–19
Bobrowicz P, Pawlak R, Correa A, Bell-Pedersen D, Ebbole DJ (2002) The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol Microbiol 45:795–804
Chismar JD, Mondala T, Fox HS, Roberts E, Langford D, Masliah E, Salomon DR, Head SR (2002) Analysis of result variability from high-density oligonucleotide arrays comparing same-species and cross-species hybridizations. Biotechniques 33:516–524
Correa A, Lewis ZA, Greene AV, March IJ, Gomer RH, Bell-Pedersen D (2003) Multiple oscillators regulate circadian gene expression in Neurospora. Proc Nat Acad Sci USA 100:13597–13602
Davis RL, de Serres D (1970) Genetic and microbial research techniques for Neurospora crassa. Methods Enzymol 27A:79–143
De Groot PWJ, Schaap PJ, Van Griensven LJLD, Visser J (1997) Isolation of developmentally regulated genes from the edible mushroom Agaricus bisporus. Microbiology 143:1993–2001
Dolan PL, Natvig DO, Nelson MA (2000) Neurospora proteome 2000. Fungal Genet Newslett 47:7–24
Dvorachek WHJ, Dolan PL, Nelson MA, Natvig DO (2001) A provisional UniGene clone set based on ESTs from Neurospora crassa. Fungal Genet Newslett 48:12
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Nat Acad Sci USA 95:14863–14868
Enard W, Khaitovich P, Klose J, Zöllner S, Heissig F, Giavalisco P, Nieselt-Struwe K, Muchmore E, Varki A, Ravid R, Doxiadis GM, Bontrop RE, Pääbo S (2002) Intra- and interspecific variation in primate gene expression patterns. Science 269:340–343
Foreman PK et al (2003) Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J Biol Chem 278:31988–31997
Galagan J et al (2003) The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859–868
Huang X, Miller M (1991) A time-efficient, linear-space local similarity algorithm. Adv Appl Math 12:337–357
Iijima N, Yoshino H, Ten LC, Ando A, Watanabe K, Nagata Y (2002) Two genes encoding fruit body lectins of Pleurotus cornucopiae: sequence similarity with the lectin of a nematode-trapping fungus. Biosci Biotechnol Biochem 66:2083–2089
Kemp HA, Sprague GFJ (2003) Far3 and five interacting proteins prevent premature recovery from pheromone arrest in the budding yeast Saccharomyces cerevisiae. Mol Cell Biol 23:1750–1763
Kim H, Metzenberg RL, Nelson MA (2002) Multiple functions of mfa-1, a putative pheromone precursor gene of Neurospora crassa. Eukaryot Cell 1:987–999
Lacourt I, Duplessis S, Abbà S, Bonfante P, Martin F (2002) Isolation and characterization of differentially expressed genes in the mycelium and fruit body of Tuber borchii. Appl Env Microbiol 68:4574–4582
Lee DW, Lee SH, Hwang HA, Kim JH, Chae KS (1996) Quantitative analysis of gene expression in sexual structures of Aspergillus nidulans by sequencing of 3’-directed cDNA clones. FEMS Microbiol Lett 138:71–76
Lewis ZA, Correa A, Schwerdtfeger C, Link KL, Xie X, Gomer RH, Thomas T, Ebbole DJ, Bell-Pedersen D (2002) Overexpression of white collar-1 (WC-1) activates circadian clock-associated genes, but is not sufficient to induce most light-regulated gene expression in Neurospora crassa. Mol Microbiol 45:917–931
Maede H et al (2004) Transcriptional analysis of genes for energy catabolism and hydrolytic enzymes in the filamentous fungus Aspergillus oryzae using cDNA microarrays and expressed sequence tags. Appl Microbiol Biotechnol 65:74–83
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor,
Masloff S, Pöggeler S, Kück U (1999) The pro1+ gene from Sordaria macrospora encodes a C6 zinc finger transcription factor required for fruiting body development. Genetics 152:191–199
Masloff S, Jacobsen S, Pöggeler S, Kück U (2002) Functional analysis of the C6 zinc finger gene pro1 involved in fungal sexual development. Fungal Genet Biol 36:107–116
Medhora M, Bousamra II M, Zhu D, Somberg L, Jacobs ER (2002) Upregulation of collagens detected by gene array in a model of flow-induced pulmonary vascular remodeling. Am J Physiol Heart Circ Physiol 282:H414–H422
Moody DE, Zou Z, McIntyre L (2002) Cross-species hybridisation of pig RNA to human nylon microarrays. BMC Genomics 3:27
Moore D (1998) Fungal morphogenesis. Cambridge University Press, Cambridge
Moore-Landecker E (1992) Physiology and biochemistry of ascocarp induction and development. Mycol Res 96:705–716
Nelson MA, Metzenberg RL (1992) Sexual development genes of Neurospora crassa. Genetics 132:149–162
Nelson MA, Kang S, Braun E, Crawford M, Dolan P, Leonard P, Mitchell J, Armijo A, Bean L, Blueyes E, Natvig D (1997) Expressed sequences form conidial, mycelial, and sexual stages of Neurospora. Fungal Genet Biol 21:348–363
Nowrousian M, Masloff S, Pöggeler S, Kück U (1999) Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol Cell Biol 19:450–460
Nowrousian M, Duffield GE, Loros JJ, Dunlap JC (2003) The frequency gene is required for temperature-dependent regulation of many clock-controlled genes in Neurospora crassa. Genetics 164:923–933
Nowrousian M, Dunlap JC, Nelson MA (2004a) Functional genomics in fungi. In: Kück U (ed) The Mycota II. Springer, Berlin-Heidelberg-New York, pp 115–128
Nowrousian M, Würtz C, Pöggeler S, Kück U (2004b) Comparative sequence analysis of Sordaria macrospora and Neurospora crassa as a means to improve genome annotation. Fungal Genet Biol 41:285–292
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Pirttila AM, McIntyre LM, Payne GA, Woloshuk CP (2004) Expression profile analysis of wild-type and fcc1 mutant strains of Fusarium verticillioides during fumonisin biosynthesis. Fungal Genet Biol 41:647–656
Pöggeler S (2000) Two pheromone precursor genes are transcriptionally expressed in the homothallic ascomycete Sordaria macrospora. Curr Genet 37:403–411
Pöggeler S, Kück U (2004) A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin. Eukaryot Cell 3:232–240
Pöggeler S, Nowrousian M, Kück U (2005) Fruiting body development in ascomycetes. In: Kües U, Fischer R (eds) The Mycota I. Springer, Berlin, Heidelberg, New York
Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM (2003) Assumption-free analysis of quantitative real-time polymerase chain (PCR) data. Neurosci Lett 339:62–66
Salvado JC, Labarere J (1991) Isolation of transcripts preferentially expressed during fruit body primordia differentiation in the basidiomycete Agrocybe aegerita. Curr Genet 20:205–210
Sims AH, Robsons GD, Hoyle DC, Oliver SG, Turner G, Prade RA, Russell HH, Dunn-Coleman NS, Gent ME (2004) Use of expressed sequence tag analysis and cDNA microarrays of the filamentous fungus Aspergillus nidulans. Fungal Genet Biol 41:199–212
Wang Z, Dooley TP, Curto EV, Davis RL, VandeBerg JL (2004) Cross-species application of cDNA microarrays to profile gene expression using UV-induced melanoma in Monodelphis domestica as the model system. Genomics 83:588–599
Weber M, Harada E, Vess C, v. Roepenack-Lahaye E, Clemens S (2004) Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J 37:269–281
Yarden O, Plamann M, Ebbole D, Yanofsky C (1992) cot-1, a gene required for hyphal elongation in Neurospora crassa encodes a protein kinase. EMBO J 11:2159–2166
Zhong J, Barbour AG (2003) Cross-species hybridization of a Borrelia burgdorferi DNA array reveals infection- and culture-associated genes of the unsequenced genome of the relapsing fever agent Borrelia hermsii. Mol Microbiol 51:729–748
Zhu H, Nowrousian M, Kupfer D, Colot HV, Berrocal-Tito G, Lai H, Bell-Pedersen D, Roe BA, Loros JJ, Dunlap JC (2001) Analysis of expressed sequence tags from two starvation, time of day-specific libraries of Neurospora crassa reveals novel clock-controlled genes. Genetics 157:1057–1065
Acknowledgements
The authors thank Swenja Ellßel and Susanne Schlewinski for excellent technical assistance, Dr. Stefanie Pöggeler for primers and probes for the ppg genes, and Dr. Georg Zoidl (Bochum) for generous provision of real time PCR facilities. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 480 project A1)
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.J. Punt
Note added in proof
Note added in proof
While our manuscript was under review, Te Biesebeke and coworkers described cross-species hybridization of cDNA macroarrays from the filamentous fungus Aspergillus niger with Aspergillus oryzae targets (Te Biesebeke et al. 2005, Mol Genet Genomics, in press). Our finding that pgg transcript levels are up-regulated in the mutants pro1, pro11, and pro22 was recently supported by an investigation demonstrating that the pheromones themselves are up-regulated in the mutant strains (Mayrhofer and Pöggeler 2005, Eukaryot Cell, in press).
Rights and permissions
About this article
Cite this article
Nowrousian, M., Ringelberg, C., Dunlap, J.C. et al. Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol Genet Genomics 273, 137–149 (2005). https://doi.org/10.1007/s00438-005-1118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-005-1118-9