Abstract
Toxoplasma gondii is a widespread zoonotic protozoan that infects most species of mammals and birds, including poultry. This study aimed to investigate the course of T. gondii infection and the efficacy of diclazuril and Artemisia annua in preventing infection in experimentally infected chickens. Seventy-five 1-month-old chickens, female and male, were randomly divided into five groups (n = 15 each) as follows: (1) uninfected untreated (negative control, NC); (2) infected with T. gondii genotype II/III isolated from a wild cat (group WC); (3) infected with T. gondii genotype II isolated from a domestic cat (group DC); (4) infected with T. gondii domestic cat strain and treated with the anticoccidial diclazuril (group DC-D); and (5) infected with T. gondii domestic cat strain and treated with the medicinal plant Artemisia annua (group DC-A). Clinical signs, body temperature, mortality rate, weight gain, feed conversion ratio, hematological parameters, and the presence of T. gondii–specific IgY antibodies were recorded in all groups. Five chickens per group were euthanized 28 days post-infection (p.i.) and their brains, hearts, and breast muscle tested for T. gondii by mouse bioassay and polymerase chain reaction (PCR). No clinical signs related to the experimental infection were observed throughout the study period. T. gondii–specific antibodies were detected by day 28 p.i., but not in all infected chickens. Overall, T. gondii DNA was detected (bioassay or tissue digests) in all infected and untreated chickens (10/10), while viable parasite (bioassay) was isolated from 7 out of 10 chickens. The parasite was most frequently identified in the brain (7/10). There were no differences in the T. gondii strains regarding clinical infection and the rate of T. gondii detection in tissues. However, higher antibody titers were obtained in chickens infected with T. gondii WC strain (1:192) comparing with T. gondii DC strain (1:48). A. annua reduced replication of the parasite in 3 out of 5 chickens, while diclazuril did not. In conclusion, broiler chickens were resistant to clinical toxoplasmosis, irrespective of the strain (domestic or wild cat strain). The herb A. annua presented prophylactic efficacy by reduced parasite replication. However, further studies are required aiming at the efficacy of diclazuril and A. annua for the prevention of T. gondii infection in chickens using quantitative analysis methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii is a widespread zoonotic protozoan that infects mammals, including felids and humans, and birds. The definitive hosts, cats, and other felids are the only source of infective oocysts, while the intermediate hosts (mammals, birds, and humans) develop tissue cysts. The main sources of infection for humans are the consumption of raw or undercooked meat, as well as water and food (fruit, vegetables) contaminated with sporulated oocysts from the environment (Dubey 2010).
This apicomplexan parasite is an important pathogen with a varying prevalence in poultry, which depends on the raising system. A wide range of seroprevalence values have been reported, from 0% in broiler chickens (Rodrigues et al. 2019) to 100% in organic and backyard farm chickens (Dubey 2010). The backyard and free-range chickens represent a good indicator of soil contamination with T. gondii since they feed on the ground. Also, they play an important role in the epidemiology of T. gondii, as in the case of home slaughtering and improper offal disposal, they are the source of infection for cats (Dubey 2010). Parasite isolation studies have shown that the prevalence of viable T. gondii in chickens from commercial indoor farms is low (Dubey 2010), but can be high in backyard chickens (Dubey et al. 2004; Schares et al. 2017). Therefore, consumption of undercooked infected chicken meat can be a potential source of infection with T. gondii for humans (Dubey 2010).
Despite numerous studies on T. gondii seroprevalence and parasite isolation in chickens, only a few clinical cases of chicken toxoplasmosis were reported worldwide (Dubey 2010). The strain of T. gondii has been shown to influence the course of infection in gallinaceous birds (Dubey et al. 1993; Koethe et al. 2015). Options for treatment of toxoplasmosis in chickens are limited. For small animals (i.e., cats), drugs such as clindamycin, azithromycin, and combinations of trimethoprim and a sulfonamide are used (Elmore et al. 2010; Lappin 2010; Bresciani et al. 2016). Diclazuril is a coccidiostat used for the prophylaxis of coccidiosis in broilers (Zechner et al. 2015; Ogolla et al. 2018). As well, experimental studies in mice have shown its efficacy against toxoplasmosis (Lindsay et al. 1995). Natural products from plant extracts have exhibited an anticoccidial activity in both in vitro and in vivo models (Mirzaalizadeh et al. 2018; de Almeida et al. 2012; Drăgan et al. 2014). Sweet wormwood plant (Artemisia annua) and its derivatives have shown efficacy in experimental models of toxoplasmosis in mice (Nagamune et al. 2007; Dunay et al. 2009; Hencken et al. 2010; Islamunddin et al. 2015; Müller et al. 2015).
The current study aimed to evaluate (i) the T. gondii clinical infection in chickens experimentally infected with a low dose of cysts of different strains (genotype II/III strain vs. genotype II strain), (ii) the rate of T. gondii infection in the brain, heart, and skeletal muscle, and (iii) the efficacy of diclazuril and A. annua for the prevention of T. gondii infection in chickens.
Materials and methods
Chickens
One-day-old chickens were purchased from S.C. AVIS S.A. Vadu Crișului and housed until 1 month old in batteries in dedicated facilities of the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca (UASVM CN). The chickens were fed with standard starter and growing feed supplemented with an anticoccidial drug (diclazuril). Diclazuril was withdrawn 2 days before the start of the experiment. Water and feed were provided ad libitum and the light was continuous. The experiment was approved by the Animal Ethics Committee of UASVM CN (protocol no. 92/20.12.2017).
Experimental design
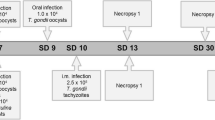
Seventy-five 1-month-old chickens were randomly divided into five groups (n = 15 each) each with three replicates of five chickens per cage, as follows: (1) uninfected untreated (negative control, NC); (2) infected with T. gondii genotype II/III isolated from a wild cat (group WC); (3) infected with T. gondii genotype II isolated from a domestic cat (group DC); (4) infected with T. gondii domestic cat strain and treated with the anticoccidial diclazuril (group DC-D); and (5) infected with T. gondii domestic cat strain and treated with the medicinal plant Artemisia annua (group DC-A) (Table 1). The day of the infection was designated as day 0, when, according to the experimental group, twelve T. gondii tissue cysts per chicken were administered by oral gavage (Table 1). Diclazuril and A. annua were introduced into the diet 2 days before experimental infection (day − 2) and continually administered until the end of the experiment (day 28 post-infection [p.i.]) (Fig. 1).
All chickens were monitored throughout the study period for any change in health status and mortality. Copro-parasitological examination by the flotation technique using a saturated salt solution (specific gravity 1.28) was performed on days 3 and 7 p.i. Body temperature (BT) was registered daily during the first-week p.i. Chickens were individually weighed at days 0, 7, and 28 p.i., to calculate the body weight gain (BWG). The quantity of feed given to the chickens was weighed daily per cage, and the feed conversion ratio (FCR) was calculated as the ratio between the amount of feed consumed and the weight gain of the chickens (Pop et al., 2015).
Blood samples were collected from 7 chickens per group on days 0, 3, 7, and 28 p.i. The samples were analyzed for hematological parameters (days 0, 7, and 28) and direct (DNA) (days 3 and 7) and indirect (specific IgY antibodies) (days 0, 7, and 28) detection of T. gondii. Then, 5 chickens per group were euthanized on day 28 p.i. The brain, heart, and skeletal muscle were collected from each chicken and analyzed by mouse bioassay and PCR.
T. gondii strains and experimental infection
T. gondii strains isolated by mouse bioassay from a wild cat (Felis silvestris), and a domestic cat (Felis catus), were used for experimental infections (Table 1).
The wild cat strain was obtained from a road-killed animal. All heart was collected and artificial digestion performed (for details, refer to the “Mouse bioassay” section). Two mice were inoculated and euthanized by cervical dislocation at 4 weeks p.i. Mouse brains were examined by microscopy for the presence of T. gondii cysts and analyzed by PCR for confirmation. Then, the isolated strain was genotyped by the multiplex nested PCR-RFLP technique (Su et al. 2006; Khan et al. 2005), with the use of the following markers: GRA6, altSAG2, BTUB, APICO, C22, C29-2, PK1, and CS3. PCR products were digested with appropriate restriction enzymes for the different markers. The products obtained after digestion were visualized by electrophoresis using a 3% agarose gel.
The domestic cat was presented to our clinic with diarrhea and apathy. T. gondii–like oocysts were observed on parasitological examination of the feces, and T. gondii DNA was identified by PCR. The oocysts were sporulated in 2.5% potassium dichromate and then orally administered to four mice. The strain was previously genotyped by the PCR-RFLP technique and identified as type II (ToxoDB number 1) (Nedișan et al., 2019).
The T. gondii cysts used for experimental infection of chickens were obtained after the second passage in mice for each strain. Briefly, ten mice per strain were inoculated intraperitoneally (i.p.) with mouse brain cysts and after 1 month, the mice were euthanized. The brains were recovered and homogenized with a saline solution. A 20-μL drop of the homogenate was placed on a microscope slide and the cysts counted under a microscope (Djurković-Djaković et al. 2005). Finally, the number of T. gondii cysts in the suspension was adjusted to 12 cysts/0.5 mL (Table 1).
Medication
Diclazuril (Clinacox® 0.5%, Huvepharma) and A. annua (leaf powder) were used for the prevention of T. gondii infection in chickens. Diclazuril was administered in the chicken diet to group DC-D at a concentration of 1.2 ppm (1.2 g/1000 kg feed) delivering 0.15 mg diclazuril/kg body weight (FAO/WHO, 2000).
A. annua plants were dried in the shade at ambient temperatures (20 °C). After drying, leaves were manually separated and ground to obtain A. annua leaf powder (Drăgan et al. 2014). The concentration of artemisinin (1.706% w/w) was evaluated by HPLC 3 months before the experiment (Ferreira and Gonzalez 2009). Broiler feed was prepared by adding 4 kg of A. annua leaf powder per 1000 kg of feed (0.4%) and fed daily to group DC-A. The amount of artemisinin in feed supplemented with 0.4% A. annua leaf powder was 68.24 ppm (Allen et al. 1997; Pop et al. 2015; Györke et al. 2019) delivering 8.53 mg artemisinin/kg body weight/day (FAO/WHO, 2000). The feed supplemented with diclazuril and A. annua was administered 2 days before the experimental infection (day − 2) until the end of the experiment (day 28 p.i.).
Hematological parameters
Red blood cell (RBC) count, hemoglobin (Hb) concentration, hematocrit (PCV), and the total and differential white blood cell (WBC) counts were performed on days 0, 7, and 28 p.i. The concentration of Hb was determined by the spectrophotometer method (ELISA Bio-Rad 1100 microplate reader). After adding 4% ammonia solution to the blood sample, the absorbance was measured at 540 nm.
The PCV was established using the microhematocrit method. The capillary tubes were centrifuged at 12,000 rpm for 5 min. The values were determined with the aid of a microhematocrit reader.
The RBC and WBC counts were carried out manually by the hemocytometer method using the Natt-Herrick solution modified by the Prochaska (Ognean and Cernea 2011).
Serology
Specific IgY antibodies against T. gondii were evaluated on days 0, 7, and 28 p.i. by MAT, TgSAG1-ELISA, and immunoblot using p30 antigen. Serum samples were sent to ANSES - Laboratoire de Santé Animale (Maisons-Alfort, France) for MAT and to Friedrich-Loffler-Institut (Germany) for TgSAG1-ELISA, and immunoblot. A positive sample in any of the used methods was considered positive in total serology.
MAT
T. gondii–specific IgY antibodies were detected by the modified agglutination test (MAT) (Desmonts and Remington 1980). Formalin-fixed whole RH tachyzoites were used as antigens. The antigen was provided by the National Reference Centre for Toxoplasmosis in Reims, France (Villena et al. 2012). The starting serum dilution was 1:6, and the sera were titrated to end-point in two-fold dilutions. All sera reactive at a serum dilution of 1:24 were considered positive (Dubey et al. 1993).
TgSAG1-ELISA
Chicken sera were also tested for antibodies against T. gondii tachyzoite surface antigen TgSAG1 as described by Schares et al. (2017). Affinity-purified TgSAG1 of T. gondii tachyzoites was diluted in bicarbonate buffer (0.1 M, pH 8.3) and used at a concentration of 30 ng/mL to sensitize ELISA plates. The plates were then washed with PBS supplemented with 0.05% (v/v) Tween® 20 (Serva, Heidelberg, Germany) (PBST). A blocking step with 1% casein in PBST (CasPBST; 30 min, 37 °C) followed. The plates were emptied and washed and 100 μL of serum, 1:200 diluted in CasPBST, was added for 30 min, 37 °C. After serum incubation and washing, a species-specific conjugate (goat anti-chicken IgY (H&L) peroxidase (POD), synonymous with anti-chicken IgY (H&L) POD, Rockland Immunochemicals, Dianova, Hamburg, Germany) was diluted 1:4000 in CasPBST. After washing with PBS-T (thrice) and distilled water (once), reactions were visualized using 1% tetra-methyl-benzidine (TMB) with 0.012% (v/v) H2O2 as the substrate. After 13–15 min at 37 °C, the reaction was stopped by the addition of 50 μL of 2 M H2SO4, and the O.D. in each well was read at 450 nm. ELISA index values (I) were calculated for each sample (S) based on the means of two O.D. values: IS = (O.D.S − O.D.NC)/(O.D.PC − O.D.NC). A cut-off optimized for maximum diagnostic specificity was applied (ELISA index 0.242) as previously described for the TgSAG1-ELISA (Schares et al. 2017).
Immunoblot using p30 antigen
The sera were analyzed according to the protocol described by Schares et al. (2017). Briefly, the p30 surface antigen was obtained from T. gondii RH strain cell culture–derived tachyzoites by affinity chromatography using the monoclonal antibodies. Purified p30 was incubated in non-reducing sample buffer (2% [w/v] sodium dodecyl sulfate (SDS), 10% [v/v] glycerol, 62 mM Tris-HCl, pH 6.8) for 1 min (94 °C), separated in 12% (w/v) SDS polyacrylamide mini gels, and transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Germany). After the transfer, membranes were blocked using PBS-TG (PBS with 0.05% (v/v) Tween 20 (Sigma-Aldrich, Germany) and 2% (v/v) liquid fish gelatine (Serva, Germany)) and cut into 50 strips and examined. Chicken sera were diluted 1:100 in PBS-TG. Peroxidase-conjugated anti-chicken IgY (H + L) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was used diluted 1:500 in PBS-TG. Positive and negative controls were used.
Mouse bioassay
Five of the fifteen chickens from each group were euthanized on day 28 p.i. Whole brain, whole heart, and half of the breast muscle were sampled separately from each chicken (n = 75). The tissue samples were bioassayed in 6–8-week-old female mice, CD1 line (one mouse/tissue; n = 75). Mice were housed and maintained following the regulations on the welfare and protection of laboratory animals, according to the national Romanian law 43/2014 and EU Directive 2010/63/EU. All mouse bioassays reported in this publication were approved by the Animal Ethics Committee of UASVM Cluj-Napoca (protocol no. 93/20.12.2017).
Each tissue sample was weighed, ground, and homogenized with digestion solution (0.25% trypsin from swine pancreas, Sigma-Aldrich code 93613; EDTA 0.025%; PBS) (50 mL for 20 g tissue). Tissue digestion was performed at 37 °C for 90 min. The digest was then centrifuged at 3000 rpm for 10 min. The supernatant was removed, and the sediment washed twice with PBS in repeated centrifugations. Finally, the supernatant was removed and the sediment (digest pellet) reconstituted in 2 mL of PBS, to which 200 μL of the antibiotic solution was added (penicillin-streptomycin, Sigma-Aldrich code P0781) (Montoya et al. 2009).
One mouse per tissue was i.p. inoculated with 1 mL tissue digest. Following inoculation, mice were housed in standard cages. Each cage was identified by the sample code and the date of inoculation. Inoculated mice were monitored for clinical signs. At 4 weeks p.i., mice were euthanized by cervical dislocation and brains harvested. Each mouse brain was checked by microscopy for the presence of T. gondii cysts (Paștiu et al. 2019) and analyzed by PCR (25 mg of the homogenized whole brain) for confirmation (Homan et al. 2000).
PCR assays
The buffy coat (WBCs) (days 3 and 7 p.i.), brain, heart, and breast muscle digests from chickens (day 28 p.i.) and the brain homogenates from the mouse bioassays were analyzed by conventional PCR. WBCs were isolated by centrifugation at 1500–2000g for 10–15 min at room temperature. After centrifugation, the buffy coat containing the WBCs was collected with a plastic transfer pipet.
The DNA extraction was performed from 100 μL of the buffy coat, digest, and mouse brain homogenate using the commercial Isolate II Genomic DNA Kit (Bioline) according to the manufacturer’s instructions.
All DNA samples were analyzed with conventional PCR targeting the 529-bp DNA fragment of T. gondii using Tox4 (5′-CGCTGCAGGGAGGAAGACGAAAGTTG-3′) and Tox5 (5′-CGCTGCAGACACA GTGCATCTGGATT-3′) specific primers (Homan et al. 2000).
Amplification was performed in a final volume of 25 μL consisting in 5 μL PCR Master Mix (12.5× Green PCR Master Mix), 10 pmol of each primer (Tox4 and Tox5), 4 μL of sample DNA, and 15 μL of ultrapure water. Positive (T. gondii domestic cat strain) and negative controls (ultrapure water) were used in each set of reactions. Amplification was performed with the C1000TM Thermal Cycler (Bio-Rad). The amplification program consisted of one initial cycle at 95 °C (5 min) and 37 cycles of denaturation at 95 °C (30 s) followed by hybridization (annealing) at 60 °C (30 s), extension at 72 °C (1 min), and a final extension cycle at 72 °C (5 min). In the end, PCR products (8 μL) were electrophoresed in 1.5% agarose gel in TAE buffer and stained with SYBR® Safe DNA gel stain (Invitrogen). Electrophoresis conditions were 100 V and 400 mA for 30 min. in TAE buffer. DNA fragments were visualized under UV light on an image analyzer (Bio-Rad BioDoc-It™ Imaging System) and compared to a 100-bp molecular weight marker (GeneRuler 100 bp DNA Ladder, Fermentas).
Statistical analysis
Arithmetic mean and standard error of the mean were calculated for numerical data. The data distribution was assessed by the Shapiro-Wilk test for normal distribution. If data followed a normal distribution, one-way analysis of variance with Tukey-Kramer as a post hoc test was used to examine differences within each experimental group at different time points, and repeated measures analysis of variance was used to compare experimental groups at specific time points (0, 7, and 28 days p.i.). In the case of the non-normal distribution of the data, the non-parametric Kruskal-Wallis with Conover as post hoc tests and the Friedman test were used. The level of significance was set at 0.05. All statistical analyses were performed with the MedCalc Statistical Software version 19.0.7.
The degree of agreement between serological methods (MAT, TgSAG1-ELISA, immunoblot) was measured by Cohen’s kappa (k) statistic in EpiTools (Watson and Petrie 2010; Sergeant 2018). Also, the proportions of the positive and negative agreements were calculated. The level of confidence was set at 0.95 and population status as unknown/mixed. The strength of agreement was defined based on the k value: < 0.00 poor agreement; 0.00–0.20 slight agreement; 0.21–0.40 fair agreement; 0.41–0.60 moderate agreement; 0.61–0.80 substantial agreement; and 0.81–1.00 almost perfect agreement (Landis and Koch 1977).
Results
T. gondii genotypes
The domestic cat strain was type II (ToxoDB number 1) and it was previously published (Nedișan et al. 2019). The wild cat strain, according to the RFLP analysis, was identified to belong to genotype II/III (Table 2).
T. gondii infection
T. gondii DNA was detected by PCR in the blood on day 3 p.i. in all experimentally infected groups, but at a different rate (Table 3). The group infected with wild cat strain (WC) presented a higher rate (71.4%; 5/7) of detection comparing with the group infected with domestic cat strain (DC) (28.5%; 2/7). The rate of T. gondii DNA detection decreased on day 7 p.i. in all groups (Table 3).
The results of T. gondii detection in chicken tissues (brain, heart, and breast muscle) on day 28 p.i. are presented in Table 4. T. gondii cysts were not identified by microscopic examination of the mouse brains.
Overall, T. gondii DNA was detected by PCR (in bioassay or in tissue digest) in all infected and untreated chickens regardless of the parasite strain. By bioassay, the rate of detection was of 80% (4/5) in chickens infected with DC strain and of 60% (3/5) in those infected with WC strain. Overall, the brain was the tissue of predilection for T. gondii in chickens 70% (7/10), followed by heart 40% (4/10) and breast 40% (4/10). However, in three chickens, T. gondii DNA was not detected in the brain, but in the heart or breast muscle.
None of the experimentally infected chickens had detectable T. gondii antibodies on day 7 p.i., and not all the chickens on day 28 p.i. regardless of the parasite strain (Table 5). The control group remained negative during the experiment. The total rate of seroconversion in infected groups was 57.2% and different accordingly to the serological test: 35.7% by MAT, 19.2% by TgSAG1-ELISA, and 42.3% by immunoblot. There was no statistically significant difference (χ(12) = 3.45, P = 0.327) among T. gondii-infected groups. The highest serum titer was 1:48 for groups infected with T. gondii DC strain and 1:192 for group infected with T. gondii WC strain (Table 6).
Substantial agreement was observed between MAT and TgSAG1-ELISA, and moderate agreement for the other serological tests pairs (Table 7). Four out of 16 seropositive chickens have been detected positive in all three serological tests.
Clinical toxoplasmosis
No clinical signs related to the experimental infection were observed. None of the animals died, showed signs of apathy, or had increased body temperature (BT). At day 3 p.i., all groups, including animals from the NC, presented mild diarrhea and hematochezia. The copro-parasitological exam was negative. The BT was in the normal range (40.17–42.52) for the species in all experimental groups (Christensen et al. 2012) (Fig. 2).
Compared to control animals, all groups of infected animals irrespective of the strain or further treatment had both a lower weight gain and a higher FCR, although not statistically significant throughout the experiment (Supplementary file 1).
The erythrocyte count (RBCs) and the concentration of Hb were in the normal range of the species during the entire experiment (Supplementary file 2). No significant differences were found among groups or days of sampling. Generally, WBC decreased significantly (P < 0.001) from day 0 to day 28 p.i. in all experimental groups. In contrast, the percentage of monocytes increased from day 0 to day 28 p.i. (Supplementary file 2) in WC (F(2,17) = 0.405, P = 0.673), DC (F(2,15) = 8.625, P = 0.003), and DC-A (F(2,17) = 2.637, P = 0.101) groups and decreased in NC (F(2,16) = 0.790, P = 0.471) and DC-D (F(2,16) = 2.597, P = 0.106) groups, but no significant differences were noted among groups at any time point.
The efficacy of diclazuril and Artemisia annua
From the clinical and serological point of view, infected and treated chickens presented similar aspects with infected and untreated chickens (Supplementary files 1–2, Fig. 2, and Tables 5 and 6). Also, none of the mice presented clinical signs of toxoplasmosis and T. gondii cysts were not identified by microscopic examination of their brains.
Regarding the T. gondii DNA detection in tissues on day 28 p.i., the data varied according to the treatment (Table 4). Overall, the rate of T. gondii detection was lower in chickens treated with A. annua (40.0%; 2/5) comparing with that in untreated ones (5/5) or even with those treated with diclazuril (5/5).
Based on bioassay, the viable parasite was detected in 2 (from brain and heart) out of 5 chickens treated with A. annua and in 3 (from brain) out of 5 chickens treated with diclazuril, while in untreated ones in 4 (from the brain, heart, and breast muscle) out of 5 chickens (Table 4).
When bioassay was compared with PCR on tissue digests, in the A. annua–treated group, the detection rate was higher in bioassay. Contrary, in group treated with diclazuril, the detection rate was higher in tissue digests. In case of the untreated group, the rate of detection was similar in bioassay and tissue digests (Table 4).
Discussion
The present study was designed to assess the clinical infection of experimental infection with a low dose of cysts of two different T. gondii strains in chickens, the rate of proliferation of T. gondii in tissues, and the efficacy of the prophylaxis with two different products: an anticoccidial drug, diclazuril, and a medicinal plant, A. annua. During the study period, no clinical signs attributable to T. gondii infection were observed. However, mild diarrhea was noticed on day 3 in all experimental groups that could be linked to a dysmicrobism of the intestinal flora caused by food change from starter to growing diet. Previously, other experimental infections showed that chickens are resistant to clinical toxoplasmosis and only a few clinical cases were reported (Dubey 2010). We noticed a slight increase in the WBC and monocytes in DC and WC groups at 7 days p.i. T. gondii has the capability to replicate in chicken macrophages, representing a reservoir for the parasite (Malkwitz et al. 2013; Quéré et al. 2013). The white blood cells followed a decreasing trend in all chickens, this leukogram pattern being consistent with a stress leukogram due to endogenous steroid release in stressful conditions (Maxwell 1993; Schmidt 2015).
Anti–T. gondii antibodies were detected on day 28 p.i. and not all experimentally infected chickens seroconvert (57.2%). We cannot exclude seroconversion before, as the serology was performed on days 7 and 28 p.i. Similar results were obtained by Godoi et al. (2010) with a seroconversion of 58.3% (by MAT, and IFAT) in experimentally infected pigeons with T. gondii oocysts. The rate of seroconversion in birds seems to vary with the infective dose and parasite strain (Geuthner et al. 2014; Geuthner et al. 2019).
T. gondii DNA was detected overall in all infected and untreated chickens, irrespectively of the parasite strain. The rate of T. gondii DNA detection was comparable in bioassay (7/10) and tissue digests (8/10). Then, the T. gondii DNA was more frequently detected in the brain, than in the heart and breast muscle. It is well known that a positive PCR in a tissue does not mean a viable parasite and that only the bioassay will prove that. Based on these results, uncooked meat from an infected chicken possesses a high risk for a consumer (Geuthner et al. 2019; Rodrigues et al. 2019).
The failure to detect T. gondii DNA in the same chicken in all examined tissues can be explained by the rare and inhomogeneous distribution of tissue cysts (Opsteegh et al. 2016). Also, the rate of infection depends on the inoculation dose, the route of infection, the parasitic stage, or the individual characteristics of the isolate. We used a low dose of cysts (12/chicken) that were orally inoculated, in order to reproduce a natural condition of infection. In a study performed by Dubey and Frenkel (1973), mice infected orally with 10 tissue cysts failed to establish infection, whereas higher doses (≥ 1000) resulted in successful infection. Then, for bioassay, we used the whole brain, whole heart, and half of the breast muscle, but only one mouse was bioassayed per tissue sample (in total three mice per chicken). If more than one mouse had been used for bioassay, the percentage of T. gondii isolation from experimentally infected chickens probably would have increased. In this study, there were no detected notable differences between the domestic and wild cat strains. This can be due to the fact that they were genotype II and genotype II/III, respectively. Previous studies showed that the heart is the predilection tissue for T. gondii (Dubey 2010; Yan et al. 2010; Opsteegh et al. 2016), but we detected the parasite more frequently in the brain, and mainly in untreated chickens. In treated chickens, the rate of detection was the same in the brain and the heart. The concentration of T. gondii DNA in chicken tissues appears to be generally low. False-negative results in an unknown proportion of tissue samples cannot be excluded, even if extremely sensitive methods are applied (Hiob et al. 2017). However, combined results obtained in three different tissues by two different techniques suggest that the combination of the detection methods and on different tissues decreases the rate of false-negative results.
The administration of a drug in feed is a simple and effective way to prevent or treat diseases in large groups of animals, representing one of the two practical solutions in birds, along with the administration in water.
For centuries, A. annua has been a medicinal plant known to be an excellent antimalarial. Many other therapeutic attributes of this plant have been discovered, even coccidiostat, antimicrobial, or anticancer properties (Tajehmiri et al. 2014; Breuer and Efferth 2014).
Overall, A. annua seems to be a promising option in preventing T. gondii infection (2 chickens positive out of 5) when compared with untreated chickens (5/5), and even with those treated with diclazuril (5/5). It remains to be evaluated its efficacy in case of high-dose T. gondii infection. Also, the response to treatment can be strain dependent. A previous study showed that the susceptibility of T. gondii to atovaquone and to sulfadiazine was different according to the parasite strain (Alves and Vitor 2005).
When bioassay results are compared, diclazuril seems not to interfere with overall parasite dissemination, but rather with its viability (3 positive chickens out of 5). In vitro studies on cell cultures have shown that treatments with diclazuril resulted in > 97% reduction in tachyzoite counts (Lindsay and Blagburn 1994). Also, in vivo studies revealed that diclazuril in doses between 0.5 and 10 mg/kg/day alone or in combination with pyrimethamine increases the survival rate of mice in acute toxoplasmosis, but did not prevent the formation of tissue cysts (Lindsay et al. 1995). Diclazuril was originally developed for prophylaxis of chicken coccidiosis in a dose of 1 ppm (0.125 mg/kg body weight). We used a dose of diclazuril of 0.15 mg/kg body weight and it was given 2 days before experimental infection. Also, the absorption of diclazuril from the gut is limited, 90% of the drug being excreted in the feces within 24 h.
Further studies are required aiming at the chicken’s response to T. gondii infection with different doses of cysts and with different strains. As regarding the efficacy of diclazuril and A. annua for prevention of T. gondii infection in chickens, further studies are needed using quantitative analysis of the parasite and different experimental models (dose of infection, strains, dose of the product).
In conclusion, broiler chickens were resistant to clinical toxoplasmosis, irrespective of the strain (domestic or wild cat strain). There were no differences in the rate of T. gondii detection in chickens depending on the parasite strain. Moreover, both strains replicated in all infected, but were detected at a higher rate in the brain. Not all infected chickens did seroconvert on day 28 p.i. regardless of the strain and serological test. The herb A. annua and the coccidiostat diclazuril revealed a prophylactic efficacy; however, further studies are required aiming at the efficacy of diclazuril and A. annua for the prevention of T. gondii infection in chickens using quantitative analysis methods.
Data availability
Not applicable.
References
Allen PC, Lydon J, Danforth H (1997) Effects of components of Artemisia annua on coccidia infections in chickens. Poult Sci 76(8):1156–1163. https://doi.org/10.1093/ps/76.8.1156
Alves CF, Vitor RWA (2005) Efficacy of atovaquone and sulfadiazine in the treatment of mice infected with Toxoplasma gondii strains isolated in Brazil. Parasite 12(2):171–177. https://doi.org/10.1051/parasite/2005122171
Bresciani KDS, Galvão ALB, de Vasconcellos AL, dos Santos TR, Kaneto CN, Viol MA, Gomes JF, Bilsland E (2016) Epidemiological aspects of feline toxoplasmosis. Arch Vet Sci 21(2):1–8. https://doi.org/10.5380/avs.v21i2.39997
Breuer E, Efferth T (2014) Treatment of iron-loaded veterinary sarcoma by Artemisia annua. Nat Prod Bioprospect 4(2):113–118. https://doi.org/10.1007/s13659-014-0013-7
Christensen K, Vizzier Thaxton Y, Thaxton JP, Scanes CG (2012) Changes in body temperature during growth and in response to fasting in growing modern meat type chickens. Br Poult Sci 53(4):531–537. https://doi.org/10.1080/00071668.2012.715744
de Almeida GF, Horsted K, Thamsborg SM, Kyvsgaard NC, Ferreira JF, Hermansen JE (2012) Use of Artemisia annua as a natural coccidiostat in free-range broilers and its effects on infection dynamic and performance.Vet. Parasitol 186(3–4):178–187. https://doi.org/10.1016/j.vetpar.2011.11.058
Desmonts GE, Remington JS (1980) Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol 11(6):562–568
Djurković-Djaković O, Nikolić A, Bobić B, Klun I, Aleksić A (2005) Stage conversion of Toxoplasma gondii RH parasites in mice by treatment with atovaquone and pyrrolidine dithiocarbamate. Microbes Infect 7(1):49–54. https://doi.org/10.1016/j.micinf.2004.09.016
Drăgan L, Györke A, Ferreira JF, Pop IA, Dunca I, Drăgan M, Mircean V, Dan I, Cozma V (2014) Effects of Artemisia annua and Foeniculum vulgare on chickens highly infected with Eimeria tenella (phylum Apicomplexa). Acta Vet Scand 56:22. https://doi.org/10.1186/1751-0147-56-22
Dubey JP (2010) Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health 57(1):60–73. https://doi.org/10.1111/j.1863-2378.2009.01274
Dubey JP, Frenkel JK (1973) Experimental toxoplasma infection in mice with strains producing oocysts. J Parasitol 59(3):505–512
Dubey JP, Ruff MD, Camargo ME, Shen SK, Wilkins GL, Kwok OC, Thulliez P (1993) Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am J Vet Res 54:1668–1672
Dubey JP, Levy MZ, Sreekumar C, Kwok OCH, Shen SK, Dahl E, Thulliez P, Lehmann T (2004) Tissue distribution and molecular characterization of chicken isolates of Toxoplasma gondii from Peru. J Parasitol 90(5):1015–1018. https://doi.org/10.1645/GE-329R
Dunay IR, Chan WC, Haynes RK, Sibley LD (2009) Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob Agents Chemother 53(10):4450–4456. https://doi.org/10.1128/aac.00502-09
Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP (2010) Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol 26(4):190–196. https://doi.org/10.1016/j.pt.2010.01.009
FAO/WHO (2000) Food additives. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives
Ferreira JFS, Gonzalez JM (2009) Analysis of underivatized artemisinin and related sesquiterpene lactones by high-performance liquid chromatography with ultraviolet detection. Phytochem Anal 56:91–97. https://doi.org/10.1002/pca.1101
Geuthner AC, Koethe M, Ludewig M, Pott S, Schares G, Daugschies A, Bangoura B (2014) Persistence of Toxoplasma gondii tissue stages in poultry over a conventional fattening cycle. Parasitol 141:1359–1364. https://doi.org/10.1017/s003118201400078x
Geuthner AC, Koethe M, Ludewig M, Pott S, Schares G, Maksimov P, Daugschies A, Bangoura B (2019) Development of an in vivo model for toxoplasma gondii infections in chickens and turkeys simulating natural routes of infection. Vet Parasitol 276:108956. https://doi.org/10.1016/j.vetpar.2019.108956
Godoi FSLD, Nishi SM, Pena HFDJ, Gennari SM (2010) Toxoplasma gondii: diagnosis of experimental and natural infection in pigeons (Columba livia) by serological, biological and molecular techniques. Rev Bras Parasitol Vet 19(4):237–243. https://doi.org/10.1590/S1984-29612010000400009
Gyӧrke A, Pop LM, Mircean M, Kalmár Z, Tăbăran AF, Paștiu AI, Dumitrache MO, Magdaș C, Balea A, Bărburaș D, Mircean V, Cozma V (2019) Metabolic and tissular effects of artemisinin supplemented diets in broiler chicken. Pol J Vet Sci 22(2):297–304. https://doi.org/10.24425/pjvs.2019.129220
Hencken CP, Jones-Brando L, Bordón C, Stohler R, Mott BT, Yolken R, Woodard LE (2010) Thiazole, oxadiazole, and carboxamide derivatives of artemisinin are highly selective and potent inhibitors of Toxoplasma gondii. J Med Chem 53(9):3594–3601. https://doi.org/10.1021/jm901857d
Hiob L, Koethe M, Schares G, Goroll T, Daugschies A, Bangoura B (2017) Experimental Toxoplasma gondii and Eimeria tenella co-infection in chickens. Parasitol Res 116(11):3189–3203. https://doi.org/10.1007/s00436-017-5636-2
Homan WL, Vercammen M, De Braekeleer J, Verschueren H (2000) Identification of a 200-to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol 30(1):69–75. https://doi.org/10.1016/S0020-7519(99)00170-8
Islamuddin M, Chouhan G, Farooque A, Dwarakanath BS, Sahal D, Afrin F (2015) Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS Negl Trop Dis 9(1):3321. https://doi.org/10.1371/journal.pntd.0003321
Khan A, Taylor S, Su C, Mackey AJ, Boyle J, Cole R, Glover D, Tang K, Paulsen IT, Berriman M, Boothroyd JC (2005) Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res 33(9):2980–2992. https://doi.org/10.1093/nar/gki604
Koethe M, Straubinger RK, Pott S, Bangoura B, Geuthner AC, Daugschies A, Ludewig M (2015) Quantitative detection of Toxoplasma gondii in tissues of experimentally infected turkeys and in retail Turkey products by magnetic-capture PCR. Food Microbiol 52:11–17. https://doi.org/10.1016/j.fm.2015.06.005
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Lappin MR (2010) Update on the diagnosis and management of Toxoplasma gondii infection in cats. Top Companion Anim Med 25(3):36–141. https://doi.org/10.1053/j.tcam.2010.07.002
Lindsay DS, Blagburn BL (1994) Activity of Diclazuril against Toxoplasma gondii in cultured cells and mice. Am J Vet Res 55(4):530–533
Lindsay DS, Rippey NS, Blagburn BL (1995) Treatment of acute Toxoplasma gondii infections in mice with Diclazuril or a combination of Diclazuril and pyrimethamine. J Parasitol 81:315–318. https://doi.org/10.2307/3283944
Malkwitz I, Berndt A, Daugschies A, Bangoura B (2013) Long-term investigations on Toxoplasma gondii-infected primary chicken macrophages. Parasitol Res 112(9):3115–3122. https://doi.org/10.1007/s00436-013-3486-0
Maxwell MH (1993) Avian blood leucocyte responses to stress. World Poultry Sci J 49:34–43. https://doi.org/10.1079/WPS19930004
Mirzaalizadeh B, Sharif M, Daryani A, Ebrahimzadeh MA, Zargari M, Sarvi S, Mehrzadi S, Rahimi MT, Mirabediny Z, Golpour M, Montazeri M (2018) Effects of Aloe vera and Eucalyptus methanolic extracts on experimental toxoplasmosis in vitro and in vivo. Exp Parasitol 192:6–11. https://doi.org/10.1016/j.exppara.2018.07.010
Montoya A, Miro G, Mateo M, Ramirez C, Fuentes I (2009) Detection of Toxoplasma gondii in cats by comparing bioassay in mice and polymerase chain reaction (PCR). Vet Parasitol 160:159–162. https://doi.org/10.1016/j.vetpar.2008.10.029
Müller J, Balmer V, Winzer P, Rahman M, Manser V, Haynes RK, Hemphill A (2015) In vitro effects of new artemisinin derivatives in Neospora caninum-infected human fibroblasts. Int J Antimicrob Agents 46(1):88–93. https://doi.org/10.1016/j.ijantimicag.2015.02.020
Nagamune K, Beatty WL, Sibley SD (2007) Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot Cell 6(11):2147–2156. https://doi.org/10.1128/ec.00262-07
Nedișan ME, Ursache AL, Dumitrache MO, Mircean V, Cozma-Petruț A, Gyӧrke A (2019) Intestinal toxoplasmosis in cats treated with Procox (case report). Sci Parasitol 20(1–2):19–24
Ognean L, Cernea LC (2011) Practical applications in animal physiology. Ed. Academic Pres, Cluj-Napoca
Ogolla KO, Gathumbi PK, Waruiru RM, Okumu PO, Chebet J, Kitala PM (2018) Efficacy of sulphachloropyrazine, amprolium hydrochloride, trimethoprim-sulphamethoxazole, and Diclazuril against experimental and natural rabbit coccidiosis. J Vet Med 5402469:1–11. https://doi.org/10.1155/2018/5402469
Opsteegh M, Schares G, Blaga R, van der Giessen J (2016) Experimental studies of Toxoplasma gondii in the main livestock species (GP/EFSA/BIOHAZ/2013/01) Final report. EFSA Support Public EN-995:161. https://doi.org/10.2903/sp.efsa.2016.EN-995
Paştiu AI, Cozma-Petruț A, Mercier A, Balea A, Galal L, Mircean V, Pusta DL, Bogdan L, Györke A (2019) Prevalence and genetic characterization of Toxoplasma gondii in naturally infected backyard pigs intended for familial consumption in Romania. Parasit Vectors 12(1):586. https://doi.org/10.1186/s13071-019-3842-8
Pop L, Györke A, Tǎbǎran AF, Dumitrache MO, Kalmár Z, Magdaş C, Mircean V, Zagon D, Balea A, Cozma V (2015) Effects of artemisinin in broiler chickens challenged with Eimeria acervulina, E. maxima and E. tenella in battery trials. Vet Parasitol 214(3–4):264–271. https://doi.org/10.1016/j.vetpar.2015.10.011
Quéré P, Pierre J, Hoang MD, Esnault E, Domenech J, Sibille P, Dimier- Poisson I (2013) Presence of dendritic cells in chicken spleen cell preparations and their functional interaction with the parasite Toxoplasma gondii. Vet Immunol Immunopathol 153:57–69. https://doi.org/10.1016/j.vetimm.2013.02.007
Rodrigues FT, Moreira FA, Coutinho T, Dubey JP, Cardoso L, Lopes AP (2019) Antibodies to Toxoplasma gondii in slaughtered free-range and broiler chickens. Vet Parasitol 271:31–53. https://doi.org/10.1016/j.vetpar.2019.06.007
Schares G, Bangoura B, Randau F, Goroll T, Ludewig M, Maksimov P, Opsteegh M (2017) High seroprevalence of Toxoplasma gondii and probability of detecting tissue cysts in backyard laying hens compared with hens from large free-range farms. Int J Parasitol 47(12):765–777. https://doi.org/10.1016/j.ijpara.2017.07.003
Schmidt S (2015) Top 5 leukogram patterns. Available at: <Available at: http://www.cliniciansbrief.com >.
Sergeant ESG (2018) Epitools epidemiological calculators. Ausvet. Available at: http://epitools.ausvet.com.au
Su C, Zhang X, Dubey JP (2006) Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: a high resolution and simple method for identification of parasites. Int J Parasitol 36(7):841–848. https://doi.org/10.1016/j.ijpara.2006.03.003
Tajehmiri A, Issapour F, Moslem MN, Lakeh MT, Kolavani MH (2014) In vitro antimicrobial activity of Artemisia annua leaf extracts against pathogenic bacteria. Adv Stud Biol 6(3):93–97. https://doi.org/10.12988/asb.2014.4525
Villena I, Durand B, Aubert D, Blaga R, Geers R, Thomas M, Perret C, Alliot A, Escotte-Binet S, Thébault A, Boireau P (2012) New strategy for the survey of Toxoplasma gondii in meat for human consumption. Vet Parasitol 183(3–4):203–208. https://doi.org/10.1016/j.vetpar.2011.08.001
Watson PF, Petrie A (2010) Method agreement analysis: a review of correct methodology. Theriogenology 73:1167–1179. https://doi.org/10.1016/j.theriogenology.2010.01.003
Yan C, Yue CL, Yuan ZG, Lin RQ, He Y, Yin CC, Xu MJ, Song HQ, Zhu XQ (2010) Molecular and serological diagnosis of Toxoplasma gondii infection in experimentally infected chickens. Vet Parasitol 173:179–183. https://doi.org/10.1016/j.vetpar.2010.07.011
Zechner G, Bauer C, Jacobs J, Goossens L, Vertenten G, Taylor MA (2015) Efficacy of Diclazuril and toltrazuril in the prevention of coccidiosis in dairy calves under field conditions. Vet Rec 176:126–126. https://doi.org/10.1136/vr.102237
Acknowledgments
AG, RB and GS are part of the TOXOSOURCES consortium, supported by funding from the European Union’s Horizon 2020 Research and Innovation programme under grant agreement No 773830: One Health European Joint Programme.
Funding
This work was supported by the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca through internal research grant number 6142/10.04.2017 and by COST Action FA 1408 through 2 STSM in the Institute for Medical Research, Belgrade, Serbia, and National Veterinary School of Alfort, UMR BIPAR, ANSES, INRA, University Paris-Est, Maisons-Alfort Animal Health Laboratory, Maisons-Alfort, France.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.G., M.E.N., and V.C.; methodology: M.E.N., C.Ș., Z.K., Z.F., A.B., A.T.N., G.S., I.K., and I.V.; formal analysis: M.E.N., A.G., C.Ș., R.B., V.M., and G.S.; writing—original draft preparation: M.E.N. and A.G.; writing—review and editing: A.G, G.S., O.D.D., and I.V. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The experiment was approved by the Animal Ethics Committee of UASVM CN (protocol no. 92/20.12.2017).
Consent to participate
Not applicable.
Code availability
Not applicable.
Additional information
Section Editor: Berit Bangoura
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Nedişan, M.E., Györke, A., Ştefănuţ, C.L. et al. Experimental infection with Toxoplasma gondii in broiler chickens (Gallus domesticus): seroconversion, tissue cyst distribution, and prophylaxis. Parasitol Res 120, 593–603 (2021). https://doi.org/10.1007/s00436-020-06984-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06984-x