Abstract
The widespread apicomplexan parasites Toxoplasma gondii (T. gondii) and Eimeria tenella (E. tenella) are important pathogens with high prevalence in poultry. The aim of our study was the investigation of mutual influences in co-infected chickens, focusing on immune response and course of infection. Two separate trials were performed using in total 96 1-day-old chickens, divided into four study groups: group NC (negative control, uninfected), group PC-T (oral or intramuscular infection with T. gondii oocysts (trial 1) or tachyzoites (trial 2), respectively), group PC-E (oral infection with E. tenella (trial 1) or E. tenella and Eimeria acervulina (trial 2)), and group TE (co-infection). T. gondii and Eimeria infections were validated by different parameters, and cytokine expression in the gut and spleen was investigated. T. gondii-specific antibodies were detected earliest 4 days post infection (p.i.) by immunoblot and direct DNA detection was possible in 22.1% of all tissue samples from infected chickens. Eimeria spp. merogony seemed to be enhanced by co-infection with T. gondii, interestingly without marked differences in oocyst excretion between co-infected and Eimeria spp. mono-infected chickens. An increase of messenger RNA (mRNA) expression of Th1- (IFN-γ, IL-12, TNF-α) and Th2-related cytokines (IL-10) mainly in groups PC-E and TE was observed, however, without statistically significant differences between co-infection and single infection with Eimeria. In conclusion, most of the measurable immune response could be attributed to Eimeria infection. To the best of our knowledge, this is the first report on co-infection experiments of T. gondii with Eimeria spp. in chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The apicomplexan parasites Toxoplasma gondii (T. gondii) and Eimeria tenella (E. tenella) are widespread (Dubey 2010; McDougald 1998). Both replicate intracellularly, and all released endogenous and exogenous parasite stages are immunogenic. The immune response of infected animals to these coccidian species is described to be highly similar (Frölich et al. 2012; Yun et al. 2000).
T. gondii has been found in almost all mammals including humans and many avian species (Dubey 2008) and is considered as an important zoonosis. Sexual reproduction takes place in the guts of felids (definitive hosts), eventually causing excretion of oocysts in cat feces. After systemic infection of any warm-blooded intermediate host, T. gondii replicates asexually as tachyzoites. In immunocompetent hosts, this phase is followed by formation of dormant tissue cysts containing bradyzoites. Upon oral ingestion, these tissue cysts are infective to other susceptible hosts. In chickens, the seroprevalence of T. gondii in organic and backyard farms reaches up to 100% (Dubey 2010). However, as there are only a few case reports on toxoplasmosis in chickens, these birds are known to be hardly susceptible to clinical disease (Dubey 2010).
E. tenella is a major cause of intestinal coccidiosis in chickens. In Sweden, coccidiosis caused by E. tenella and Eimeria maxima was demonstrated in up to 31% of litter-based, high stocking density farm systems (Lundén et al. 2000). In the host, development of E. tenella is restricted to the ceca and typical clinical signs are diarrhea, mucosal lesions, depressed weight gain, and high mortality. Thus, substantial economic losses are caused to the poultry industry by that infection (McDougald and Fitz-Coy 2013). Also, secondary infections are common (McDougald 1998), additionally increasing the economic losses.
Eimeria acervulina (E. acervulina) is also a frequently encountered species in chickens causing coccidiosis and poor feed conversion and is described as mildly pathogenic (McDougald and Fitz-Coy 2013).
Because of the high prevalence of both T. gondii and E. tenella, it can be assumed that co-infections in chickens with T. gondii and E. tenella occur frequently in the field. A mutual influence or co-stimulation of the immune response appears possible due to the existence of closely related antigens and similar invasion mechanisms. However, specific cross-immunity is unlikely to occur because even between closely related Eimeria species in chickens no cross-immunity was observed (Rose 1987).
To the best of our knowledge, no experimental studies about co-infections with T. gondii and E. tenella in chickens have been published. Zou et al. (2011) studied T. gondii as a vaccine vector for coccidiosis in chickens and demonstrated a limited protective effect against E. tenella. Reversely, Tang et al. (2016) reported partial protection against T. gondii infection in chickens and mice induced by transgenic E. tenella (Et-TgSAG1). In both cases, homologous antigens were used to confer protection. It is already known that mixed infections with bacterial pathogens like clostridia cause distinct modulations on the immune reaction to Eimeria infection (Alnassan et al. 2013).
The aim of the present study is to investigate whether such mutual influence also occurs during concurrent infection with two apicomplexan parasites, T. gondii and E. tenella, that lead to either generalized (T. gondii) or localized (E. tenella) infection. While the study mainly focused on immune response and course of infection, clinical parameters, oocyst excretion, and distribution of T. gondii in tissues were examined additionally.
Materials and methods
Experimental design
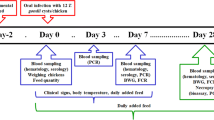
The study was divided into two subsequent trials, trial 1 and trial 2. In total, 96 Hubbard I 757 broiler chickens were purchased as 1-day-old chickens from an organic farm (Biogeflügelzucht und Brüterei Hetzenecker Küken, Neumarkt-Sankt Veit, Germany (trial 1), Brüterei Ludwig Hölzl, Moosburg, Germany (trial 2)). Chickens were reared in floor pens. They received a diet without antibiotics and anticoccidials and had water access ad libitum. On study day (SD) 7, the chickens were divided into four groups (n = 12 per group; see Table 1) and transferred group-wise to eight different wire cages, i.e., two cages per group. The first study group remained uninfected as negative control (group NC), the second was infected with E. tenella (trial 1) or E. tenella and E. acervulina (trial 2) (group PC-E), the third group with T. gondii only (group PC-T), and the fourth group was infected with both Eimeria spp. and T. gondii (group TE). From SD 12 until the end of the study (SD 30), fecal samples were collected daily for determination of Eimeria spp. oocyst counts. Blood samples were taken weekly for detection of T. gondii-specific antibodies. For evaluation of intestinal alterations, half of each animal group was necropsied on SD 13, the other half on SD 30/31. Lesion scoring was performed, and tissue samples were collected for analysis of cytokine expression and T. gondii detection (see below). A timeline of the study is shown in Fig. 1.
Eimeria infection
The Houghton strain of E. tenella (kindly provided by Dr. D. P. Blake, Royal Veterinary College, University of London, Hatfield, UK) was used less than 6 months after in vivo passage as previously described (Alnassan et al. 2013). Infection dose was adjusted after oocysts had been quantified using a modified McMaster method (see Eimeria spp. oocyst counts).
In trial 1, all chickens of the study groups PC-E and TE were inoculated with 2.5 × 104 oocysts of E. tenella on SD 7. In trial 2, the inoculum contained a mixture of 2.5 × 104 oocysts of E. tenella and 1.87 × 104 oocysts of E. acervulina, which had been collected after an in vivo passage. Oocysts were inoculated by a disposable plastic Pasteur pipette (polyethylene) directly into the crop. In previous trials, the chosen E. tenella infection dose was determined as suitable to induce subclinical or clinical coccidiosis in most animals (data not shown). E. acervulina infection dose of around 2.0 × 104 oocysts should induce only mild coccidiosis (McDougald and Fitz-Coy 2013) and were used for experimental mixed Eimeria infections in broiler chickens before (Pop et al. 2015). A successful Eimeria spp. infection was validated by detection of consistent oocyst excretion and specific lesion determination during intestinal lesion scoring.
T. gondii infection
T. gondii type III strain NED oocysts (Howe and Sibley 1995) were used in trial 1. Chickens of the groups PC-T and TE were inoculated orally on SD 9 with 1.0 × 104 oocysts into the crop by using a plastic Pasteur pipette. Oocysts were isolated from cat feces. Similar oocyst infection doses were used in several studies before (Kaneto et al. 1997; Dubey et al. 1993). In contrast, the chickens of trial 2 were infected on SD 10 with 2.5 × 105 T. gondii tachyzoites of type II strain ME 49 (Lunde and Jabobs, 1983) intramuscularly. Commonly used infection doses for experimental T. gondii infections in chickens are 5.0 × 105 (Kaneto et al. 1997) or 1.0 × 106 tachyzoites (Geuthner et al. 2014). Because we used very young chickens, which were partly infected with E. tenella and E. acervulina (group TE), we applied a lower infection dose in order to prevent mortality. Tachyzoites for infection of the birds were grown in HFF cells cultivated with IMDM medium (PAA, Pasching, Austria), 5% fetal calf serum, 1% penicillin/streptomycin, and 1% amphotericin B at 37 °C, 5% CO2 as described before (Zintl et al. 2009). To harvest the parasites, supernatant of cell culture was centrifuged at 2000×g for 5 min. The pellet was resuspended in 1 ml PBS, and tachyzoites were counted using a Neubauer chamber to adjust the infection dose. Viability was microscopically determined by morphology and locomotion of the tachyzoites. Success of infection was determined by immunoblot and PCR. Animals inoculated with T. gondii, which remained negative in both assays, were excluded from statistical analysis.
Clinical observations
Clinical observation was carried out twice daily for all chickens throughout the experiment. Fecal consistency and mortality were particularly considered. Individual body weights were assessed on SD 2, SD 7, SD 14, SD 21, and SD 28 in all groups.
Lesion scoring
On SD 13 and SD 30/31, ceca of necropsied chickens were scored for macroscopical lesions typical for E. tenella infection (according to Johnson and Reid 1970). In trial 2, the duodenum was additionally scored because the inoculum also contained E. acervulina. The lesion score (LS) system ranged from 0 to 4, score 0 was recorded when no lesions were observed whereas score 4 corresponded to maximum lesions. Mucosa smears were scored microscopically at × 100 magnification for the number of Eimeria stages as follows: 0, no parasites; 1, < 50 parasite stages; 2, 50 to 250 stages; and 3, > 250 stages per field of view. Each score represents the average of five fields of view per smear.
Eimeria spp. oocyst counts
Between SD 0 and SD 11, birds were monitored for accidental Eimeria infection by fecal samples collected from 10 different locations of the floor pen litter or from beneath the cages. From SD 12 (5 days after experimental Eimeria spp. infection in the respective groups) until the end of the study period, fecal samples were examined daily to monitor the course of infection. The number of oocysts per gram of feces (opg) was assessed using a modified McMaster method as described before (Long and Rowell 1958; Alnassan et al. 2013). One hundred grams of bulk samples taken from each cage was homogenized with 50 ml water. Six grams of the feces–water mix was suspended with 15 ml of saturated sodium chloride solution and transferred into a graduated cylinder via a funnel with a tea strainer. Saturated sodium chloride solution was added up to 60 ml, and the suspension was mixed thoroughly on a magnetic stirrer for 2 min. Aliquots were then transferred into McMaster chambers, and oocysts under two McMaster grids (150 μl) per sample were counted after 2 min. Opg was calculated by multiplying the mean count for two counting grids per sample with 100.
Detection of T. gondii-specific antibodies by immunoblot
Starting at SD 7, blood samples were taken from chickens weekly (Vena ulnaris). Individual blood samples were centrifuged at 3000×g for 15 min. The serum was stored at − 20 °C until processing. T. gondii-specific antibodies (IgY type) in the sera were determined by immunoblot. For immunoblotting, T. gondii RH tachyzoites were used as described (Azevedo et al. 2010). Purified tachyzoites (2 × 108 per minigel) were incubated in non-reducing sample buffer (2% [w/v] sodium dodecyl sulfate (SDS), 10% [v/v] glycerol, 62 mM TrisHCl, pH 6.8) for 1 min (94 °C), separated in 12% [w/v] SDS polyacrylamide minigels of 60 × 70 × 1 mm size, and transferred to PVDF membranes (Immobilon-P, Millipore) (Azevedo et al. 2010). After the transfer, membranes were blocked using PBS-TG (PBS with 0.05% (v/v) Tween 20 (Sigma) and 2% (v/v) liquid fish gelatine (Serva, Germany)) and cut into 50 stripes and examined as described below. Serum was diluted 1:10 in PBS-TG. Reactivity of sera with bands of 20, 30, 35, and 43 kDa Mr was recorded (Huskinson et al. 1989). A reaction was regarded as positive, if at least two of these bands were recognized and questionable if only one band reacted. Birds which showed only questionable results throughout the observation period were regarded negative. Peroxidase-conjugated antichicken IgG (H + L) (Rockland Immunochemicals Inc., Limerick, PA, USA) was used diluted 1:500 in PBS-TG.
T. gondii detection in tissue samples by PCR
For detection of T. gondii DNA, tissue samples of the brain, heart, femoral, and breast muscle were taken during necropsy on SD 13 and SD 30/31, respectively, and stored at − 20 °C until examination.
Samples were homogenized individually using commercial household blenders (La Moulinette, Tefal Groupe SEB, Offenbach, Germany) for femoral and breast muscles and a mortar and pestle for brain tissue. Hearts were cut into small pieces with surgical scissors.
DNA extraction from 25 mg of each tissue sample was performed applying QIAamp DNA Mini Kit® (Qiagen, Hilden, Germany) following the manufacturer’s instructions. DNA concentration was adjusted to 80–200 ng template DNA per PCR reaction. In every PCR batch, a positive control consisting of DNA from cell culture-derived ME49 tachyzoites and three negative controls (two aliquots of water and one aliquot of DNA elution buffer) were carried along. A direct PCR reaction, followed by a nested PCR (nPCR), was performed as described by Zöller et al. (2013). Briefly, the 25 μl mastermix for a direct PCR reaction consisted of 0.5 U Dream Taq Green DNA Polymerase and 10× Dream Taq Green Buffer, 200 μM of each dNTP, 3 mM MgCl2, 0.4 μM of each primer Tg1 (5′-AAA AAT GTG GGA ATG AAA GAG-3′), and Tg2 (5′-ACG AAT CAA CGG AAC TGT AAT-3′) (Thermo Fisher Scientific, Schwerte, Germany), amplifying the 469-bp fragment. In order to enhance sensitivity, a subsequent nPCR was performed as described before (Zöller et al. 2013) using the primers Tgnested1 (5′-CGC TAATGT GTT TGC ATA GG-3′) and Tgnested2 (5′-GGC ACG TCT CTT GTT CTT CT-3′) and 2.5 μl of the previously amplified PCR product as template. The target fragment length for the nPCR was 375 bp. Gel electrophoresis with 1.5% agarose gel was performed using 10 μl of the nPCR products. Afterwards, the gel was stained with ethidium bromide and DNA bands were visualized under UV light.
For magnetic capture PCR (MC-PCR), up to 100 g of sample was used, cut into pieces (ca. 2 × 2 × 1.5 cm), and homogenized in Stomacher bags with filters (femoral and breast muscle) or 50 ml tubes (heart, brain). Cell lysis buffer contained 10 nM Tris–HCl, 5 mM EDTA, 200 nM NaCl, 0.2% sodium dodecyl sulfate (SDS), and 20 mg/ml proteinase K. DNA extraction and real-time PCR were performed as previously described (Koethe et al. 2015) with slight modifications: 25 μl mastermix contained 2.5 μl DNA, 2× Maxima Probe/ROX qPCR Master Mix (Thermo Fisher Scientific, Schwerte, Germany), 900 nM of each Primer, and 200 nM Tox–TP1 probe.
Cytokine expression profile
Samples of the duodenum, ceca, and spleen were collected on both necropsy time points and stored at − 80 °C until RNA extraction. Expression of the cytokines interleukin 10 (IL-10), IL-12, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) was evaluated by specific reverse transcriptase quantitative polymerase chain reactions (RT-qPCR).
Total RNA extraction and cDNA synthesis
For RNA extraction, the RNeasy® Mini Kit (Qiagen, Hilden, Germany) was used. Briefly, 25 mg of each individual tissue was mixed with 600 μl RLT buffer and 10 μl ß-mercaptoethanol (Sigma GmbH, Taufkirchen, Germany). Homogenization was performed in the MagNA Lyser (Roche GmbH, Mannheim, Germany) with glass beads (diameter 0.75–1 mm) at 6500 rpm for 50 s. After full speed centrifugation (10,000 rpm for 3 min), the supernatant was collected in 1.5 ml tubes and the RNA was isolated according to the manufacturer’s instructions. The RNA concentration was measured with a spectrophotometer (Eppendorf BioPhotometer, Eppendorf, Germany). For complementary DNA (cDNA) synthesis, approximately 1200 ng/μl RNA was used per sample. Reverse transcription into cDNA was done by Revert-Aid® first strand cDNA synthesis kit (Thermo Fisher Scientific, Schwerte, Germany) as described by Hong et al. (2006). The mixture was incubated 5 min at 25 °C, followed by 60 min at 42 °C, and a final heating reaction at 70 °C for 5 min.
qPCR
The messenger RNA (mRNA) expression of cytokines was quantified by a qPCR assay (Park et al. 2008) which was slightly modified as described below. Transcript levels of genes were normalized to those of the reference genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as described by Park et al. (2008) and additionally for glucose-6-phosphate dehydrogenase (G6PDH). Sequences of the primers used for qPCR are listed in Table 2.
The qPCR assay was performed according to Hong et al. (2006). Ten microliters of SYBR Green master mix (Thermo Fisher Scientific, Schwerte, Germany), 6.6 μl water, 0.4 μl ROX solution, 2 μl cDNA template, and 0.5 μl of the corresponding primers were used.
The following thermal profile setup was applied: initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min and termination at 95 °C for 1 min, 55 °C for 30 s and 95 °C for 30 s. Reference target stability and fold change differences were calculated using qBase+ 2.3 (Biogazelle NV, Gent, Belgium).
The fold change in cytokine mRNA expression was calculated for each group compared to the NC group for the respective necropsy day.
Statistical methods
The collected data were analyzed by means of IBM SPSS Statistics 22 ® (IBM, New York, USA). Test for normal distribution was conducted for all parameters by Kolmogorov–Smirnov test. To analyze data for group differences, ANOVA was applied and followed by Bonferroni or Dunnett’s T3 test for normally distributed data. Kruskal–Wallis test and successive Mann–Whitney U test were performed in case of non-normally distributed data. Because of only two bulk fecal samples per study group per SD, statistical comparison of opg values was performed over the complete study period.
Results
Clinical signs, fecal consistency, and mortality
In trial 1, no clinical disease attributable to the experimental infection was observed throughout the study period. Fecal consistency was physiological or soft in all study groups. Although one animal died in each of the groups PC-T, PC-E, and NC, this was not related to E. tenella or T. gondii infection, as evidenced by necropsy.
Though, in trial 2, individual chickens of the groups PC-E and TE exhibited mild apathy and reduced general condition from SD 14 to SD 17. In the same study groups, liquid and hemorrhagic diarrhea was observed between SD 12 and SD 15. Apart from these findings, fecal consistency was physiological during the study period. Birds of the groups NC and PC-T did not show any clinical signs and physiological fecal consistency. One chicken died on SD 13 (group TE) without infection-related pathological findings.
Body weight
Mean body weight comparison did not reveal significant differences between the four study groups in trial 1 (see electronic supplementary material, Fig. S1). However, in trial 2 (electronic supplementary material, Fig. S2), body weight was statistically significantly lower in Eimeria spp. infected chickens (PC-E, TE) compared to NC and PC-T on SD 14 (ANOVA, Dunnett’s T3 post hoc test, P < 0.05). Although not statistically significant, this effect was observed by trend also on subsequent SD 21 and SD 28. Body weight gain was calculated for the different weighing intervals (Table 3). In trial 1, body weight gains of groups PC-E and TE were lower than in groups NC and PC-T on SD 14, though there was no significant difference. Accordingly, there were significant lower body weight gains in trial 2 on SD 14 in Eimeria spp.-infected chickens (PC-E, TE) than in NC and PC-T (Mann–Whitney U test, P < 0.05); however, body weight gains converged subsequently on SD 21 and SD 28.
Pathological examination
Lesion scoring
In trial 1, no intestinal lesions were recorded on SD 13 except for one bird of group PC-T and two birds of group NC that showed some scattered focal erosions of unknown origin. On SD 30 groups, PC-E and TE displayed mild E. tenella-specific lesions (Table 4).
In trial 2, Eimeria spp.-infected chickens developed distinct lesions in the ceca on SD 13 with median scores of 3 recorded for both groups PC-E and TE (Mann–Whitney U test, P < 0.05 compared to NC and PC-T; Table 4). Group PC-E animals showed mild lesions in the ceca on SD 31. E. acervulina-specific lesions were seen in groups PC-E and TE on SD 13 with median values of 2 (Mann–Whitney U test, P < 0.05 compared to NC and PC-T; electronic supplementary material, Table S1). No Eimeria-associated pathological changes were noticed in groups NC and PC-T in both necropsies in both trials.
Microscopical scoring
In trial 1, Eimeria stages were detected on SD 13 only in group TE chicken (median score 1; Table 4) whereas this was the case in both Eimeria infected groups on SD 30 (median score of 1 both in PC-E and TE, Mann–Whitney U test, P < 0.05 for group TE compared to groups NC and PC-T and for group PC-E compared to NC). In trial 2, median scores in the ceca were higher on SD 13 than in trial 1 (median score of 2 in PC-E and TE; Mann–Whitney U test, P < 0.05 compared to NC and PC-T; Table 4), corroborating the results of lesion scoring. On SD 31, group TE animals had a lower median score of 1, while no Eimeria stages were seen in any of the other groups. In duodenal scrapings, Eimeria stages were seen on SD 13 in group PC-E (median score 1) and TE (median score 2) (Mann–Whitney U test, P < 0.05 for group TE compared to all other groups; electronic supplementary material, Table S1). In general, most of the stages noticed during microscopical scoring were meronts and to a lower extent gamonts, zygotes, and oocysts. No Eimeria stages were observed in the duodenum of any of the birds of groups NC and PC-T.
Eimeria spp. oocyst counts
In both trials, animals of study groups NC and PC-T did not shed oocysts throughout the observation period. In trial 1 (Fig. 2), oocyst excretion started on SD 13 in both E. tenella-infected groups with peak excretion of 9.1 × 105 opg in group PC-E and 9.2 × 104 opg in group TE observed on SD 23. Shedding of E. tenella oocysts in trial 2 (Fig. 3) started on SD 12 in group PC-E and TE with maximum values on SD 14 (PC-E 2.4 × 105 opg, TE 3.2 × 105 opg). The excretion of E. acervulina oocysts was recorded separately (data not shown) with a comparable curve shape like shedding of E. tenella oocysts. Maximum of excretion was observed on SD 13 for group PC-E (4.9 × 106 opg) and on SD 14 for group TE (6.2 × 106 opg).
Based on all daily observed opg values, study group PC-E had a significantly higher oocyst excretion over the study period (mean 6.4 × 104 ± 1.6 × 105 opg) in trial 1 compared to co-infection group TE (mean 1.3 × 104 ± 2.1 × 104 opg) (Mann–Whitney U test, P < 0.05), whereas in trial 2, there was no significant difference in opg values of the Eimeria spp.-infected groups PC-E and TE.
T. gondii-specific antibodies
Animals of the groups NC and PC-E did not seroconvert in trial 1 whereas four chickens of group PC-T (80%) and of group TE (66.6%) became seropositive for T. gondii from SD 21 (12 days p.i.) onwards. One bird of group TE (16.6%) had a questionable result on SD 28 (19 days p.i.). In trial 2, all birds of group PC-T seroconverted on SD 21 (11 days p.i.) and chickens of the co-infected group TE seroconverted between SD 14 and SD 28 (4–18 days p.i.). Four birds of group PC-E showed very weak reactions with just one band at 43 kDa; because none of these birds recognized additional bands throughout the observation period these birds were regarded as serologically T. gondii negative, too. Results of individual animals are listed in Table 5.
PCR detection of T. gondii
For detection of T. gondii DNA, direct PCR followed by nPCR and MC-PCR were performed (Table 5). In trial 1, four out of six chickens (67.0%) of group TE and one out of five chickens (20.0%) of group PC-T were tested positive by nPCR 21 days after T. gondii oocyst infection (SD 30). Positive tested samples were the breast muscle (40.0%), brain (20.0%), heart (20.0%), and femoral muscle (20.0%). All birds of groups NC and PC-E were negative for T. gondii DNA. By means of MC-PCR, three chickens of group PC-T were additionally tested positive in the heart, brain tissue, or breast muscle. One chicken of group PC-T revealed positive nPCR and MC-PCR results.
In contrast, in trial 2, no tissue sample was tested positive in any group by nPCR after T. gondii tachyzoite infection. However, two positive birds were detected in group PC-T (33.3%) and one in group TE (20.0%) by MC-PCR for the heart or brain tissue 21 days p.i. (SD 31).
Cytokine expression profile
Cytokine expression was assessed as fold change for each study group compared to the mRNA expression levels in NC group by tissue and necropsy day for the ceca (Fig. 4), spleen (Fig. 5), and duodenum (electronic supplementary material, Fig. S3). Displayed P values were calculated by ANOVA. A summary of significantly altered data is shown in Table 6.
IFN-γ
In trial 1, IFN-γ levels were significantly increased on SD 13 in the ceca in all infected groups (PC-E, PC-T, TE) compared to NC (P < 0.05). On SD 30, group PC-E showed still significantly higher IFN-γ mRNA expression compared to group NC and PC-T (P < 0.05; Fig. 4). Group TE showed significantly increased IFN-γ on SD 30 in the spleen compared to group NC (P < 0.05).
In trial 2, significantly higher IFN-γ expression levels were found in the cecal tissue on SD 13 and SD 31 in the groups PC-E and TE (33 fold changes) compared to PC-T and NC (P < 0.05; Fig. 4). Additionally, there were significantly increased expression levels in the duodenum in groups PC-E and TE compared to NC and PC-T and in PC-T compared to NC (SD 13, P < 0.05). On SD 31, group TE showed still significantly higher levels compared to groups NC and PC-T (P < 0.05; electronic supplementary material, Fig. S3). In the spleen tissue, significantly higher expression levels were observed on SD 13 in group PC-T compared to PC-E (P < 0.05; Fig. 5).
IL-12
No significant group differences were seen in trial 1 regarding IL-12 mRNA expression levels. However, in trial 2, significantly higher concentrations were found in ceca in groups PC-E and TE compared to those determined for NC and PC-T (P < 0.05) on SD 13 (Fig. 4). Additionally, duodenum and spleen displayed significantly higher expression levels in group PC-E compared to PC-T (P < 0.05) on SD 13 (electronic supplementary material, Fig. S3; Fig. 5).
TNF-α
In trial 1, both E. tenella-infected groups PC-E and TE showed significantly higher TNF-α mRNA expression levels on SD 13 in the spleen compared to group NC (P < 0.05; Fig. 5). There were no statistically significant differences between any of the groups in the ceca or duodenum.
IL-10
In trial 1, IL-10 expression levels in the ceca of group PC-E animals were statistically significantly increased on SD 30 (Fig. 4) in comparison to all other groups (P < 0.05). No significant differences were detected in spleen tissue. In trial 2, both Eimeria spp.-infected groups (PC-E, TE) showed significantly higher IL-10 mRNA expression in ceca and duodenum on SD 13 than group NC and PC-T (P < 0.05; Fig. 4; electronic supplementary material, Fig. S3). Furthermore, groups PC-E and TE had significantly higher IL-10 expression levels in the spleen than NC animals (SD 13, P < 0.05; Fig. 5). On SD 31, only for ceca, significantly higher expression levels were measured in group PC-E compared to NC and group TE compared to NC and PC-T (P < 0.05).
Discussion
Co-infections in chickens with the widespread parasites T. gondii and E. tenella can be considered as putatively common events in the field. Nonetheless, no studies about T. gondii and Eimeria spp. co-infections in chickens are available to the best of our knowledge. One report of Mason et al. (2015) described that lapine T. gondii infection was associated with relatively heavy infections by Eimeria stiedae in wild rabbits in Scotland.
Innate as well as adaptive immune responses play an important role during infections with apicomplexan parasites (Yun et al. 2000; Gaddi and Yap 2007). Therefore, the aim of our current study was to evaluate the influence of both parasites on the host reaction and to investigate potential interactions in the course of infection.
In the present study, we verified the validity of T. gondii infection indirectly by T. gondii-specific antibodies (immunoblot) and directly by parasite DNA detection using nPCR and MC-PCR. In trial 1, one of the chickens of each of the groups PC-T and TE revealed negative results by immunoblot and PCR and was excluded from statistical analysis. Detectable antibody levels were observed earliest 4 days p.i. in co-infected birds of trial 2 and the other seropositive chickens between 11 and 19 days p.i. Similar results were observed by Yan et al. (2010) and Dubey et al. (1993) who detected T. gondii-specific antibodies within 2 weeks p.i. by ELISA and MAT. A scientific report from the European Food Safety Authority (EFSA) describes antibody response to T. gondii within 2 to 3 weeks p.i. (Opsteegh et al. 2016). We detected mainly antigenic bands with molecular weights of 20 kDa (tachyzoite surface antigen, SAG2 (P22), (Prince et al. 1990), 30 kDa (major tachyzoite surface antigen, SAG1 (P30), Burg et al. 1988), 35 kDa (P35, Aubert et al. 2000), and 43 kDa (SAG3 (P43), Cesbron-Delauw et al. 1994). However, DNA detection was successful only in 55.0% of seropositive chickens. Similar results were reported by others with 53.4 or 33.3% of serologically positive chickens being found PCR positive (Opsteegh et al. 2016; Geuthner et al. 2014).
In total, 22.1% of all tissue samples from infected chickens were PCR positive after around 3 weeks of infection. Geuthner et al. (2014) described even lower DNA detection rates of 2.1% in tissues from experimentally infected chickens. The detection limit of the performed MC-PCR was 102 parasite genomes per 100 g sample.
The reason for T. gondii DNA detection failure in many chicken samples might be explained by the sparse and inhomogeneous distribution of tissue cysts. For positive chickens, a concentration of only one tissue cyst per 50 g tissue was expected (Opsteegh et al. 2016). Generally, this is critical especially for parasite detection by nPCR, as only a limited amount of tissue (25 mg) can be used for DNA extraction and only a subset of this sample is used for the following PCR reaction. It was also suggested that the tissue cyst load changes over time and DNA detection may be more difficult in chickens recently subjected to primary infection (Opsteegh et al. 2016). This is in accordance to Yan et al. (2010) who detected T. gondii DNA by PCR earliest 21 days p.i. in chickens and our finding that T. gondii infection could not be always confirmed in the tissue of our seropositive chickens. The tissue most likely to produce positive results was the heart muscle, followed by the breast muscle, brain, and femoral muscle. This is in concordance with previous findings in chickens (Opsteegh et al. 2016; Dubey 2010; Yan et al. 2010). T. gondii DNA concentration in the tissues appears to be generally low and false-negative results in an unknown proportion of the tissue samples cannot be excluded, even if highly sensitive methods like MC-PCR are applied. However, we believe that the combination of serological and molecular data is suited to sufficiently confirm successful invasion by T. gondii for both trials, at least for those chickens which were considered for statistical analysis.
Successful Eimeria infection was confirmed by oocyst excretion, lesion scores and evaluation of mucosal scrapings. Typical depression in body weight gain was recorded 7 days p.i. for both groups PC-E and TE. This is not surprising and reflects the acute disease caused by E. tenella (Williams 1996). The more pronounced clinical findings in trial 2 were possibly due to additional E. acervulina infection, although E. acervulina by itself is considered to be less pathogenic than E. tenella (McDougald 1998; Soulsby 1982). Altogether, lower body weight gain was obviously related to Eimeria infection with no or only marginal contribution by T. gondii.
Interestingly, in trial 1, oocyst excretion was higher in group PC-E compared to co-infected group TE. The peak of excretion was observed about 1 day after two prepatent period intervals indicating that there was a reinfection of birds inside the cages via feces residuals. Higher excretion after challenge infection may be attributed to the early challenge time point. In trial 2, E. tenella oocyst excretion expectedly started 5 to 6 days p.i. and followed a similar pattern in both Eimeria mono- or co-infected groups PC-E and TE. It can be assumed that T. gondii effects on the manifestation of E. tenella infection depend on the route of infection. We considered this by comparing co-infected chickens either orally infected with T. gondii oocysts (trial 1) or intramuscularly infected with tachyzoites (trial 2). It appeared that oral T. gondii infection somewhat depressed Eimeria replication whereas this was not the case following parenteral infection. A study by Cui et al. (2016) demonstrated higher oocyst excretion of E. tenella in chickens co-infected with subgroup J avian leucosis virus (ALV-J) than in Eimeria mono-infected chickens. In addition, significantly lower body weights and significantly higher cecal lesion scores in co-infected birds were detected, suggesting acceleration of pathogenicity of E. tenella, probably due to immunosuppression exerted by avian virus infection (Cui et al. 2016). An increase of pathogenic effects has also been reported in E. tenella and reticuloendotheliosis virus (REV) co-infected chickens (Motha and Egerton 1984). For T. gondii co-infections, Santiago et al. (1999) discovered that this parasite inhibits antigen-specific Th2 immune response and tissue inflammation in BALB/C mice co-infected with Leishmania major. Reduction of Th2 responses has also been reported by Liesenfeld et al. (2004) in C57BL/6 mice co-infected with Nippostrongylus brasiliensis as well as modulations in inflammatory response and clinical outcome in BALB/C mice suffering from Helicobacter felis–T. gondii co-infections (Stoicov et al. 2004). In contrast, no differences in dynamics of specific serum antibody development in T. gondii mono-infected and T. gondii–Trichinella spiralis co-infected pigs were detected (Bokken et al. 2012).
Regarding E. tenella-specific lesions in the ceca, there was no significant difference between the co-infected group TE and the mono-infected group PC-E in both trials. However, we detected more Eimeria meronts by microscopical scoring (Table 4) at most necropsies in the co-infected group as compared to PC-E although this observation was not statistically evident (P > 0.05, Mann–Whitney U test). It appears that T. gondii infection has a boosting or beneficial influence on the (asexual) E. tenella reproduction but on a level that was not related to more severe cecal lesions. Strikingly oocyst excretion was similar in mono- and co-infected groups. In contrast, studies on Eimeria spp.- and Clostridium perfringens-co-infected chickens by Park et al. (2008) and Bangoura et al. (2014) demonstrated more severe intestinal lesions in the co-infected groups than in birds only infected with Eimeria spp. Giambrone et al. (1977) described more severe enteric lesions due to Eimeria infection in the presence of infectious bursal disease virus infection. Similar results were reported by Cui et al. (2016) in specific pathogen-free chickens co-infected with E. tenella and ALV-J. In our study, systemic infection with another apicomplexan parasite did not induce more severe lesions in the gut, although a tendency to higher numbers of Eimeria meronts was observed in the co-infected group. It may be hypothesized that the observed increase in parasite reproduction was too low to induce obvious increase in tissue lesions and may be more relevant in situations of high infection pressure. However, this assumption needs to be addressed in further studies.
Eimeriosis induces a cell-mediated as well as a humoral immune response in poultry (Yun et al. 2000; Daszak 1999). The immune reaction of chickens to T. gondii infection is less studied. It is known that not only, e.g., macrophages, monocytes, and dendritic cells play an important role in the control of T. gondii, but they also support parasite reproduction and dissemination in the host (Jones et al. 1975; Quéré et al. 2013; Malkwitz et al. 2013). IFN-γ (Laurent et al. 2001; Rothwell et al. 2000) as well as IL-10 upregulation (Rothwell et al. 2004; Haritova and Stanilova 2012) were observed in chicken intestine and spleen during Eimeria infection representing activation of local and systemic Th1 and Th2 immune response.
Lesions during intestinal eimeriosis not only are caused by direct host cell damage but are also attributable to inflammation (Michael and Hodges 1971; Lillehoj and Trout 1996), i.e., host reaction. Immune reaction against toxoplasmosis like the Th1 response in mammals (Dupont et al. 2012) may contribute not only to immunoprotection but also to pathology of the disease. Cytokine expression measurement is a valuable tool to evaluate immune response and inflammatory reaction to parasitic infections in chickens (Ovington et al. 1995).
Investigations of Park et al. (2008) and Bangoura et al. (2014) into cytokine responses in chickens co-infected with Eimeria spp. and C. perfringens resulted in increase of both Th1 (IFN-γ and IL-12) and Th2 (IL-10) immune response with elevated cytokine mRNA expression levels in co-infected birds compared to mono-infection with Eimeria. Results of our study indicate an increase of Th1-related cytokines like IFN-γ, IL-12, and TNF-α especially in the ceca of Eimeria spp.-infected animals whereas Th2-related cytokine IL-10 expression was elevated in Eimeria mono-infected as well as in co-infected chickens. Parenteral and oral infection with T. gondii tachyzoites or oocysts, respectively, similarly affected cytokine expression in the ceca and spleen. This is assumed to reflect generalized infection of the host irrespective of the route of infection. This is in accordance with previous studies in turkey (Zöller et al. 2013; Bangoura et al. 2013). Since the effect of mono-infection with T. gondii turned out to be marginal throughout, the observed immune reaction in co-infected chickens is obviously mainly directed against Eimeria. Immune reaction in the ceca was more pronounced compared to spleen or the duodenum. Local inflammatory reaction in the ceca complements the cytokine expression data. Typical cecal lesions were particularly seen when highest Th1-cytokine upregulation was observed.
In summary, it can be stated that none of the infected chickens developed symptoms related to clinical toxoplasmosis. The relatively small number of T. gondii positive tested birds indicates a low concentration of T. gondii in the examined tissues. Clinical Eimeria infection with high oocyst excretion and typical intestinal lesions occurred mainly in trial 2 when inoculum additionally contained E. acervulina.
Eimeria merogony seemed to be more pronounced in co-infected chicken, although this was not statistically evident. Host reaction to concurrent infections with both parasites like pathology and immune response did not differ substantially from Eimeria mono-infections. Vice versa, Eimeria displayed no effect on the quantity of T. gondii positive tissue samples or on the clinical course of T. gondii infection. Although this first study about co-infection with two common apicomplexan parasites in chickens did not reveal major mutual effects, it appears reasonable to consider such interactions in further experimental setups.
References
Alnassan AA, Shehata AA, Kotsch M, Schrödl W, Krüger M, Daugschies A, Bangoura B (2013) Efficacy of early treatment with toltrazuril in prevention of coccidiosis and necrotic enteritis in chickens. Avian Pathol 42:482–490
Aubert D, Maine GT, Villena I, Hunt JC, Howard L, Sheu M, Brojanac S, Chovan LE, Nowlan SF, Pinon JM (2000) Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol 38:1144–1150
Azevedo SS, Pena HF, Alves CJ, Guimaraes Filho AA, Oliveira RM, Maksimov P, Schares G, Gennari SM (2010) Prevalence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in swine from Northeastern Brazil. Rev Bras Parasitol Vet 19:80–84
Bangoura B, Zöller B, Koethe M, Ludewig M, Pott S, Fehlhaber K, Straubinger RK, Daugschies A (2013) Experimental Toxoplasma gondii oocyst infections in turkeys (Meleagris gallopavo). Vet Parasitol 196(3–4):272–277
Bangoura B, Alnassan AA, Lendner M, Shehata AA, Krüger M, Daugschies A (2014) Efficacy of an anticoccidial live vaccine in prevention of necrotic enteritis in chickens. Exp Parasitol 145:125–134
Bokken GC, van Eerden E, Opsteegh M, Augustijn M, Graat EA, Franssen FF, Görlich K, Buschtöns S, Tenter AM, van der Giessen JW, Bergwerff AA, van Knapen F (2012) Specific serum antibody responses following a Toxoplasma gondii and Trichinella spiralis co-infection in swine. Vet Parasitol 184(2–4):126–132
Burg JL, Perelman D, Kasper LH, Ware PL, Boothroyd JC (1988) Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol 141:3584–3591
Cesbron-Delauw MF, Tomavo S, Beauchamps P, Fourmaux MP, Camus D, Capron A, Dubremetz JF (1994) Similarities between the primary structures of two distinct major surface proteins of Toxoplasma gondii. J Biol Chem 269(23):16217–16222
Cui N, Wang Q, Shi W, Han L, Wang J, Ma X, Li H, Wang F, Su S, Zhao X (2016) Synergy of subgroup J avian leukosis virus and Eimeria tenella to increase pathogenesis in specific-pathogen-free chickens. Vet Immunol Immunopathol 177:42–47
Daszak P (1999) Zoite migration during infection: parasite adaptation to host defences. Parasitol Today 15(2):67–72
De Boever S, Vangestel C, De Backer P, Croubels S, Sys SU (2008) Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet Immunol Immunopathol 122(3–4):312–317
Dubey JP (2008) The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol 55:467–475
Dubey JP (2010) Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses and Public Health 57:60–73
Dubey JP, Ruff MD, Camargo ME, Shen SK, Wilkins GL, Kwok OC, Thulliez P (1993) Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am J Vet Res 54:1668–1672
Dupont CD, Christian DA, Hunter CA (2012) Immune response and immunopathology during toxoplasmosis. Semin Immunopathol 34(6):793–813
Frölich S, Entzeroth R, Wallach M (2012) Comparison of protective immune responses to apicomplexan parasites. J Parasitol Res 2012:852591
Gaddi PJ, Yap GS (2007) Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol 85(2):155–159
Geuthner AC, Koethe M, Ludewig M, Pott S, Schares G, Daugschies A, Bangoura B (2014) Persistence of Toxoplasma gondii tissue stages in poultry over a conventional fattening cycle. Parasitology 141:1359–1364
Giambrone JJ, Anderson WI, Reid WM, Eidson CS (1977) Effect of infectious bursal disease on the severity of Eimeria tenella infections in broiler chicks. Poult Sci 56:243–246
Haritova AM, Stanilova SA (2012) Enhanced expression of IL-10 in contrast to IL-22B mRNA in poultry with experimental coccidiosis. Exp Parasitol 132:378–382
Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP (2006) Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet Immunol Immunopathol 114:209–223
Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172:1561–1566
Huskinson J, Stepick-Biek PN, Araujo FG, Thulliez P, Suzuki Y, Remington JS (1989) Toxoplasma antigens recognized by immunoglobulin G subclasses during acute and chronic infection. J Clin Microbiol 27:2031–2038
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36
Jones TC, Len L, Hirsch JG (1975) Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med 141:466–482
Kaneto CN, Costa AJ, Paulillo AC, Moraes FR, Murakami TO, Meireles MV (1997) Experimental toxoplasmosis in broiler chicks. Vet Parasitol 69(3–4):203–210
Koethe M, Straubinger RK, Pott S, Bangoura B, Geuthner AC, Daugschies A, Ludewig M (2015) Quantitative detection of Toxoplasma gondii in tissues of experimentally infected turkeys and in retail turkey products by magnetic-capture PCR. Food Microbiol 52:11–17
Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M (2001) Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun 69:2527–2534
Liesenfeld O, Dunay IR, Erb KJ (2004) Infection with Toxoplasma gondii reduces established and developing Th2 responses induced by Nippostrongylus brasiliensis infection. Infect Immun 72(7):3812–3822
Lillehoj HS, Trout JM (1996) Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev 9:349–360
Long PL, Rowell JG (1958) Counting oocysts of chicken coccidia. Lab Pract 7:515–518
Lunde MN, Jabobs L (1983) Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol 69:806–808
Lundén A, Thebo P, Gunnarsson S, Hooshmian-Rad P, Tauson R, Uggla A (2000) Eimeria infections in litter-based, high stocking density systems for loose-housed laying hens in Sweden. Br Poult Sci 41:440–447
Malkwitz I, Berndt A, Daugschies A, Bangoura B (2013) Long-term investigations on Toxoplasma gondii-infected primary chicken macrophages. Parasitol Res 112(9):3115–3122
Mason S, Dubey JP, Smith JE, Boag B (2015) Toxoplasma gondii co-infection with diseases and parasites in wild rabbits in Scotland. Parasitology 142(11):1415–1421
McDougald LR (1998) Intestinal protozoa important to poultry. Poult Sci 77:1156–1158
McDougald L, Fitz-Coy S (2013) Coccidiosis: protozoal infections. In: Swayne DE (ed) Diseases of poultry, 13th edn. Blackwell, New York, pp 1148–1166
Michael E, Hodges RD (1971) The pathogenic effects of Eimeria acervulina: a comparison of single and repeated infections. Vet Rec 89(12):329–333
Motha MX, Egerton JR (1984) Influence of reticuloendotheliosis on the severity of Eimeria tenella infection in broiler chickens. Vet Microbiol 9:121–129
Nang NT, Lee JS, Song BM, Kang YM, Kim HS, Seo SH (2011) Induction of inflammatory cytokines and Toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet Res 42:64
Opsteegh M, Schares G, Blaga R, van der Giessen J, on behalf of the consortium (2016) Experimental studies of Toxoplasma gondii in the main livestock species (GP/EFSA/BIOHAZ/2013/01) Final report. EFSA Support Public 2016 EN-995:161
Ovington KS, Alleva LM, Kerr EA (1995) Cytokines and immunological control of Eimeria spp. Int J Parasitol 25(11):1331–1351
Park SS, Lillehoj HS, Allen PC, Park DW, FitzCoy S, Bautista DA, Lillehoje EP (2008) Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis 52:14–22
Pop L, Györke A, Tǎbǎran AF, Dumitrache MO, Kalmár Z, Magdaş C, Mircean V, Zagon D, Balea A, Cozma V (2015) Effects of artemisinin in broiler chickens challenged with Eimeria acervulina, E. maxima and E. tenella in battery trials. Vet Parasitol 214(3–4):264–271
Prince JB, Auer KL, Huskinson JF, Parmley SF, Araujo FG, Remington JS (1990) Cloning, expression and cDNA sequence of surface antigen p22 from Toxoplasma gondii. Mol Biochem Parasitol 43:97–106
Quéré P, Pierre J, Hoang MD, Esnault E, Domenech J, Sibille P, Dimier-Poisson I (2013) Presence of dendritic cells in chicken spleen cell preparations and their functional interaction with the parasite Toxoplasma gondii. Vet Immunol Immunopathol 153(1–2):57–69
Rose ME (1987) Immunity to Eimeria infections. Vet Immunol Immunopathol 17(1–4):333–343
Rothwell L, Muir W, Kaiser P (2000) Interferon-gamma is expressed in both gut and spleen during Eimeria tenella infection. Avian Pathol 29:333–342
Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P (2004) Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol 173:2675–2682
Santiago HC, Oliveira MA, Bambirra EA, Faria AM, Afonso LC, Vieira LQ, Gazzinelli RT (1999) Coinfection with Toxoplasma gondii inhibits antigen-specific Th2 immune responses, tissue inflammation, and parasitism in BALB/c mice infected with Leishmania major. Infect Immun 67(9):4939–4944
Soulsby EJL (1982) Helminths, arthropods and protozoa of domesticated animals, 7th edn. Lea & Febiger, Philadelphia, p 634
Stoicov C, Whary M, Rogers AB, Lee FS, Klucevsek K, Li H, Cai X, Saffari R, Ge Z, Khan IA, Combe C, Luster A, Fox JG, Houghton J (2004) Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J Immunol 173(5):3329–3336
Tang X et al (2016) Transgenic Eimeria tenella as a vaccine vehicle: expressing TgSAG1 elicits protective immunity against Toxoplasma gondii infections in chickens and mice. Sci. Rep 6:29379. https://doi.org/10.1038/srep29379
Williams RB (1996) The ratio of the water and food consumption of chickens and its significance in the chemotherapy of coccidiosis. Vet Res Commun 20:437–447
Yan C, Yue CL, Yuan ZG, Lin RQ, He Y, Yin CC, MJ X, Song HQ, Zhu XQ (2010) Molecular and serological diagnosis of Toxoplasma gondii infection in experimentally infected chickens. Vet Parasitol 173:179–183
Yun CH, Lillehoj HS, Lillehoj EP (2000) Intestinal immune responses to coccidiosis. Dev Comp Immunol 24(2–3):303–324
Zintl A, Halova D, Mulcahy G, O’Donovan J, Markey B, DeWaal T (2009) In vitro culture combined with quantitative TaqMan PCR for the assessment of Toxoplasma gondii tissue cyst viability. Vet Parasitol 164:167–172
Zöller B, Koethe M, Ludewig M, Pott S, Daugschies A, Straubinger RK, Fehlhaber K, Bangoura B (2013) Tissue tropism of Toxoplasma gondii in turkeys (Meleagris gallopavo) after parenteral infection. Parasitol Res 112:1841–1847
Zou J, Huang XX, Yin GW, Ding Y, Liu XY, Wang H, Chen QJ, Suo X (2011) Evaluation of Toxoplasma gondii as a live vaccine vector in susceptible and resistant hosts. Parasit Vectors 4:168
Acknowledgements
The authors would like to thank all members of the Institute of Parasitology, University Leipzig, especially Dr. Alaa Alnassan and Dr. Ahmed Thabet, for the consistent technical and practical support. We acknowledge the excellent technical assistance of Andrea Bärwald.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The in vivo trials have been conducted in compliance with the current European and national legal requirements under registration and permission by the responsible authority (Landesdirektion Leipzig, file no. TVV 59/13).
Electronic supplementary material
ESM 1
(DOCX 400 kb).
Rights and permissions
About this article
Cite this article
Hiob, L., Koethe, M., Schares, G. et al. Experimental Toxoplasma gondii and Eimeria tenella co-infection in chickens. Parasitol Res 116, 3189–3203 (2017). https://doi.org/10.1007/s00436-017-5636-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5636-2