Abstract
Turkeys are known to be natural hosts for the zoonotic protozoan parasite Toxoplasma gondii. The objective of the present study was to gain further knowledge of possible predilection sites of T. gondii infection in this species after parenteral application of tachyzoites. A total of 38 turkeys were infected with different doses of T. gondii tachyzoites. Birds were killed either 6 to 8 or 10 to 12 weeks after the experimental infection. Fourteen different tissues per bird were investigated by a nested polymerase chain reaction (PCR) for the presence of the parasites’ DNA. T. gondii DNA was found in any type of tissue analysed; in 86.1 % of all infected birds, at least one sample was tested positive. Over all intravenously infected birds, 15.4 % of all analysed samples contained T. gondii DNA. Most frequently affected tissues were liver (43.3 % positive samples), breast muscle (26.7 % positive samples) and heart (20.0 % positive samples), while the brain was less frequently positive (6.7 %). The number of positive tissues varied from zero to seven tissues per animal with at least one T. gondii-positive edible tissue sample in 80 % of all intravenously infected birds. Still, the results did not indicate defined target tissues or a cyst distribution pattern. Nonetheless, edible organs were most frequently parasitised. The number of positive findings did not differ between the early and the late examination time points. Therefore, a persistence of the tissue stages until the end of the study (12 weeks after infection) is concluded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii, a zoonotic protozoan parasite, has the ability to infect all warm-blooded animals including humans and occurs ubiquitously worldwide. Reportedly, up to one third of the human population is infected with this parasite (Tenter et al. 2000). In many cases, the course of disease is asymptomatic; however, an infection with T. gondii may cause severe diseases in humans such as encephalitis, neurological disorders, chorioretinitis as well as abortions (Montoya and Liesenfeld 2004; da Silva and Langoni 2009).
Human infections often result from the uptake of bradyzoite-containing cysts from raw or undercooked meat of infected animals (Kijlstra and Jongert 2009).
The formation of these tissue cysts occurs regularly in animals including birds whereas quantity, localization and duration of persistence vary among the different species.
Regarding food-delivering animals, toxoplasmosis in turkeys is quite underexplored up to date. In previous studies, turkeys were infected experimentally with either tachyzoites or oocysts of different T. gondii strains (Drobeck et al. 1953; Dubey et al. 1993a; Sedlák et al. 2000) and viable tissue cysts were found up to 9 weeks after infection. In combination with several reports of high seroprevalence rates, e.g. 10.0 to 70.6 % in the USA (Lindsay et al. 1994; Quist et al. 1995), 29.4 to 59.5 % in Egypt (El-Massry et al. 2000; Harfouch and Tahoon 2010) or up to 18.4 % in Germany (Koethe et al. 2011), it can be assumed that turkeys might play a role in the transmission of T. gondii to humans.
Turkey meat is consumed at a consistently high level, and, lately, nonheated turkey meat products such as raw sausages or dry-cured turkey ham were introduced onto the market in different countries.
However, the potential consumer’s risk to acquire a T. gondii infection via turkey meat and its products has not been explored sufficiently so far. Therefore, we designed this study to determine the T. gondii cyst presence in 14 different organs and tissues after T. gondii infection. Dependence of tissue cyst formation on infectious dose and time point after infection with T. gondii tachyzoites, respectively, was investigated.
This artificial infection model using tachyzoites to infect the turkeys was chosen to characterise the maximum effects (worst case scenario) that can be induced by the parasite when it is applied parenterally bypassing the intestinal barrier. The two time points of examination were chosen to determine if the parasite is able to persist over a time span of more than 9 weeks after infection. To our knowledge, data referring to longer observation periods are not available, though turkeys are regularly kept for longer periods, e.g. for 15 to 17 weeks (females) or 21 to 22 weeks (males), respectively, in European intensive rearing systems (Mitterer-Istyagin et al. 2011).
Material and methods
Study animals and husbandry
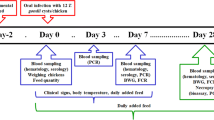
A total of 38 turkeys (British United Turkey Big 6, 29 male and 9 female birds) were used for the experiments and kept in four batches under identical conditions. The experiments complied with the current legal regulations and were approved by the responsible local authority (trial no. TVV 50/07, Landesdirektion Leipzig, Germany). Animals were stabled on the first day after hatching and raised on solid floor with straw bedding at the Institute of Parasitology, University of Leipzig, Germany.
The birds were kept groupwise and had free access to water and commercial poultry feed pellets (deuka Wild-und Ziergeflügelfutter, Deutsche Tiernahrung Cremer, Duesseldorf, Germany; without anticoccidials) supplemented during the first 6 weeks with skim milk powder and minerals (Korvimin ZVT + Reptil, WDT, Garbsen, Germany). Animals underwent daily health observations. The turkeys were slaughtered either 6 to 8 weeks (“early”) postinfection (p.i.) or 10 to 12 weeks (“late”) p.i. During the study, two animals died from aspergillosis and clostridiosis, respectively, and were excluded from the analysis. From all animals, blood samples were drawn directly before infection and in weekly intervals afterwards.
Serotiters specific for T. gondii were determined by a kinetic enzyme-linked immunosorbent assay as previously described (Koethe et al. 2011) to assess seroconversion. In addition, uninfected control animals were kept in parallel to each infection group in the same pen (see Table 1).
Infection
The infection material consisted of cell culture-derived tachyzoites of the genotype II strain ME49 (Lunde and Jabobs 1983) passaged on Vero cells (ECACC catalogue no. 84113001). Tachyzoites were counted light-microscopically in a Neubauer chamber at 200-fold magnification to adjust the infectious doses. After a rearing period of 4 to 8 weeks, the turkeys were infected with different doses of T. gondii tachyzoites (ranging from 1 × 106 to 3 × 107) which were suspended in approx. 0.5 ml of sterile isotonic 0.9 % sodium chloride solution (B. Braun Melsungen AG, Melsungen, Germany).
Thirty animals were inoculated intravenously (i.v.) in the vena cutanea ulnaris. Regarding the remaining six birds, tachyzoites were applied intramuscularly (i.m.) in the breast muscle (n = 3) or by a combination of i.v. and i.m. infection (n = 3) to investigate if the tissue stage distribution pattern is basically influenced by the route of tachyzoite inoculation.
The study design regarding the different infection groups is delineated in Table 1.
Sample processing
At the dissection, the following organs were sampled: breast muscle, thigh muscle, drumstick muscle, heart, liver and gizzard (altogether regarded as the edible organs) as well as the brain, lung, kidneys, spleen, proventriculus, intestine, pancreas and testicles (if applicable). Each organ (except for skeletal muscle) was homogenised in total using a commercial chopper (La Moulinette, Tefal Groupe SEB, Offenbach, Germany). For skeletal muscles, adequate samples were taken from different locations (approx. 300 g per muscle) and processed separately for each muscle in the same way.
Afterwards, the homogenates were stored in microcentrifuge tubes at −20 °C until deoxyribonucleic acid (DNA) was extracted. DNA extraction was performed using the Qiagen® Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Control samples were extracted exemplarily from uninfected animals to assess if accidental spreading of T. gondii DNA occurred during the sample preparation (called in-process controls). Therefore, samples from all organs of three infected and three uninfected birds were extracted alternately, i.e. the brain sample of an infected animal followed by the brain sample of an uninfected animal and so on. Unfortunately, some of the control animals showed seroconversion during the study (data not shown); thus, seronegative animals from similar infection trials with a similar study design were used for generation of the in-process controls.
Additionally, squash preparations of random samples (n = 70) from infected animals (edible organs and intestine) were analysed for the presence of tissue stages by light microscopy at 400-fold magnification.
DNA analysis
DNA was subjected to a conventional polymerase chain reaction (PCR) for T. gondii detection. A basic PCR was run to specifically detect the Toxoplasma B1 gene according to Jalal et al. (2004) using the primer set Tg1 (5′-AAA AAT GTG GGA ATG AAA GAG-3′) and Tg2 (5′-ACG AAT CAA CGG AAC TGT AAT-3′) amplifying a 469-bp fragment. In order to enhance sensitivity, a subsequent nested PCR was performed using the primers Tgnested1 (5′-CGC TAA TGT GTT TGC ATA GG-3′) and Tgnested2 (5′-GGC ACG TCT CTT GTT CTT CT-3′) which we designed using Primer3 software (http://primer3.sourceforge.net) and tested for specificity by in silico alignments in the NCBI Basic Local Alignment Search Tool, Nucleotide BLAST® (BLASTN) database (http://www.ncbi.nlm.nih.gov/BLAST). The target fragment length for the nested PCR was 375 bp.
The master mix for the basic PCR reaction consisted of 0.5 U GoTaq® Flexi Polymerase and 1× GoTaq® Flexi Buffer (Promega GmbH, Mannheim, Germany), 200 μM of each dNTP (Fermentas, St. Leon Rot, Germany), 3 mM MgCl2, 0.4 μM of each primer (Tg1 and Tg2), and 80 to 200 ng total template DNA of the respective organ per 25 μl reaction. Nested PCR was performed accordingly with 2.5 μl of the basic PCR reaction yield as template. Both basic and nested PCR were carried out in the iCycler® Thermal Cycler (Bio-Rad, Hercules, CA, USA). The reaction conditions comprised a preheating (2 min at 94 °C) followed by 35 cycles (nested PCR, 25 cycles) of 1 min at 94 °C, 40 s at 55 °C and 1 min at 72 °C, and a single final extension step of 5 min at 72 °C. For every reaction, approx. 12 μl of the PCR product were analysed by gel electrophoresis on a 1.5 % agarose gel.
The gel was stained with ethidium bromide, and bands were visualised by ultraviolet light. Samples were evaluated as T. gondii-positive if bands were seen after either PCR reaction. Each PCR assay included, as positive control, template DNA extracted from cell-derived ME49 tachyzoites as well as water as negative control. Random in-process controls were performed for DNA extraction and PCR (see Supplementary Data, Fig. 5).
A serial dilution of a tachyzoite DNA standard was prepared, and it revealed a detection limit of a DNA equivalent of five tachyzoites per millilitre for the nested PCR (see Fig. 1). Random samples (n = 8) of positive nested PCR reactions underwent sequencing analysis (IZKF, University of Leipzig, Germany).
PCR image of sensitivity titration (T. gondii DNA equivalents: lane 1 = 5 × 106/ml, lane 2 = 5 × 105/ml, lane 3 = 5 × 104/ml, lane 4 = 5 × 103/ml, lane 5 = 5 × 102/ml, lane 6 = 50/ml, lane 7 = 5/ml; PC, positive control; NC, negative control; M, marker; band at approx. 950 bp refers to unspecific PCR products)
Statistical analysis
For statistical evaluation of the data obtained from strictly i.v. infected animals, statistical units were formed by infection dose (by categories low, medium or high) or examination time point (early vs. late). The collected data were regarded as ordinal-scaled (single organs) or metric-scaled (sums of organs).
Statistical tests were performed using the PASW Version 18.0.0 software package (SPSS Inc., Chicago, Illinois, USA). The Kolmogorov–Smirnov test for normal distribution revealed that all data were nonnormally distributed. Therefore, Kruskal–Wallis test, Friedman test and Mann–Whitney U test were applied for grouped variables. The Spearman rank correlation test was performed to explore possible correlations. Group differences and correlations with p values of less than 0.05 were defined as statistically significant.
For i.m. and i.m. plus i.v. infected animals, a descriptive comparison to i.v. infected animals was drawn due to the low case number.
Results
No clinical signs or cases of death due to toxoplasmosis were observed in any bird irrespective of the infectious dose. A serological conversion between 6 and 14 days after infection was detected for all turkeys.
In squash preparations of one liver and two breast muscle samples of three different birds, we found tissue cysts (with a diameter of approximately 50 μm) by light microscopic examination. In 31 of all 36 infected birds (86.1 %), T. gondii DNA was detected in at least one organ by PCR analysis (for individual animal data, see Supplementary Data, Table 3). Sequences of eight random samples from positive nested PCR reactions were analysed (IZKF, University of Leipzig) followed by BLASTN analysis and corresponded to the T. gondii B1 gene sequence with an identity level of 99 %. Analysis of the exemplarily performed in-process controls, i.e. extracted negative control samples, revealed that all of them were negative. Thus, there was no T. gondii DNA spreading between the analysed samples due to sample processing (see Supplementary Data, Fig. 5).
Analysis of i.v. infections
Twenty-five (83.3 %) of the strictly i.v. infected turkeys (n = 30) were tested positive for the parasite DNA. T. gondii DNA was detected in 15.4 % of all analysed samples (n = 428) and in every type of tissue (see Fig. 2). Altogether, the number of affected organs per turkey ranged between zero and seven (see Fig. 3). The liver was the most frequently infected organ (43.3 % of the birds), followed by breast muscle (26.7 % of the birds) and the heart (20.0 % of the birds; see Fig. 2).
Most (80.0 %) of the T. gondii-positive turkeys were positive in at least one edible tissue (see Fig. 4). The study design permitted the summary of the different infectious doses in infectious dose categories high, medium and low for further analysis since the distribution of the applied doses was even between both time points of examination p.i. (“early” vs. “late”; Spearman r = 0.167, p = 0.376).
Impact of the time point of examination p.i.
There was no significant difference in the number of T. gondii-positive turkeys in regard to the time point of examination; all of the ten early slaughtered i.v. infected animals and 15 of the 20 late slaughtered animals were tested positive for T. gondii.
However, the median number of parasitised tissues was significantly higher (p = 0.002) at the early examination time point with a median value of 3 (first quartile = 2; third quartile = 5.75) than at the late examination time point with a median value of 1 (first quartile = 0.75; third quartile = 2). Particularly, nonedible organs contributed to this observation, while edible organs were statistically similarly infected at both time points.
Impact of the infection dose category
The applied infectious dose of tachyzoites did not influence the total number of T. gondii-positive birds since all birds were tested positive except for three birds of the lowest infection dose of 1 × 106 tachyzoites i.v. and two birds of the highest infection dose of 2 × 107 tachyzoites i.v. (see Supplementary Data, Table 3). Equally, the number of affected tissues per animal did not depend on the infectious dose (Spearman r = 0.074, p = 0.697). Regarding the implemented dose categories, also no significant impact of the category on the frequency of infection of single organs or the total number of infected organs (p > 0.05) was seen. For the total sum of positive organs, the median value varied between the dose categories: low dose accounted for a median of 1 (first quartile = 0.75; third quartile = 2), medium dose for a median of 3 (first quartile = 1; third quartile = 3.5) and high dose for a median of 2 (first quartile = 1; third quartile = 2), respectively. Regarding the number of T. gondii-positive edible organs, all groups displayed a median value of 1 organ (first quartile = 0 for low and high dose, or 1 for medium dose, respectively; third quartile = 1.25 (low dose), 2.5 (medium dose) or 1 (high dose), respectively).
Infection rate by tissues
Analysis of samples of all tachyzoite-infected turkeys by Friedman test and Wilcoxon test revealed a statistically significantly higher T. gondii infection rate in the liver compared to the brain, proventriculus, lung, spleen and testicles (p < 0.01). The proventriculus was more often affected by the parasite than the brain (p = 0.046). All other statistical comparisons did not reveal significant differences in regard to the infection rate of distinct tissues though the percentages varied slightly (see Fig. 2). The different investigated types of skeletal muscles (thigh, drum stick and breast muscle) and the heart were tested by Spearman correlation test regarding the coincidence of positivity between them as histologically similar tissues. No correlation of the positivities of different muscles could be found.
Alternative infection routes
In all turkeys infected intramuscularly or by a combination of i.v. and i.m. infection, at least one T. gondii DNA-containing tissue sample was identified. The number of affected organs per animal ranged from one to three (i.m.) and two to five (i.v./i.m.), respectively. Regarding the infection of edible tissues, positive samples were found in both animal groups. However, breast muscle as application site was tested positive for T. gondii in one of three animals per infection route. The parasite distribution over the single organs is presented in details in Table 2.
Discussion
Toxoplasmosis in turkeys is of importance because turkeys are food-producing animals, which are often kept on straw beddings or outdoors with contact to potentially contaminated environments. Therefore, the present study was performed to investigate the tissue stage presence and distribution in infected animals under well-defined experimental conditions. Genotypic characterisation of T. gondii isolates stated a predominance of type II strains in Europe (Schares et al. 2008). Thus, we used the type II strain ME49 for infections. The artificial model based on tachyzoite infections was chosen to exclude the intestinal barrier and assess the maximal parasite effects.
We demonstrated reproducible valid infections of turkeys via parenteral infection. Clinical signs were not observed despite the relatively high dose of up to 3 × 107 tachyzoites administered. This is consistent with the findings of Dubey et al. (1993a) who demonstrated that experimentally oocyst-infected turkeys remained clinically normal but in contrast to experiences in mammalian species like mice which developed severe meningitis after infection with ten tissue cysts of the ME49 strain (Ferguson et al. 1994). Anyhow, occasional cases of death are also described in naturally infected wild and domestic turkeys (Schulte 1954; Howerth and Rodenroth 1985; Quist et al. 1995).
The infected birds in our study developed tissue stages, some of which were identified microscopically as tissue cysts. Dubey et al. (1993a) could prove infectivity of tissue stages in turkeys earlier. In our study, infectivity of the detected stages was not assessed, and not all T. gondii DNA findings can be unobjectionably correlated with the presence of tissue cysts. Nonetheless, positive PCR findings on the B1 gene are species-specific and can be directly linked with the presence of T. gondii stages (Kompalic-Cristo et al. 2007). The present study did not reveal defined target organs; any organ was tested positive at least once. Most frequently, T. gondii was detected in the liver and muscles (including heart), though only for the liver that certain predominance, compared to other tissues, could be observed regarding the detection of T. gondii DNA. While Dubey et al. (1993a) described the presence of infective stages in skeletal muscles and the heart, they did not find such stages in the brain. Quist et al. (1995) observed parasitic stages in the liver, kidney and spleen of a deceased naturally infected wild turkey. Thus, there seems to be no predictable pattern in organ affection in T. gondii-infected turkeys in contrast to well-investigated animal species like mice (Disko et al. 1978) or chicken (Beauregard et al. 1965; Dubey et al. 1993b; Kaneto et al. 1997) where the parasite shows a significant affinity to the brain.
Based on the low case number in our study, it seems that the i.m. infection directly into the breast muscle did not necessarily induce T. gondii stages at the injection site. This indicates that haematogenic distribution might precede cyst development.
Kaneto et al. (1997) found more tissues containing infective T. gondii stages after inoculation of chickens with higher doses of tachyzoites compared to lower-dose parasite inocula. In our trial, the administered dose of the parasite did not exhibit a direct influence on the number of T. gondii-positive tissues per animal. But this only refers to the infection rate of the parasite detection while we did not perform a quantitative assay to assess the amount of tissue cysts present. Additionally, we have to note that only a small portion of each tissue could be subjected to DNA isolation and PCR, which implicates that the true tissue stage prevalence may be distinctly higher than our results indicate. Nonetheless, our study demonstrated a high percentage of parasitised tissues after experimental infection of turkeys with T. gondii tachyzoites which persisted at least until 12 weeks, when the study was ended and which is within the range of a conventional fattening cycle of turkeys (Mitterer-Istyagin et al. 2011).
Conclusion
Though the chosen infection route was artificial, considering the frequent affection of edible tissues, a potential risk for consumers of undercooked turkey meat products from naturally T. gondii-infected animals may exist. In general, the ingestion of meat (products) containing viable tissue cysts is a major source of human infection (Kijlstra and Jongert 2009). Thus, a further investigation of turkey toxoplasmosis via natural infection routes will be complemented.
References
Beauregard M, Magwood SE, Bannister GL, Robertson A, Boulanger P, Ruckerbauer GM, Appel M (1965) A study of Toxoplasma infection in chickens and cats on a family farm. Can J Comp Med Vet Sci 29:286–291
da Silva RC, Langoni H (2009) Toxoplasma gondii: host-parasite interaction and behavior manipulation. Parasitol Res 105:893–898
Disko R, Braveny I, Greutélaers MT (1978) Experimental studies on the affinity of Toxoplasma gondii to various organs of mice. Zentralbl Bakteriol Orig A 242:565–571
Drobeck HP, Manwell RD, Berstein E, Dillon RD (1953) Further studies of toxoplasmosis in birds. Am J Hyg 58:329–339
Dubey JP, Camargo ME, Ruff MD, Wilkins GC, Shen SK, Kwok OC, Thulliez P (1993a) Experimental toxoplasmosis in turkeys. J Parasitol 79:949–952
Dubey JP, Ruff MD, Camargo ME, Shen SK, Wilkins GL, Kwok OC, Thulliez P (1993b) Serologic and parasitologic responses of domestic chickens after oral inoculation with Toxoplasma gondii oocysts. Am J Vet Res 54:1668–1672
El-Massry A, Mahdy OA, El-Ghaysh A, Dubey JP (2000) Prevalence of Toxoplasma gondii antibodies in sera of turkeys, chickens, and ducks from Egypt. J Parasitol 86:627–628
Ferguson DJ, Huskinson-Mark J, Araujo FG, Remington JS (1994) A morphological study of chronic cerebral toxoplasmosis in mice: comparison of four different strains of Toxoplasma gondii. Parasitol Res 80:493–501
Harfouch M, Tahoon A (2010) Seroprevalence of Toxoplasma gondii antibodies in domestic ducks, free-range chickens, turkeys and rabbits in Kafr El-Sheikh Governorate Egypt. J Egypt Soc Parasitol 40:295–302
Howerth EW, Rodenroth N (1985) Fatal systemic toxoplasmosis in a wild turkey. J Wildl Dis 21:446–449
Jalal S, Nord CE, Lappalainen M, Evengård B, ESCMID Study Group on Toxoplasmosis (2004) Rapid and sensitive diagnosis of Toxoplasma gondii infections by PCR. Clin Microbiol Infect 10:937–939
Kaneto CN, Costa AJ, Paulillo AC, Moraes FR, Murakami TO, Meireles MV (1997) Experimental toxoplasmosis in broiler chicks. Vet Parasitol 69:203–210
Kijlstra A, Jongert E (2009) Toxoplasma-safe meat: close to reality? Trends Parasitol 25:18–22
Koethe M, Pott S, Ludewig M, Bangoura B, Zöller B, Daugschies A, Tenter AM, Spekker K, Bittame A, Mercier C, Fehlhaber K, Straubinger RK (2011) Prevalence of specific IgG-antibodies against Toxoplasma gondii in domestic turkeys determined by kinetic ELISA based on recombinant GRA7 and GRA8. Vet Parasitol 180:179–190
Kompalic-Cristo A, Frotta C, Suárez-Mutis M, Fernandes O, Britto C (2007) Evaluation of a real-time PCR assay based on the repetitive B1 gene for the detection of Toxoplasma gondii in human peripheral blood. Parasitol Res 101:619–625
Lindsay DS, Smith PC, Blagburn BL (1994) Prevalence and isolation of Toxoplasma gondii from wild turkeys in Alabama. J Helminthol Soc Wash 61:115–117
Lunde MN, Jabobs L (1983) Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol 69:806–808
Mitterer-Istyagin H, Ludewig M, Bartels T, Krautwald-Junghanns ME, Ellerich R, Schuster E, Berk J, Petermann S, Fehlhaber K (2011) Examinations on the prevalence of footpad lesions and breast skin lesions in B.U.T. Big 6 fattening turkeys in Germany. Part II: Prevalence of breast skin lesions (breast buttons and breast blisters). Poult Sci 90:775–780
Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet 363:1965–1976
Quist CF, Dubey JP, Luttrell MP, Davidson WR (1995) Toxoplasmosis in wild turkeys—a case-report and serologic survey. J Wildl Dis 31:255–258
Schares G, Vrhovec MG, Pantchev N, Herrmann DC, Conraths FJ (2008) Occurrence of Toxoplasma gondii and Hammondia hammondi oocysts in the faeces of cats from Germany and other European countries. Vet Parasitol 152:34–45
Schulte F (1954) Toxoplasmose – Encephalitis beim Geflügel. Dtsch Tierärztl Wochenschr 61:482–484
Sedlák K, Literák I, Vitula F, Benák J (2000) High susceptibility of partridges (Perdix perdix) to toxoplasmosis compared with other gallinaceous birds. Avian Pathol 29:563–569
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258
Acknowledgments
The authors thank the zoonosis network Toxonet01, especially the working groups of Gereon Schares (Friedrich-Loeffler-Institute, Wusterhausen, Germany) and Carsten Lüder (Institute for Medical Microbiology, Georg-August-University, Göttingen, Germany) for practical support. The work was funded by the German Ministry for Education and Research (BMBF) within the network Toxonet01 (grant 01KI0762).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zöller, B., Koethe, M., Ludewig, M. et al. Tissue tropism of Toxoplasma gondii in turkeys (Meleagris gallopavo) after parenteral infection. Parasitol Res 112, 1841–1847 (2013). https://doi.org/10.1007/s00436-013-3337-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3337-z